Green microfluidic synthesis of monodisperse silver nanoparticles via genetic algorithm optimization†
Abstract
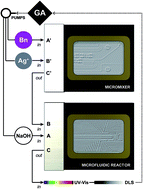
A scalable and green procedure for the microfluidic flow synthesis of monodisperse silver nanoparticles is reported. Beetroot extract is used both as a reducing and growth-regulating agent. A multi-objective genetic algorithm was used to automate the optimization of the reaction and reduce sample polydispersity observed in previous reports. The proposed methodology ensures high-quality nanoparticles in a rapidly manner and devoid of human skill or intuition, essential for method standardization and implementation.

 Please wait while we load your content...
Please wait while we load your content...