Modulation of bactericidal action in polymer nanocomposites: light-tuned Ag+ release from electrospun PMMA fibers†
I. Morenoa,
N. Navascuesa,
S. Irusta*ab and
J. Santamaria*ab
aDept. Chemical Engineering, Nanoscience Institute of Aragon (INA), University of Zaragoza, 50018 Zaragoza, Spain. E-mail: xsirusta@unizar.es; jesus.santamaria@unizar.es
bNetworking Research Center of Bioengineering, Biomaterials and Nanomedicine (CIBER-BBN), Maria de Luna, 11, 50018 Zaragoza, Spain
First published on 5th August 2016
Abstract
While silver is widely regarded as a potent antibacterial material, controlling the rate of release of the bactericidal agents (silver ions) remains a challenge. When silver nanoparticles are used as precursors, the release process involves oxidation and dissolution in the surrounding environment. Here we show that it is possible to tune the rate of silver release from polymer matrices containing silver precursors by a simple UV irradiation step. To demonstrate this, silver-containing polymer (PMMA) fibers have been produced by electrospinning, then subjected to different levels of exposure to UV radiation and fully characterized in terms of silver state, presence of nanoparticles, Ag+ release rates and bactericidal power. The as-spun fibers contained mainly silver as Ag+, while samples exposed to UV radiation displayed progressively higher proportions of Ag0 (XPS analysis). Nanoparticles consisting of metallic silver were present in UV-exposed samples, and their size increased with irradiation time. The Ag+ release rates (and the bactericidal action of the nanofibers) were found to directly depend on the degree of UV exposure: a fast Ag+ release was observed for non-irradiated samples, which during the release experiment delivered roughly three times as much silver than samples subjected to 24 h UV exposure.
Introduction
Bacterial colonization affects a variety of materials including medical devices, wound dressings, industrial pipes, food packaging and separation membranes.1 This serious problem has prompted an intense search for new antimicrobial agents, especially those capable of fighting film-forming bacteria. Different bactericidal agents in the form of nanomaterials have been studied as alternative materials to address this problem. Carbon nanotubes have been incorporated into polyurethane,2 surface engineered gold nanoparticles have been tested in biofilm disruption,3 and even nanostructuration of black silicon has been used against bacteria.4But among all the alternative bactericidal agents investigated, silver has a prominent role. The antiseptic properties of silver against a very broad spectrum of bacteria, yeasts and fungi have been known for centuries. Nowadays, the use of silver to control microbial proliferation is contemplated for a wide variety of materials used in daily life, ranging from textiles to stainless steel coatings in home appliances and food-contact.5 The efficiency of silver in medical applications is widely reported in literature and it is used in fields such as urology, dentistry, general surgery and orthopaedics,6 and in a diversity of medical devices that includes endotracheal tubes, catheters, surgical meshes, dressings and dental fillings.7
In spite of the above, there are significant questions regarding the efficient use of Ag in bactericidal applications. In particular, it has been shown that there is no direct correlation between the total amount of silver present and the observed bactericidal effect.8 Instead, the bactericidal efficiency of silver was univocally related to the bioavailability of Ag+ ions. Thus, AgNO3 presented a high bactericidal effect at low doses, while Ag nanoparticles with sizes around 100 nm did not show activity, due to the slow oxidation of silver and subsequent release of Ag+ ions.8 Similarly, a high antimicrobial efficiency was observed even at low Ag loadings for Ag-containing zeolites, where Ag+ could be rapidly released by exchange of other cations into the zeolite network.9
However, if Ag+ ions are responsible for the bactericidal activity of silver, their presence at suitable concentrations in the desired environment must be guaranteed at all times. Thus, if all silver is immediately available (e.g., when present as AgNO3), a strong bactericidal effect will be obtained initially, but once the Ag+ ions are used up, sequestered, washed or removed by other processes, there will be no protection against further bacterial colonization. From this point of view, it would be more efficient to have a tuned Ag+ delivery, with a fast initial release followed by a slower release to ensure a sustained presence of Ag+ ions.
Different application scenarios may demand different release profiles and therefore in general it seems highly desirable to have the capacity to tune the release of Ag+ ions at will. For example, treating of wounds requires silver release during hours or days, but dental materials should show antimicrobial properties for several months.10 For food packaging, extensive research has been performed to control release of the active agent in the polymeric network. Thus, while a fast release of silver ions can be achieved by using a silver salt, the limitation is the instability of free silver ions, that are known to be easily inactivated by many different physical or chemical factor.3 Ag nanoparticles (AgNPs) are able to provide a more sustained release of fresh Ag+ ions as AgNPs become oxidized and release Ag+, a process favoured by the presence of dissolved dioxygen and protons.
One efficient approach to tune the Ag+ release rate is to embed the silver species inside a polymer matrix.11 The structure of polymers can be engineered to harbour the silver species (and hinder their aggregation) and to favour, or to retard, the diffusion of the Ag+ produced. This structure does not have to be static: silver has been immobilized in smart polymers, which can undergo phase transition under external stimuli, affecting Ag+ release.10
Electrospinning as a technique of polymer processing is ideally suited for release applications, since it produces films made of micro or nanofibers, with a very high surface to volume ratio and short diffusion lengths. The properties of electrospun nanofibers including fiber diameter, specific surface, polymer composition and porosity can significantly modulate the drug and/or ions release profiles.12 In addition, the micro and nanofibers obtained by electrospinning can be easily loaded with the desired precursors during the preparation process.
Poly(methyl methacrylate) (PMMA) is among the most important materials for biomedical applications, due to its good biocompatibility and mechanical strength.7,13 PMMA can be endowed with antimicrobial properties by doping it with silver in its different bioactive forms, including ions, oxide and silver nanoparticles. Ag/PMMA materials significantly reduced bacterial growth and prevented biofilm formation.7,14–16 In addition, the hydrophobic character of this polymer and the relatively low electrical conductivity in most solvents ensures consistent nanofiber formation and extends the electrospinning operation window in terms of the applied voltages and nanofiber diameters obtained.17
The most straightforward way of incorporating AgNPs into a polymer structure is the chemical reduction of a silver salt precursor by a reducing agent. This can easily be done after nanofiber synthesis, inducing the in situ formation of AgNPs on the surface of the electrospun fibers. However, the AgNPs synthesized in this way are prone to aggregation, which decreases their antibacterial properties. Because of this, alternative procedures have been developed to deposit the AgNPs. Thus for instance, Ar plasma was applied to chitosan/poly(ethylene oxide) nanofibers containing silver nitrate as a precursor to induce the formation of AgNPs primarily on the nanofiber surface.18 Another possibility is to use UV irradiation, a simple well-known method of reducing Ag+ to obtain the nanoparticles, even inside polymer matrices.19,20
In this work, we have used electrospinning to produce PMMA fibers loaded with silver nitrate, then different levels of exposure to UV irradiation were employed to induce the gradual reduction of silver ions into AgNPs. In this way, for the first time we have shown that it is possible to use light to control the Ag+/Ag0 proportions on a polymer nanofiber. Since Ag+ is immediately available for release while Ag0 requires a slower oxidation process to deliver Ag+ ions, changing the Ag+/Ag0 allows tuning the Ag+ release rate and the corresponding bactericidal activity over time.
Experimental
Materials
Silver nitrate (AgNO3, 99.8% Panreac), acetone (Pan-reac), poly(methyl methacrylate) (PMMA, Mw – 120![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, Sigma Aldrich) and N,N-dimethylformamide (DMF, 99.8% Sigma-Aldrich) were used to synthesize the silver/PMMA fibers. Tryptone Soy Broth (TSB, Cultimed, Panreac), Tryptone Soy Agar (TSA, Cultimed, Panreac) and Dulbecco's Phosphate Buffer Saline (DPBS, Biowest) were used as media for the bactericidal tests.
000, Sigma Aldrich) and N,N-dimethylformamide (DMF, 99.8% Sigma-Aldrich) were used to synthesize the silver/PMMA fibers. Tryptone Soy Broth (TSB, Cultimed, Panreac), Tryptone Soy Agar (TSA, Cultimed, Panreac) and Dulbecco's Phosphate Buffer Saline (DPBS, Biowest) were used as media for the bactericidal tests.
Fibers preparation
Two different solutions were used, solution A was prepared by dissolving AgNO3 in a 50 v/v% mixture of acetone and DMF under magnetic stirring for 5 minutes, then PMMA (10 wt%) was added and the solution was stirred overnight. Solution B consists of 25 wt% PMMA in DMF. An Yflow 2.2 D500 electrospinner with a coaxial setup was used, solution A was fed to the core needle with a flow rate of 1 ml h−1; solution B was fed in the shell at 0.5 ml h−1. The coaxial needle was placed 15 cm from a flat collector. In order to obtain homogeneous films, the needle was moved in 2 dimensions (left-right, front-rear) to cover all the electrospinning area. A Vilber VL-215L UV lamp (30 W/365 nm) was used to reduce the AgNO3 to metallic nanoparticles. The lamp was place at 5 cm from the sample for 2, 6 or 24 h. One sample was reduced with ascorbic acid to assure the complete reduction of the silver nitrate reduction. The prepared materials were named XAgYhUV, where X is the AgNO3 concentration (mg ml−1) in the solution and Y is the UV exposure time. One sample without silver nitrate (PMMA) was prepared for comparison.Characterization
SEM (scanning electron microscopy) images were taken after coating the samples with a Pt using a FEI Inspect equipment. TEM (transmission electron microscopy) images were taken using a FEI Tecnai F30 equipment. The size distribution statistics were obtained by measuring at least 200 fibers or particles in different images. Release experiments were carried out in a continuous device, 10 mg of fibers were placed in a filter and distilled water was passed at 1 ml min−1 using a Shimadzu LC-10AT VP pump. Samples collected at different times were measured in a Microwave Plasma Atomic Emission Spectrometer (Agilent MP-AES 4100) in order to obtain the amount of silver released. The total silver load was obtained from the remaining weight after calcination. The fibers were dissolved in DMF and the obtained solution was measured using a Varian 50Probe UV-Vis spectrophotometer. X-ray diffraction measurements of Ag/PMMA fiber were obtained using a Panalytical Empyrean diffractometer and were used, together with UV-Vis measurements (Varian 50Probe UV-Vis spectrometer), to monitor the formation of silver nanoparticles inside the fibers with UV irradiation. Mettler Toledo TGA/SDTA 851 equipment was used for thermal analysis of composites.The antimicrobial activity was tested against S. aureus using disk diffusion method. Mixtures of Tryptone Soy agar in 1 L distilled water at pH 7.2 as well as the empty Petri plates were autoclaved. The agar medium was then cast into the Petri plates and cooled in laminar airflow. Direct colony suspension method was used to prepare the inoculum, suspending between 3 and 5 colonies of S. aureus in PBS, the turbidity was adjusted to 0.5 MCFarland standard turbidity. Finally, 0.2 ml of the suspension was spread in each agar plate and 1.9 mg samples disks (14 mm diameter) were planted onto the agar plates. All the plates were incubated at 37 °C for 24 h, following which the zone of inhibition was measured. The bactericidal tests were performed using a modification of ASTM E-2180 (Standard Test Method for Determining the Activity of Incorporated Antimicrobial Agents In Polymeric or Hydrophobic Materials). Briefly, 0.5 ml of an overnight stationary growth phase of S. aureus ATCC 6538 bacteria in TSB were diluted in 500 ml sterile TSA solution at 40 °C. 0.5 ml of this solution was placed in each well of a 12-well plate. Then, 3.8 mg samples with the size of the well (20 mm diameter) were placed on the TSA and then incubated at 37 °C during 24 h in the dark. After incubation, the samples with the agar were places in a tube with 10 ml of sterile TSB. The tubes were immersed in an ultrasonic bath (50 kHz) during 1 min and stirred with vortex for 1 min. Afterwards, seven dilutions 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 of each tube were prepared and three 25 μl drops of each dilution were spread on TSA plates. Viable bacterial colonies were counted after incubation overnight at 37 °C for the different times. For each test, three controls (neat PMMA fibers) and three samples were used.
10 of each tube were prepared and three 25 μl drops of each dilution were spread on TSA plates. Viable bacterial colonies were counted after incubation overnight at 37 °C for the different times. For each test, three controls (neat PMMA fibers) and three samples were used.
Results and discussion
Ag/PMMA fibers characterization
Fig. 1 shows SEM images and diameter distribution for two samples of as-spun fibers loaded with different concentrations of silver nitrate in the inner needle solution, together with silver-free polymer fibers obtained under the same conditions. The introduction of silver nitrate causes a concentration-dependent decrease in the fiber diameter and on the width of the diameter size distribution, reaching average diameter values of 937 nm for 40 mg ml−1 AgNO3 in the solution. Both silver-containing fibers have similar diameter distributions, this is related to the increase of conductivity of the spinning solution caused by the presence of the silver salt (Table 1) since high solution conductivity values allows a higher degree of stretching in electrospinning jets.21 The silver loading in the fibers estimated by weight difference after burning the as-spun fibers was very close to the theoretical value (Table 1).| Sample | AgNO3 concentration mg ml−1 | Conductivity (μS cm−1) | Fibers diameter (nm) | Ag loading (wt%) | |
|---|---|---|---|---|---|
| Theoretical | Experimental | ||||
| PMMA | — | 1.2 ± 0.10 | 1536 ± 247 | — | — |
| 20Ag0hUV | 20 | 990.32 ± 5.03 | 971 ± 114 | 1.96 | 1.91 ± 0.12 |
| 40Ag0hUV | 40 | 1743.99 ± 8.19 | 937 ± 76 | 3.84 | 3.64 ± 0.10 |
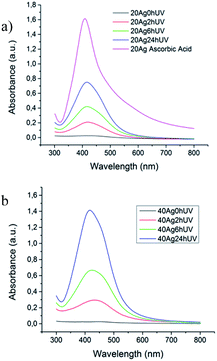
The generation of silver nanoparticles from the precursor salt under UV-Vis irradiation could be followed by UV-Vis spectroscopy. The color of the resulting fibers turned gradually to yellow under irradiation, suggesting the formation of AgNPs.22 This was confirmed by the evolution of the absorption spectra of samples prepared with 20 mg ml−1 of silver nitrate subjected to different irradiation times (Fig. 2). As-spun fibers exhibited near no absorption in the wavelength range around 415 nm characteristic of AgNPs.23
However, a clear absorption band at 415 nm is already observed after a 2 h irradiation and its intensity increased with irradiation time, gradually tending to the pattern of complete reduction of silver nitrate, obtained with the sample reduced with ascorbic acid, also included in Fig. 2a. On the other hand, as could be expected the absorbance of the sample 40Ag is higher due to the higher concentration of silver nanoparticles produced for a given UV irradiation time (Fig. 2b).
Additional evidence of the formation of silver nanoparticles was obtained from XRD analysis. Fig. 3 shows the diffractograms of sample 20Ag with different irradiation times. The pure polymer fibers pattern, added for comparison, show broad peaks at 19, 30 and 42°,24 similar to those of the non-irradiated sample. After 2 h UV irradiation a small but discernible signal is already present at 38.22°, in good correspondence with the (111) plane of center cubic (fcc) silver structure25 (JCPDS card no. 04-0783). As in the case of the silver plasmon band, this peak increases in intensity with increasing irradiation time. 40Ag fibers presented similar XRD patterns (Fig. 1 ESI†).
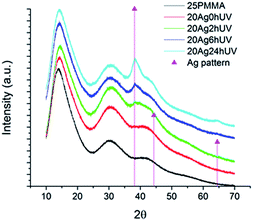 | ||
| Fig. 3 XRD patterns of the pristine polymer and 20Ag fibers sample under different irradiation time. | ||
Monitoring the evolution of the samples irradiated for different lengths of time using TEM analysis was more complex due to the difficulties in distinguishing AgNO3 particles from silver particles in the STEM micrographs since they are present as bright spots of similar appearance (Fig. 4). In spite of this, analysis of the particle size distribution shows that there is a clear increase of particles size with irradiation time, together with a certain broadening of the particle size distribution (Table 2).
 | ||
| Fig. 4 STEM images of 20Ag sample and particles size distribution after different irradiation times for: (a) as spun fibers; (b) 2 h irradiation; (c) 6 h irradiation; (d) 24 h irradiation. | ||
| Irradiation time (h) | UV-Vis maximum (nm) | Particle size (nm) |
|---|---|---|
| 0 | — | 2.0 ± 0.3 |
| 2 | 419.3 | 2.3 ± 0.5 |
| 6 | 416.8 | 3.1 ± 0.7 |
| 24 | 415.6 | 4.9 ± 1.2 |
The analysis of particle size distribution data indicates that the average size of the particles in the as-synthesized fibers (mainly assigned to silver nitrate) is around 2 nm, progressively increasing to around 5 nm with irradiation time.
This suggests that, simultaneously to the reduction process that transforms Ag+ to Ag0, diffusion of Ag+ ions also takes place, in a ripening process that increases the size of the larger particles at the expense of the smaller ones (particles under 2 nm practically disappear after 24 h of irradiation, while a new population of particles with sizes 4–9 nm appears).
TEM images also show that in spite of this process a good particle distribution is maintained, with a low degree of agglomeration. Under the conditions employed, the electrospinning process assures a good dispersion of silver precursor in the as spun fibers. The silver nanoparticles are formed in situ, from the silver nitrate precursor. Once the nanoparticles are formed, their movement is hindered by the polymer matrix, and this helps to avoid agglomeration. The good dispersion observed after irradiation confirms that the ripening process takes place through Ag+ ion migration, rather than through movement of whole particles.
XPS analysis was carried out to confirm the chemical sate of silver in the fibers and the progressive reduction under UV illumination. The survey spectra (Fig. 2 ESI†) showed only characteristic peaks of O, C and Ag. Fig. 5 shows the XPS spectra of 40Ag sample after different irradiation times. The most concentrated sample was used to these analyses in order to increase the sensitivity of the analysis. The curve fitting analysis of the Ag 3d region of the as-spun sample shows two peaks at 368.2 and 374.3 eV corresponding to the 3d5/2 and 3d3/2 respectively and are associated to cationic silver as can be seen from the Ag spectrum of the silver nitrate. Therefore the silver present in the as spun fibers is essentially Ag+, indicating that the electrospinning process itself does not result in a reduction of silver, under the conditions employed. Peaks obtained for samples after irradiation are broader due to the presence of a second peak at a higher binding energy, which is related to metallic silver. The spectrum obtained for a standard silver film confirms binding energies of 368.9 and 374.9 eV for the 3d level of Ag0. According to spectrum curve fitting the samples irradiated for 6 and 24 h presented a concentration of Ag+ of 67 and 14% respectively. These results show that the degree of reduction of the silver precursor can be tuned by controlling the irradiation time. As we will show next, this has direct consequences on the rate of Ag+ release.
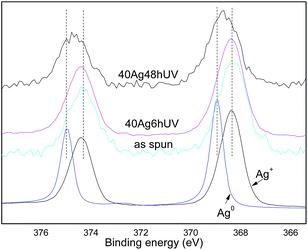 | ||
| Fig. 5 Ag 3d core level of sample 40Ag with different irradiation times, silver nitrate and metallic silver. | ||
For the times employed, polymer ageing was negligible. Thus, after UV irradiation for 24 h, no effect on the polymer matrix could be found by TGA analysis (Fig. 3 ESI†). The slight weight increase observed in the irradiated sample at low temperature could be related to oxidation of metallic silver.
Silver release
The cumulative release of silver (including both, silver ions and nanoparticles) from fibers irradiated during different periods of time is shown in Fig. 6 as mean value ± standard deviation. Release curves present similar patterns, but the release rates are very different for fibers with different UV treatments. As predicted, the higher release rate was found for the as spun fibers, since most of the Ag is already in the cationic form, as shown by UV-Vis and XPS results, and Ag+ ions are easily leached from the polymer fibers. As silver becomes reduced, the Ag+ release rate decreases: the formed Ag nanoparticles act as silver reservoirs that slowly dissolve and leach out silver ions to the medium. Fig. 6 shows that around 60% of the silver load is released after 4 hours, a value that is roughly halved after only 6 h of UV exposure. | ||
| Fig. 6 Silver cumulative release from 20Ag fibers under UV irradiation for different periods of time and fibers reduced by ascorbic acid. | ||
If the release experiment is prolonged to 8 hours, as spun fibers are able to release 72% of the silver load, a value that is progressively reduced with UV irradiation to less than 26% for a 24 h exposure. This value is not far from that of fibers chemically reduced using ascorbic acid, that release around 20% of the silver load.
In addition to the state of the silver, the size increase observed for UV-exposed samples also retards the release process. The release of soluble silver from Ag surfaces is primarily a heterogeneous oxidation reaction involving the cooperative effects of dissolved oxygen and protons. Liu et al.26 demonstrated that size of nanoparticles has a dominant effect on soluble silver release. Therefore both the higher proportion of Ag0 and the increase in Ag nanoparticle size would be responsible for the slower release of Ag+ that takes place in samples with higher irradiation time.
Antibacterial properties
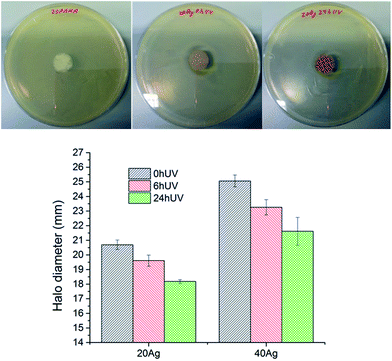
Unlike antibiotics, silver is toxic to multiple components of bacterial cell metabolism, and there is abundant evidence that Ag+ cations in water, PBS or culture media are effective against S. aureus bacteria strains.27The antibacterial activity of AgPMMA nanofibers was investigated using the disk-diffusion method. Disc of fibers were placed on agar plates, which were seeded with S. aureus. As shown in Fig. 7, Ag-free PMMA did not produces any zone of inhibitions as was previously reported,28 while the 20Ag0hUV possesses good antibacterial activity against S. aureus (zone of inhibition is 20.69 ± 0.32 mm).
The inhibition zone decreased to 18.18 mm after irradiation of the sample for 24 h due to the lower release of silver ions from this sample. A summary of the results of zone of inhibition experiments against S. aureus is shown in Fig. 7. The observed trends are expected, with larger inhibition zones at the same irradiation time for samples with a higher silver load, and a consistent decrease of inhibition zone with irradiation time for a given Ag content.
In agreement with the silver release profile studies (Fig. 6), the fibers irradiated for the longest time (24 h) showed the smallest zone of inhibition because of the formation of silver nanoparticles and the corresponding decrease in the release of Ag+ ions.
Finally, the bactericidal effect of the prepared materials was also analyzed using the ASTM E-2180 standard, a test appropriate for polymeric or hydrophobic materials (Fig. 8). Pure PMMA had the same bacterial count as the control sample, confirming that the polymer itself does not present any bactericidal effect.
 | ||
| Fig. 8 log of S. aureus concentration in TSA after incubation with samples at 37 °C during 24 h in the dark. | ||
Non irradiated samples presented the highest bacteria count reduction, 40Ag0hUV showed about 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log reductions within the 24 h exposure time while only a 2
log reductions within the 24 h exposure time while only a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log reduction was observed for 20Ag with a lower silver load. This was expected since in non-irradiated samples silver is immediately available as bactericidal Ag+. The bactericidal effect decreased with the increasing UV irradiation time, the slower release from the irradiated samples leads to lower bactericidal effect. But even after 24 h irradiation 2 and 1
log reduction was observed for 20Ag with a lower silver load. This was expected since in non-irradiated samples silver is immediately available as bactericidal Ag+. The bactericidal effect decreased with the increasing UV irradiation time, the slower release from the irradiated samples leads to lower bactericidal effect. But even after 24 h irradiation 2 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log reduction were achieved with 40Ag and 20Ag samples respectively.
log reduction were achieved with 40Ag and 20Ag samples respectively.
Conclusions
PMMA fibers of around 950 nm diameter loaded with different concentrations of silver nitrate were obtained by electrospinning. The degree of the precursor reduction and the silver nanoparticles formation can be tuned by controlling the UV irradiation time. The Ag particles size increases from 2 to almost 5 nm under irradiation. The bactericidal effect on S. aureus depends not only on the silver load but also on the irradiation time. This method constitute a flexible way for optimizing Ag/polymer formulations for the performance targets of a variety of medical and consumer antimicrobial applications.Acknowledgements
This research was supported by the European Research Council (ERC-Advanced Grant HECTOR) (267626). The microscopy work was conducted at the Laboratorio de Microscopias Avanzadas at Instituto de Nanociencia de Aragon—Universidad de Zaragoza. I. M. acknowledges support by the Aragon Government (B008/12).References
- L. Guo, W. Yuan, Z. Lu and C. M. Li, Colloids Surf., A, 2013, 439, 69–83 CrossRef CAS.
- H. C. Shi, H. Y. Liu, S. F. Luan, D. Shi, S. J. Yan, C. M. Liu, R. K. Y. Li and J. H. Yin, RSC Adv., 2016, 6, 19238–19244 RSC.
- K. Giri, L. R. Yepes, B. Duncan, P. K. Parameswaran, B. Yan, Y. Jiang, M. Bilska, D. F. Moyano, M. A. Thompson, V. M. Rotello and Y. S. Prakash, RSC Adv., 2015, 5, 105551–105559 RSC.
- X. F. Zhai, C. T. Sun, K. Li, F. Guan, X. Q. Liu, J. Z. Duan and B. R. Hou, RSC Adv., 2016, 6, 46081–46088 RSC.
- A. Martínez-Abad, J. M. Lagarón and M. J. Ocio, Int. J. Food Microbiol., 2014, 174, 39–46 CrossRef PubMed.
- R. Raho, F. Paladini, F. A. Lombardi, S. Boccarella, B. Zunino and M. Pollini, Mater. Sci. Eng., C, 2015, 55, 42–49 CrossRef CAS PubMed.
- S. Silver, FEMS Microbiol. Rev., 2003, 27, 341–353 CrossRef CAS PubMed.
- P. Lalueza, M. Monzon, M. Arruebo and J. Santamaria, Mater. Res. Bull., 2011, 46, 2070–2076 CrossRef CAS.
- P. Lalueza, M. Monzon, M. Arruebo and J. Santamaria, Chem. Commun., 2011, 47, 680–682 RSC.
- R. Elashnikov, O. Lyutakov, Y. Kalachyova, A. Solovyev and V. Svorcik, React. Funct. Polym., 2015, 93, 163–169 CrossRef CAS.
- N. Alissawi, V. Zaporojtchenko, T. Strunskus, T. Hrkac, I. Kocabas, B. Erkartal, V. S. K. Chakravadhanula, L. Kienle, G. Grundmeier, D. Garbe-Schoenberg and F. Faupel, J. Nanopart. Res., 2012, 14, 928 CrossRef.
- H. Yang, P. F. Gao, W. B. Wu, X. X. Yang, Q. L. Zeng, C. Li and C. Z. Huang, Polym. Chem., 2014, 5, 1965–1975 RSC.
- T. Zhang, G. Xu, O. Regev and F. D. Blum, J. Colloid Interface Sci., 2016, 461, 128–135 CrossRef CAS PubMed.
- M. Cecilia Cruz, G. Ruano, M. Wolf, D. Hecker, E. Castro Vidaurre, R. Schmittgens and V. Beatriz Rajal, Chem. Eng. Res. Des., 2015, 94, 524–537 CrossRef PubMed.
- O. Lyutakov, Y. Kalachyova, A. Solovyev, S. Vytykacova, J. Svanda, J. Siegel, P. Ulbrich and V. Svorcik, J. Nanopart. Res., 2015, 17, 120 CrossRef.
- S. Morrison, A. Singh, J. Rousseau, M. Walker, A. Nazarali, E. Crawford, B. Brisson, W. C. Sears and J. S. Weese, Am. J. Vet. Res., 2015, 76, 395–401 CrossRef CAS PubMed.
- H.-T. Chen, H.-L. Lin, I.-G. Chen and C. Kuo, ACS Appl. Mater. Interfaces, 2015, 7, 9479–9485 CAS.
- D. Annur, Z.-K. Wang, J.-D. Liao and C. Kuo, Biomacromolecules, 2015, 16, 3248–3255 CrossRef CAS PubMed.
- M. Basuny, I. O. Ali, A. Abd El-Gawad, M. F. Bakr and T. M. Salama, J. Sol-Gel Sci. Technol., 2015, 75, 530–540 CrossRef CAS.
- H. W. Lu, S. H. Liu, X. L. Wang, X. F. Qian, J. Yin and Z. K. Zhu, Mater. Chem. Phys., 2003, 81, 104–107 CrossRef CAS.
- I. Moreno, N. Navascues, S. Irusta and J. Santamaria, J. Catal., 2015, 329, 479–489 CrossRef CAS.
- M. N. Boroumand, M. Montazer, F. Simon, J. Liesiene, Z. Šaponjic and V. Dutschk, Appl. Surf. Sci., 2015, 346, 477–483 CrossRef CAS.
- A. Regiel, S. Irusta, A. Kyziol, M. Arruebo and J. Santamaria, Nanotechnology, 2013, 24, 015101 CrossRef PubMed.
- N. D. Singho, M. R. Johan and N. A. C. Lah, Nanoscale Res. Lett., 2014, 9, 42 CrossRef PubMed.
- J. Puišo, I. Prosyčevas, A. Guobienė and S. Tamulevičius, Mater. Sci. Eng., B, 2008, 149, 230–236 CrossRef.
- J. Liu, D. A. Sonshine, S. Shervani and R. H. Hurt, ACS Nano, 2010, 4, 6903–6913 CrossRef CAS PubMed.
- M. Amberg, P. Rupper, R. Storchenegger, M. Weder and D. Hegemann, Nanomedicine: Nanotechnology, Biology and Medicine, 2015, 11, 845–853 CrossRef CAS PubMed.
- J. Song, H. Kang, C. Lee, S. H. Hwang and J. Jang, ACS Appl. Mater. Interfaces, 2012, 4, 460–465 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra16876k |
| This journal is © The Royal Society of Chemistry 2016 |