Rational design of a novel mitochondrial-targeted near-infrared fluorescent pH probe for imaging in living cells and in vivo†
Peng Wang*,
Jinxin Huang and
Yueqing Gu*
Department of Biomedical Engineering, School of Engineering, China Pharmaceutical University, Nanjing, 210009, PR China. E-mail: wangpeng159seu@hotmail.com
First published on 3rd October 2016
Abstract
Intracellular pH plays an important role in cellular behaviors and pathological conditions. Sensing and monitoring intracellular pH changes in living systems by near-infrared fluorescence probes is essential. In this study, a novel mitochondrial-targeted near-infrared fluorescent pH probe was rationally designed and synthesized. Based on the protonation/deprotonation of the hydroxyl and sulfonic group on the hemicyanine skeleton, probe NIR-F1 exhibits excellent sensitivity and selectivity to pH. Moreover, NIR-F1 could target the mitochondria and monitor pH changes in living cells and living mice. The results indicate that NIR-F1 is a reliable NIR fluorescent pH probe in vivo, giving it potential for biological applications.
1. Introduction
Intracellular pH is a critical parameter that relates closely to many biological processes including cell metabolism, apoptosis, proliferation, ion transport and homeostasis, enzyme activity, and endocytosis.1–4 The maintenance of an appropriate pH inside individual cell plays an important role in their normal physiology. However, abnormal extracellular/intracellular pH changes would influence the progression of various pathophysiologic states and cause diseases such as Alzheimer's disease, cancer, and cardiopulmonary problems.5–7 The cellular pH gradient in tumors is usually different from normal tissue, which can be employed as a general tumor biomarker and provide an exploitable opportunity for the treatment of cancer.8 Therefore, the monitoring and sensing intracellular pH values could provide essential information for further understanding the pathophysiologic processes closely relevant to pH.Today many detection methods have been used to measure pH value, including acid–base indicator titration and potentiometric titration.9,10 Of the several methods available to determine pH, fluorescence probes has several advantages including rapid response time, high signal-to-noise ratio, noninvasiveness, and excellent pH sensitivity. A number of molecular probes for cellular pH sensing have been developed in the past.11,12 However, the above mentioned fluorescent probes suffered from short wavelength emission and are not applicable for use in biological systems. Compared to most other conventional fluorescent probes, those that rely on near-infrared (NIR) fluorescence possess unique advantages for tracing molecular processes in vitro and in vivo.13–15 Because complex biological samples usually contain endogenous components that will produce a high autofluorescence background, and thus make the common fluorescent probes ineffective for assaying biotargets in these systems without sample pretreatment. NIR fluorescence makes it easy to discriminate the probe signal from the background fluorescence around 400–500 nm. At present, few NIR fluorescent probe has been developed for the sensing intracellular pH, and most of the fluorescent probes respond to neutral pH ranging from 6 to 8.16–18 Thus, the development of a high sensitive NIR fluorescent probe with wide range for monitoring intracellular pH is still in demand.
In this work, we designed and developed probe NIR-F1, a new NIR fluorescent probe for monitoring intracellular pH. Hydroxyl and sulfonic groups were employed as the two pH sensing sites and combined with hemicyanine skeleton. With the variation of the pH from acidity to alkalinity, the probe NIR-F1 exhibits turn-on fluorescence for the enhancement of the ICT efficiency. In addition, the semiheptamethine can also be served as a mitochondria-targeted group.19 And we presented the application of NIR-F1 in pH detection and imaging various pH environments in biological samples. The results indicated that NIR-F1 exhibited high sensitivity and excellent selectivity, and was capable of monitoring pH changes in real time. Moreover, NIR-F1 was mitochondria targeted and could sense mitochondrial pH in living cells. The results also showed that NIR-F1 could image pH changes in living mice with perfect tissue penetration and without interference of bioautofluorescence.
2. Experimental section
2.1 Materials and instruments
All reagents and solvents were used as received without further purification. Compound IR-820 (sodium 4-(2-((E)-2-((E)-2-chloro-3-((E)-2-(1,1-dimethyl-3-(4-sulfonatobutyl)-1,3-dihydro-2H-benzo[e]indol-2-ylidene)ethylidene)cyclohex-1-en-1-yl)vinyl)-1,1-dimethyl-1H-benzo[e]indol-3-ium-3-yl)butane-1-sulfonate) was achieved by previous report.20 Thin-layer chromatography (TLC) analysis was performed on silica gel plates, and column chromatography was conducted over silica gel (mesh 200–300, Qingdao Ocean Chemicals). UV-vis spectrophotometer (JH754PC, Shanghai, China) was used for the absorption measurements. Perkin Elmer LS55 was utilized for fluorescence spectra detection. Laser confocal fluorescence microscopy (FluoView™, FV1000, Olympus, Japan) was used for cell imaging. 1H-NMR spectrum was taken on Bruker Advance 300 MHz spectrometer, δ values are in ppm relative to TMS. Mass data (ESI) were recorded by quadruple mass spectrometry. Due to the somewhat solubility of NIR-F1 series in water, stock solution (1 mM) of the probe was prepared by dissolving NIR-F1 series in DMSO. Different pH buffer solutions were prepared by using 50 mM potassium hydrogen phthalate (for pH 2.0–6.0 buffer), and potassium dihydrogen phosphate (for pH 6.1–11.0 buffer). The pH was adjusted by adding 0.1 M NaOH or 0.1 M HCl solutions. For the selectivity experiments, H2O2 was delivered from 30% aqueous solution.2.2 Synthesis and structural characterization of NIR-F1
To a flask attached with a condenser were added compound IR-820 (212 mg, 0.25 mmol), resorcinol (340 mg, 2.0 mmol), K2CO3 (280 mg, 2.6 mmol), and acetonitrile (5 mL) under N2 protection. The mixture was heated to 50 °C for 4 h, resulting in a blue solution. Solvents were removed on a rotary evaporator. The crude product was purified by silica gel chromatography (CH2Cl2/MeOH = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) to afford 55 mg (41%) of (E)-4-(2-(2-(6-hydroxy-2,3-dihydro-1H-xanthen-4-yl)vinyl)-1,1-dimethyl-1H-benzo[e]indol-3-ium-3-yl)butane-1-sulfonate (NIR-F1) as a blue solid. 1H-NMR (DMSO-d6, 400 MHz) δ: 10.81 (s, 1H), 8.63 (d, J = 15 Hz, 1H), 8.34 (t, 1H), 8.16 (m, 2H), 7.99 (d, J = 8.6 Hz, 1H), 7.73 (t, 1H), 7.62 (t, 1H), 7.48 (t, 2H), 7.00 (s, 1H), 6.83 (d, J = 6.5 Hz, 1H), 6.57 (d, J = 15 Hz, 1H), 4.53 (m, 2H), 2.67–2.73 (m, 4H), 2.57–2.61 (m, 2H), 1.97 (s, 6H), 1.84–1.89 (m, 2H), 1.24–1.25 (m, 4H). ESI-MS: 554.2 [M − H]−.
10) to afford 55 mg (41%) of (E)-4-(2-(2-(6-hydroxy-2,3-dihydro-1H-xanthen-4-yl)vinyl)-1,1-dimethyl-1H-benzo[e]indol-3-ium-3-yl)butane-1-sulfonate (NIR-F1) as a blue solid. 1H-NMR (DMSO-d6, 400 MHz) δ: 10.81 (s, 1H), 8.63 (d, J = 15 Hz, 1H), 8.34 (t, 1H), 8.16 (m, 2H), 7.99 (d, J = 8.6 Hz, 1H), 7.73 (t, 1H), 7.62 (t, 1H), 7.48 (t, 2H), 7.00 (s, 1H), 6.83 (d, J = 6.5 Hz, 1H), 6.57 (d, J = 15 Hz, 1H), 4.53 (m, 2H), 2.67–2.73 (m, 4H), 2.57–2.61 (m, 2H), 1.97 (s, 6H), 1.84–1.89 (m, 2H), 1.24–1.25 (m, 4H). ESI-MS: 554.2 [M − H]−.
2.3 Theoretical calculations
The energy levels of the highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) were determined by the density functional theory (DFT) calculation using the Gaussian 09 software. The B3LYP exchange functional employing 6-31+G(d) basis sets was used in the calculations.2.4 Cytotoxic assay
The human cell lines MCF-7 (breast cancer cells) was purchased from American Type Culture Collection (ATCC; Manassas, VA, USA). MCF-7 cells were seeded in a 96-well plate (1 × 104 cells per well).After cultivation for 24 h, NIR-F1 (DMSO dissolve first, then added it into the cell culture medium) of different concentrations were added into the wells (n = 6) and incubated for 24 h. Then stock solution of MTT (20 μL; 5 mg mL−1) was added into each well. After 4 h incubation at 37 °C, the MTT solution was replaced with 150 μL DMSO in each well. The absorbance in each well was measured at 570 nm with a multi-well plate reader. Cell viability was calculated using the following formula: cell viability = (mean absorbance of test wells − mean absorbance of medium control wells)/(mean absorbance of untreated wells − mean absorbance of medium control well) × 100%.2.5 Cell culture and confocal fluorescence imaging
MCF-7 cells were cultured in DMEM (Invitrogen) supplemented with 10% fetal bovine serum (FBS, Hyclone), 100 μg mL−1 penicillin and 100 μg mL−1 streptomycin at 37 °C in a humidified atmosphere containing 5% CO2. One day before imaging, cells were seeded in laser scanning confocal microscope (LSCM) culture dishes with a density of 5 × 105 cells per well. The dishes were subsequently incubated at 37 °C in a humidified atmosphere containing 5% CO2. For imaging at various pH, the cells were incubated with 10 μM NIR-F1 for 30 min at 37 °C, then the media was replaced with PBS buffer at various pH. The cells were sequentially incubated with the PBS buffer, 10 μM nigericin, and 5.0 μM monensin for another 30 min, and then imaged using an Olympus FV1000 confocal microscope (Ex. 653 nm, Em. 700–800 nm). Mito-Tracker Green was used to co-localize in cell imaging study. (Ex. 488 nm, Em. 495–550 nm) was set for Mito-Tracker Green.2.6 Fluorescent imaging in living mice
BALB/c were purchased from Charles River Laboratories (Shanghai, China) for in vivo imaging investigation. All animal experiments were carried out in compliance with the Animal Management Rules of the Ministry of Health of the People's Republic of China (Document no. 55, 2001) and the guidelines for the Care and Use of Laboratory Animals of China Pharmaceutical University. BALB/c mice were given an hypodermic or intraperitoneal injection of probe NIR-F1 (100 μM, in DMSO/saline = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9, v/v). Images were taken by using the NIR fluorescence imaging system. This home-built imaging system was reported in our previous works.21,22 The NIR system contains an excitation laser (λ = 660 nm), a high sensitivity NIR CCD camera (PIXIS 512B, Princeton Instrumentation) and a 700 nm long pass filter for capturing the fluorescence emission from the tissue.
9, v/v). Images were taken by using the NIR fluorescence imaging system. This home-built imaging system was reported in our previous works.21,22 The NIR system contains an excitation laser (λ = 660 nm), a high sensitivity NIR CCD camera (PIXIS 512B, Princeton Instrumentation) and a 700 nm long pass filter for capturing the fluorescence emission from the tissue.
3. Results and discussion
3.1 Design and synthesis of NIR-F1
The hemicyanine skeleton presented a significant NIR absorption and emission as reported,23 which is suitable for imaging in living cells and animals. Moreover, the protonation/deprotonation at the hydroxyl in the skeleton could lead to intramolecular charge transfer (ICT) and produce fluorescence changes, which would be feasible for pH detection. Therefore, we designed and synthesized NIR-F1 by introducing two pH sites (phenolic hydroxyl and sulfonic group) onto the hemicyanine skeleton. We hypothesized the protonation/deprotonation at the sulfonic group could further enhance the intramolecular charge transfer. As shown in Scheme 1, NIR-F1 can be easily achieved via a retro-Knoevenagel reaction by reacting NIR-820 with resorcin in the presence of K2CO3 at 50 °C for 4 h using acetonitrile as solvent. The chemical structure of NIR-F1 was confirmed by NMR and MS (ESI).3.2 Investigations of spectral properties of NIR-F1
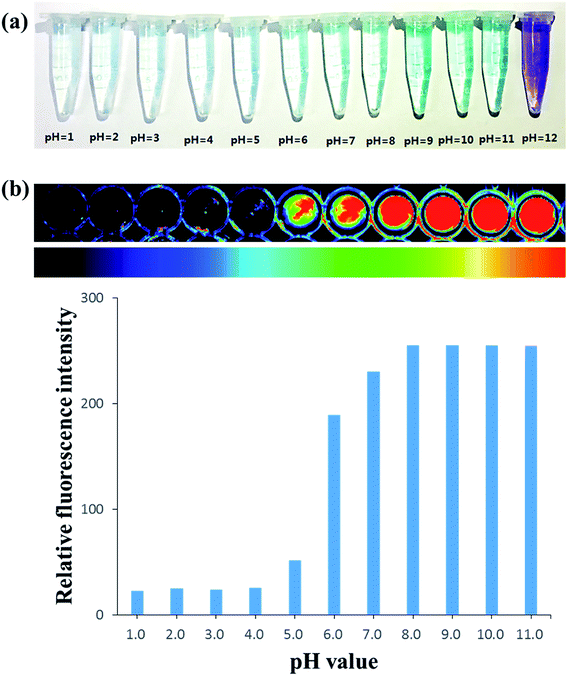
The pH-dependent absorption spectra are presented in Fig. 1a for titration in aqueous buffer. At a pH below 6.0, the probe showed two weak absorption peaks at 625 and 680 nm. And there was almost no absorption around 710 nm. Upon alkalization of NIR-F1 from 6.0 to 8.0, the absorbance at 710 nm appeared obviously. When the pH increased from 8.0 to 11.0, the absorption peak at 710 nm further enhanced and a clear shoulder peak at 650 nm was observed, which induced the color change of NIR-F1 from near colorless to light green (Fig. 2a).The fluorescence response of probe NIR-F1 to pH was illustrated in Fig. 1b. With the increase of pH values, the probe showed a concomitant increase of the fluorescence intensity at 735 nm (I735 nm). The pH changed from 2.0 to 10.0 with a more than 10-fold enhancement in the I735 nm. The inset in Fig. 1b shows the results of the nonlinear curve fitting of the emission peak at 735 nm according to a literature method, affording a pKa value of 6.79 ± 0.09. Moreover, the probe showed pH-dependence over the pH range of 6.0–8.0, with the regression equation Y = 83.414x − 359.9 (R2 = 0.9976). Next, we continued to examine the in vitro fluorescence imaging characters of probe NIR-F1 at various pH values (Fig. 2b). There was little to no fluorescence response at pH 1.0–5.0. In comparison, pH values with 6.0–12.0 can trigger NIR-F1 to produce a remarkable fluorescence signal enhancement. The results were similarly to fluorescence spectrophotometry.
3.3 Sensing mechanisms of NIR-F1
In order to better understand the fluorescence response mechanism of NIR-F1 to pH, we performed a computational study using a suit of Gaussian 09 programs [density functional theory (DFT) at the B3LYP/6-31+G(d) level]. The proposed mechanism for fluorescence changes of probe NIR-F1 was shown in Scheme 2. Three main forms of NIR-F1 might exist at pH 2.0–10.0 due to the ionization of the hydroxyl and the protonation of the sulfonate group. The structures of three forms were optimized, and their frontier molecular orbital energies were calculated (Fig. S1†). Form b could exhibit some red fluorescence because of the hemicyanine chromophore, which have conjugated π-electrons. When the pH value increases, form b was transformed to form c upon formation of a phenolate ion. Because the negative oxygen ion possesses stronger electron-donating ability than hydroxyl, the ionization of hydroxyl is essentially beneficial for enhancing the ICT efficiency. Thus, form c can emit stronger red fluorescence with the increase of pH values. Under acidic condition, the protonation of the sulfonate ions leads to the formation of form a. Then the sulfonic group weakened the fluorescence of the primordial fluorophore because of its weak electron-donating ability. The energy gaps (HOMO–LUMO) of three forms were calculated as 2.30, 2.13, and 2.02 eV, respectively. The theory calculations are in agreement with the experimental results, which rationalize the ICT process.3.4 Selectivity and cytotoxicity assay of NIR-F1
To determine other interference on the pH detection by biological molecules, we next examined the selectivity of NIR-F1 across a series of potential interferents under physiological conditions. NIR-F1 was respectively incubated with various essential ions (Na+, K+, Ca2+, Mg2+, Zn2+, Fe2+, Ba2+, Cu2+, as their chloride salts) and bioactive small molecules (cysteine, hemocysteine, glutathione, alanine, glucose, H2O2, HClO). As a result, no significant variations in the fluorescent signals were observed in the presence of these biologically relevant species (Fig. S2†), indicating that NIR-F1 has striking selectivity to H+ and can be a promising pH-sensitive probe for investigating pH-related biological processes without interference from biological environment.After having demonstrated that NIR-F1 is high sensitivity and excellent selectivity for detection of pH in buffer solution, we then explored its capacity for live cell imaging of pH changes. The cytotoxicity of NIR-F1 on MCF-7 cells were evaluated using standard cell viability protocols (MTT assay). After incubation with NIR-F1 (20.0 μM) for 24 h later the survival rate is over 85% (Fig. S3†), indicating the low cytotoxicity and good biocompatibility of NIR-F1.
3.5 Imaging of NIR-F1 in MCF-7 cells defined at various pH values
To prove NIR-F1 is suitable for pH detection in live cells, the intracellular pH of MCF-7 cells was homogenized to the surrounding medium at varied pH values from 4.0 to 8.0, and the confocal fluorescence imaging was then carried out (Fig. 3). As the pH value went up, the fluorescence emission grew higher. No fluorescence was observed at pH 4.0. While remarkable pseudocolor changes were observed between pH 6.0 and 8.0. These results indicated probe NIR-F1 might be good for monitoring slight pH changes in physiological environments.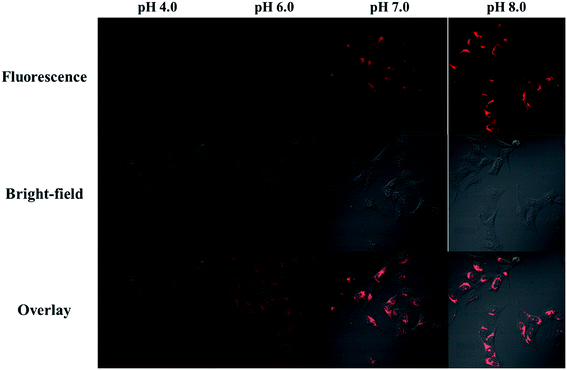 | ||
| Fig. 3 Fluorescent confocal microscopy images of NIR-F1 (10 μM) in MCF-7 cells defined at various pH values. The excitation wavelength was 653 nm and the images were collected at 700–800 nm. | ||
3.6 Mitochondria-targeting performance of NIR-F1
To evaluate the mitochondria-targeting performance of NIR-F1, MCF-7 cells were co-stained with NIR-F1 and a commercially available mitochondria-targeting dye Mito-Tracker Green. As shown in Fig. 4 and S4,† both NIR-F1 and Mito-Tracker Green display strong localized fluorescence within mitochondria. The fluorescence images of NIR-F1 and Mito-Tracker Green can be merged rather well, confirming that NIR-F1 can specifically target the mitochondria of living cells with good cell-membrane permeability. Furthermore, there are relatively few changes in the emission intensity profiles of the dyes NIR-F1 and Mito-Tracker Green (Fig. 4e–f) within the linear regions of interest (ROI; given by a blue line in Fig. 4a). The high Pearson coefficient and overlap coefficient are 0.792 and 0.949, respectively, evaluated using the conventional dye overlay method (Fig. 4h). These experiments indicate NIR-F1 can specifically localize in mitochondria of living cells.3.7 Imaging of NIR-F1 in vivo
Building on these data from our in vitro experiments, NIR-F1 was further evaluated in vivo imaging due to its superior imaging potencies, including high sensitivity and fast response ability. BALB/c mice were injected subcutaneously with saline (left side) and NIR-F1 (right side), respectively. As shown in Fig. 5a, fluorescence in the right side was very strong, which can be contributed to the fluorescence characteristic of NIR-F1 under physiological conditions. However, in the left control side, no fluorescence was observed. Further experiments were developed to confirm that probe NIR-F1 could sensitively and selectively response to different pH values in vivo. PBS with various pH values from 5.0–9.0 were injected subcutaneously to different regions of BALB/c mice, respectively. Then probe NIR-F1 was injected after 5 min. As illustrated in Fig. 5b, strong fluorescence was observed in regions with pH values of 6.0, 7.0 and 9.0. And the fluorescence increased gradually with pH values. However, little or no fluorescence was found in region with pH 5.0. To further evaluate the imaging capability of NIR-F1 in vivo, BALB/c mice were preinjected intraperitoneally with PBS solution of different pH values range 6.0 to 9.0 for 10 min to construct a mouse model with different pH microenvironment, and then treated with NIR-F1 via intraperitoneal injection (Fig. 5c). Similarly to the subcutaneous injection experiment, the fluorescence increased obviously from pH 6.0 to 9.0. The above results confirmed that the probe NIR-F1 possessed the satisfactory ability to image in complicated living organisms.4. Conclusions
In summary, a novel near-infrared fluorescence probe based on hemicyanine derivatives was designed and synthesized to detect pH values. It exhibited excellent sensitivity and low cytotoxicity. Fluorescence images of living cells indicated it can easily diffuse into mitochondria and monitor the pH changes. Moreover, fluorescent imaging of pH and its fluctuations in vivo were also achieved successfully with the use of small animal imager, demonstrating its ability for detection of pH in complex biological systems. Overall, the proposed method provides a new strategy for quantitative biology of molecular events inside other subcellular organelles, and the probe NIR-F1 might potentially be used as a clinically auxiliary tool for disease diagnosis and real-time monitoring of therapy effects.Note
The authors declare no competing financial interest.Acknowledgements
We are grateful for the financial support from the NSFC (National Nature Science Foundation of China, No. 81501529) and Fundamental Research Funds for the Central Universities (2015PY001).References
- J. R. Casey, S. Grinstein and J. Orlowski, Nat. Rev. Mol. Cell Biol., 2010, 11, 50–61 CrossRef CAS PubMed.
- R. A. Gottlieb, J. Nordberg, E. Skowronski and B. M. Babior, Proc. Natl. Acad. Sci. U. S. A., 1996, 93, 654–658 CrossRef CAS.
- V. A. Golovina and M. P. Blaustein, Science, 1997, 275, 1643–1648 CrossRef CAS PubMed.
- M. Lakadamyali, M. J. Rust, H. P. Babcock and X. W. Zhuang, Proc. Natl. Acad. Sci. U. S. A., 2003, 100, 9280–9285 CrossRef CAS PubMed.
- J. R. Thornton, Lancet, 1981, 1, 1081–1083 CrossRef CAS.
- C. C. Curtain, F. E. Ali, D. G. Smith, A. I. Bush, C. L. Masters and K. J. Barnham, J. Biol. Chem., 2003, 278, 2977–2982 CrossRef CAS PubMed.
- S. K. Ohri, J. Becket, J. Brannan, B. E. Keogh and K. M. Taylor, Annals of Thoracic Surgery, 1994, 57, 1193–1199 CrossRef CAS PubMed.
- F. Mbeunkui and D. J. Johann Jr, Cancer Chemother. Pharmacol., 2009, 63, 571–582 CrossRef PubMed.
- N. Pimpodkar, B. Surve and S. Bhise, Curr. Pharma Res., 2014, 4, 1124–1127 Search PubMed.
- K. Vahl, H. Kahlert, L. von Muhlen, A. Albrecht, G. Meyer and J. Behnert, Talanta, 2013, 111, 134–139 CrossRef CAS PubMed.
- S. Yao, K. J. Schafer-Hales and K. D. Belfield, Org. Lett., 2007, 9, 5645–5648 CrossRef CAS PubMed.
- X. Zhang, S. Y. Jing, S. Y. Huang, X. W. Zhou, J. M. Bai and B. X. Zhao, Sens. Actuators, B, 2015, 206, 663–670 CrossRef CAS.
- Q. Jin, L. Feng, D. D. Wang, J. J. Wu, J. Hou, Z. R. Dai, S. G. Sun, J. Y. Wang, G. B. Ge, J. N. Cui and L. Yang, Biosens. Bioelectron., 2016, 83, 193–199 CrossRef CAS PubMed.
- L. H. Li, W. Shi, X. F. Wu, Q. Y. Gong, X. H. Li and H. M. Ma, Biosens. Bioelectron., 2016, 81, 395–400 CrossRef CAS PubMed.
- Y. H. Li, Y. J. Wang, S. Yang, Y. R. Zhao, L. Yuan, J. Zheng and R. H. Yang, Anal. Chem., 2015, 87, 2495–2503 CrossRef CAS PubMed.
- T. Myochin, K. Kiyose, K. Hanaoka, H. Kojima, T. Terai and T. Nagano, J. Am. Chem. Soc., 2011, 133, 3401–3409 CrossRef CAS PubMed.
- B. Tang, F. Yu, P. Li, L. L. Tong, X. Duan, T. Xie and X. Wang, J. Am. Chem. Soc., 2009, 131, 3016–3023 CrossRef CAS PubMed.
- Z. R. Zhang and S. Achilefu, Chem. Commun., 2005, 5887–5889 RSC.
- C. M. Han, H. R. Yang, M. Chen, Q. Q. Su, W. Feng and F. Y. Li, ACS Appl. Mater. Interfaces, 2015, 7, 27968–27975 CAS.
- L. Strekowski, J. C. Mason, H. Lee, M. Say and G. Patonay, J. Heterocycl. Chem., 2004, 41, 227–232 CrossRef CAS.
- P. Wang, K. Wang, D. Chen, Y. B. Mao and Y. Q. Gu, RSC Adv., 2015, 5, 85957–85963 RSC.
- P. Wang, K. Wang and Y. Q. Gu, Sens. Actuators, B, 2016, 228, 174–179 CrossRef CAS.
- J. J. Zhang, L. L. Ning, J. T. Liu, J. X. Wang, B. F. Yu, X. Y. Liu, X. J. Yao, Z. P. Zhang and H. X. Zhang, Anal. Chem., 2015, 87, 9101–9107 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra22470a |
| This journal is © The Royal Society of Chemistry 2016 |