Preparation and characterization of Fe3O4@SiO2@TiO2@Pd and Fe3O4@SiO2@TiO2@Pd–Ag nanocomposites and their utilization in enhanced degradation systems and rapid magnetic separation
Hossein Khojastehab,
Masoud Salavati-Niasari*ab,
Mohammad-Peyman Mazhariab and
Masood Hamadanianab
aYoung Researchers and Elite Club, Mahabad Branch, Islamic Azad University, Mahabad, Iran
bInstitute of Nano Science and Nano Technology, University of Kashan, P.O. Box 87317-51167, Kashan, Iran. E-mail: salavati@kashanu.ac.ir; Fax: +98 315 5913201; Tel: +98 315 5912383
First published on 4th August 2016
Abstract
Two new palladium catalysts immobilized on modified magnetic nanoparticles with titanium dioxide shells (Fe3O4@SiO2@TiO2@Pd and Fe3O4@SiO2@TiO2@Pd–Ag nanocomposites) were synthesized and characterized using XRD, SEM, TEM, VSM, EDS, DRS and IR techniques. These catalytic systems showed high activity in the photodegradation of rhodamine B under UV irradiation. The activity of these catalysts was compared and the results showed a higher activity for the Fe3O4@SiO2@TiO2@Pd–Ag nanocomposite due to a synergic effect between silver and palladium. The supported catalysts have the advantage of being completely recoverable with the simple application of an external magnetic field.
1. Introduction
For a large number of fundamental research and industrial applications, heterogeneous catalysis is favored over homogeneous catalysis. This is because heterogeneous catalysts have a number of advantages such as ease of handling, simple workup and ability to be recycled.1 Despite their synthetic simplicity, heterogenized catalysts are typically less effective than their homogeneous counterparts.2 To overcome this constraint, nanoscale catalysts have been introduced. These catalysts are endowed with larger surface areas in comparison to their bulk scale. Metal and metal oxide nanostructures have been efficiently used as environmentally friendly and solid supports for many reactions such as epoxidation, coupling, MR imaging, benzylation reactions etc.1,3–5 Because of their high surface energy, nanostructures have a tendency to agglomerate and this phenomenon will decrease the number of accessible active sites. Thus, to derive maximum benefit and prevent particle aggregation, surface-modified catalysts have been designed.6By reducing the size of the support to the nanometer scale, the activity of the supported catalyst can be significantly improved compared to homogeneous catalysts immobilized on conventional supports, because of the large accessible surface area of the catalyst.7,8 The main problem of using nanoparticles as catalyst supports is their difficult separation and recycling and also, many noble metals used in homogeneous systems are very expensive and it is difficult to separate and retrieve the soluble catalyst from the reaction system for the next reaction.8 A simple solution for this is immobilization of the catalyst on nanomaterial supports to enhance their recyclability. Polymers, dendrimers and ligands have all been implemented in the immobilization of nanocatalysts.9,10
The immobilization of catalysts on nanoparticle supports is a generic procedure to produce heterogenized homogeneous catalysts. Among different nanoparticles, those which have magnetic properties are appropriate candidates for nanocatalyst supports. Magnetic separation is an attractive way to retrieve catalysts as it prevents loss of the catalyst and the reusability increases compared to other chemical or physical procedures, such as liquid–liquid extraction, chromatography, distillation, filtration or centrifugation.11 Employing magnetic supports allows the catalyst to be easily separated from the liquid reaction media using a magnet.12 This property makes the catalyst cost-effective and suitable for industrial applications.
Some magnetic nanoparticles like Fe3O4, γ-Fe2O3, NiFe2O4 and CoFe2O4 are used frequently as cores or supports for immobilizing other nanocatalysts.13–16 These nanoparticles have been utilized as catalyst supports for several organic transformations such as hydrogenation reactions, cross-coupling reactions, olefin hydroformylation, dye degradation systems and the aldol reaction, and for biocatalysts.17–27
In this work, by synthesizing Fe3O4@SiO2@TiO2@Pd and Fe3O4@SiO2@TiO2@Pd–Ag nanocomposites, we combined the advantages of heterogeneous catalysis, magnetic separation and enhanced catalytic activity of TiO2 photocatalysts for dye degradation. Due to a strong interaction between the magnetic NPs and an external applied magnetic field, the catalyst immobilized on superparamagnetic nanomaterials can be easily separated from the products, and it can be easily redispersed in the absence of a magnetic field due to the absence of “magnetic memory”.28
2. Experimental
2.1. Materials and methods
Catalyst preparation reactions were carried out under an inert argon or nitrogen atmosphere. Polyethylene glycol 400 (PEG 400), hydrochloric acid, ammonium hydroxide 30%, 2-propanol, palladium(II) acetate, silver(I) nitrate, iron(II) chloride hydrate, iron(III) chloride hydrate, tetraethylorthosilicate (TEOS), tetranormalbuthyltitanate (TNBT), acetylacetone, ethanol and methanol were purchased from the Merck company and all of them were used without further purification. Deionised water was used during all of the steps where water was needed.XRD patterns were recorded using a Philips X-ray diffractometer using Ni-filtered Cu Kα radiation. SEM images were obtained using an LEO instrument model 1455VP. Prior to taking images, the samples were coated with a very thin layer of gold to make the sample surface conducting and prevent charge accumulation. The magnetic properties of the samples were measured at room temperature using a vibrating sample magnetometer (VSM, Meghnatis Kavir Kashan Co., Kashan, Iran). FT Infrared (FT-IR) spectra were obtained with potassium bromide pellets in the range of 400–4000 cm−1 using a Nicolet-Impact 400D spectrophotometer. The UV-Vis spectra of the samples were taken on a UV-Vis spectrophotometer (Shimadzu, UV-2550, Japan) with a 400 W Osram lamp as the light source.
2.2. Synthesis of the catalyst support (Fe3O4@SiO2 nanoparticles)
The catalyst support comprised silica-coated magnetic NPs and was synthesized using a simple co-precipitation method. These nanoparticles were prepared by the chemical co-precipitation of Fe3+ and Fe2+ ions with a molar ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Typically, FeCl3·6H2O (5.838 g) and FeCl2·4H2O (2.147 g) were dissolved in 38 ml degased 0.4 molar HCl. Then, this solution was added to 300 ml of degased 25% NH4OH quickly in one portion while stirring in an ultrasonic bath. The addition of the base to the Fe2+/Fe3+ salt solution resulted in the formation of a black precipitate of MNPs immediately. Stirring in the ultrasonic bath continued for another 30 min. The black solid was collected using a magnet and washed with ethanol and distilled water three times. The sediment was then dispersed in 150 ml distilled water.
1. Typically, FeCl3·6H2O (5.838 g) and FeCl2·4H2O (2.147 g) were dissolved in 38 ml degased 0.4 molar HCl. Then, this solution was added to 300 ml of degased 25% NH4OH quickly in one portion while stirring in an ultrasonic bath. The addition of the base to the Fe2+/Fe3+ salt solution resulted in the formation of a black precipitate of MNPs immediately. Stirring in the ultrasonic bath continued for another 30 min. The black solid was collected using a magnet and washed with ethanol and distilled water three times. The sediment was then dispersed in 150 ml distilled water.
Since Fe3O4 nanoparticles are sensitive to heat and oxidative conditions, to protect and prevent oxidation of their surface, the magnetic nanoparticles were coated with a thin layer of SiO2. The SiO2 shell can effectively prevent chemical degradation and photo-dissolution of the Fe3O4 particles making the photocatalyst recyclable after multiple reaction cycles. So this action promotes the photocatalytic activity of TiO2 by decreasing the adverse influence of the magnetic core. In the second synthesis step of the silica-coated Fe3O4 nanoparticles, 50 ml of the previous suspension was added to a 250 ml solution of 2-propanol that contained ammonium hydroxide (4.5 ml). This dispersion was homogenized using ultrasonic vibration. Then, 6 ml tetraethoxysilane (TEOS) was slowly added. Finally, silica was formed on the surface of the magnetite nanoparticles through hydrolysis and condensation processes. The resulting silica-coated Fe3O4 nanoparticles were thoroughly washed with deionized water and collected using magnetic separation, followed by drying at 45 °C under vacuum for 12 h.
2.3. Synthesis of Fe3O4@SiO2@TiO2 nanocomposite
0.7 g of Fe3O4@SiO2 nanoparticles was dispersed in 150 ml of Merck ethanol and sonicated for 20 minutes. After that, 5 ml PEG 400 was added to the mixture. In a second vessel, 8 ml tetranormalbuthyltitanate was added to 20 ml of Merck ethanol and 1 ml acetylacetone and stirred using a magnetic stirrer for 10 minutes. The contents of the second vessel were added slowly to the above mixture while gently shaking using a mechanical stirrer. After 20 minutes, 2 ml deionized water was added and the final mixture was stirred at 70 °C for 12 hours. The obtained gray products were separated using an external magnet and washed thoroughly with ethanol and deionized water before drying at 60 °C. Subsequently, the powder was calcined at 400 °C under an air atmosphere for 2 hours to improve the crystalline properties of the obtained Fe3O4@SiO2@TiO2 nanoparticles. In this step, the gray powder changed color to brown. The final products were characterized using XRD, SEM, DRS and IR.2.4. Synthesis of Fe3O4@SiO2@TiO2/Pd nanocatalyst
Fe3O4@SiO2@TiO2/Pd nanoparticles were synthesized using a photodeposition method. All steps are summarized in Fig. 1. Pd(NO3)2·2H2O (0.0052 g) was dissolved in 40 ml deionized water to obtain a 0.5 mM solution and then 0.1 g Fe3O4@SiO2@TiO2 was added. The mixture was stirred using a probe sonicator for 20 minutes to disperse the magnetic nanostructures in the solvent. The mixture was conveyed to a quartz tube, 0.4 ml methanol was added and it was degased with nitrogen gas for 20 minutes. After that, the mixture in the tube was stirred under UV irradiation for 10 hours. During this step palladium ions were reduced to metallic palladium and deposited on the TiO2 surface. The final solid was separated using an external magnetic field, washed with ethanol and water three times and dried at 60 °C for 10 hours. In this process, Pd was selectively deposited on electron trapping sites. This is the key to Pd being highly deposited on Fe3O4@SiO2@TiO2 in the palladium(0) oxidation state. The process can be described as follows (Fig. 2): (1) first, electron activation with UV light will occur and the generated electron will migrate from the bulk TiO2 shell to its surface, (2) Pd2+ near the electron trapping sites is reduced to metal palladium, (3) instead of the former electron trapping sites, the resulting Pd clusters will serve as new electron sinks and after trapping electrons, these clusters will participate in the deposition of more Pd ions or in the reduction of reactants via this procedure.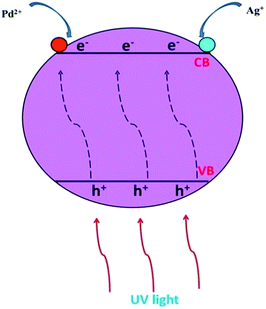 | ||
| Fig. 2 Schematic illustration of the deposition process of palladium and silver ions on the TiO2 nanoparticle surfaces. | ||
2.5. Synthesis of Fe3O4@SiO2@TiO2/Pd–Ag nanocatalyst
All circumstances were similar to the previous synthesis process. In addition to Pd, 0.0034 g Ag(NO3)2 was added to obtain a 0.5 mM solution of Ag2+. After sonication, degassing and adding methanol, the mixture was stirred under UV irradiation to photodeposit Ag and Pd on the TiO2 shell.2.6. Photocatalytic measurements
To investigate the catalytic activity of the photocatalysts, degradation of rhodamine B as a water pollutant was studied. The photocatalytic efficiency of the catalysts was investigated using a 100 ml quartz tube. 0.03 g of photocatalyst nanoparticles was mixed with 50 ml of rhodamine B solution (initial concentration of about 10 ppm). As we know, in polar solvents, RhB is known to exist in two principal forms, viz., in cationic (RhB+) or in zwitterionic form (RhB±). When the solution pH is higher than the acid dissociation constant of RhB, the carboxyl group becomes deprotonated and transformation of rhodamine B from the cationic form to the zwitterionic form will occur.But, protonation of rhodamine B enriches the hydrophobicity of the molecules and leads to an improvement in the degradation efficiency of dyes in acidic media. Also, during UV irradiation, H2O2 molecules that were formed could become highly unstable in basic media, so adding an acid like HNO3 will improve the rhodamine B photodegradation rate. Finally 1 ml HNO3 65% was added to the vessels. The resulting suspensions were stirred to obtain the maximum absorption of organic pollutant molecules on the photocatalyst surface, to make oxygen available for the reaction, and to obtain the highest level of homogeneity in the mixture. The reaction was carried out under UV lamp irradiation, and the mixture was placed inside the photoreactor in which the vessel was 40 cm away from the UV source of a 400 W Mercury lamp. After each 10 minute or 15 minute period, sampling was performed and the samples were juxtaposed to a magnet to separate the solid particles, which were subsequently analyzed using the UV-Vis spectrometer.3. Results and discussion
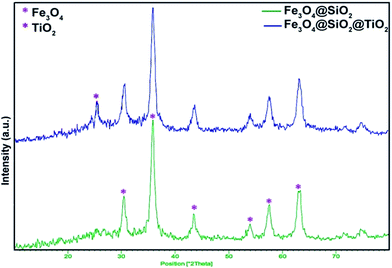
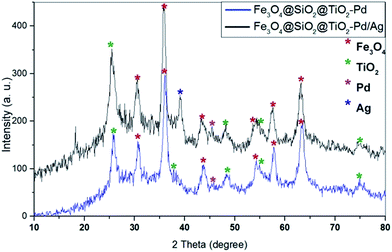
The structure of the magnetic nanoparticles was characterized using X-ray powder diffraction (XRD). Fig. 3 shows the XRD patterns of Fe3O4@SiO2 and Fe3O4@SiO2@TiO2. It can be seen that, in general, all of the powders are crystalline materials. For Fe3O4@SiO2 nanoparticles, six diffraction peaks were observed at 2θ = 30.56, 35.91, 43.61, 54.03, 57.59 and 63.19 that correspond to the crystalline Fe3O4 magnetic nanostructures (JCPDS card no. 75-0449). After interaction with titanium precursors and calcination at 400 °C, some peaks related to TiO2 emerged. The peak at 2θ = 25.5 is consistent with the values on the JCPDS card (no. 01-0562) which show the characteristics of anatase-phase of TiO2.Fe3O4@SiO2@TiO2/Pd and Fe3O4@SiO2@TiO2/Pd–Ag nanoparticles were synthesized using the photodeposition method. Previous investigations have demonstrated that palladium deposited on TiO2 nanostructure surfaces by UV deposition is mainly metallic (Pd0).28,29 It is commonly believed that the metallic state (M0) induces greater enhancement of the photocatalytic activity of TiO2 semiconductors by creating a Schottky junction between the metal and the semiconductor. The metal particle acts as a sink for photogenerated electrons and reduces the rate of their recombination with holes so that photocatalytic activity will increase.30 XRD analysis of Fe3O4@SiO2@TiO2/Pd and Fe3O4@SiO2@TiO2/Pd–Ag nanocomposites obtained using the photodeposition method confirmed that palladium and silver deposited on the supports are in the form of metallic palladium and silver and there were no peaks related to their oxide structures (Fig. 4).
As shown in the XRD patterns, the peaks at 2θ = 25.5, 48.17, 57.44 and 74.85 are consistent with the values on the JCPDS card (no. 01-0562) relating to anatase-phase TiO2. The peaks at 2θ = 39.09 and 45.33 correspond to metallic silver and palladium respectively. Since the loading of Pd is low, the diffraction peak related to Pd has a low intensity.
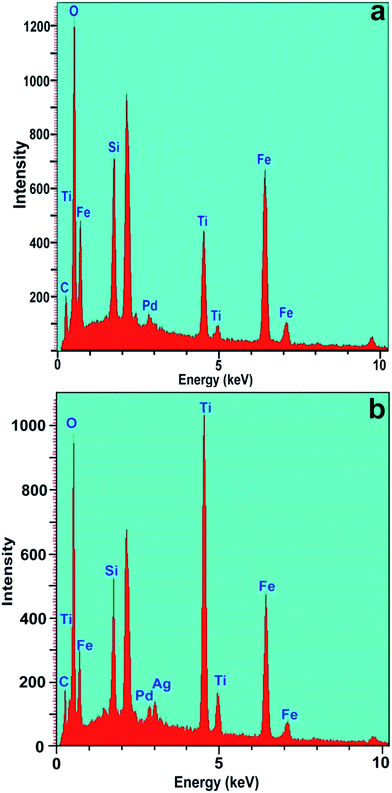
To confirm the chemical composition of the synthesized powders, elemental analysis of the chemical purity of the Fe3O4@SiO2@TiO2/Pd and Fe3O4@SiO2@TiO2/Pd–Ag samples was carried out using energy dispersive X-ray spectroscopy (EDS) analysis (Fig. 5). The EDAX spectra confirm that the targeted chemical composition was achieved in the powders. Obviously, the sample is composed of Si, O, Fe, Ti, Pd and Ag. So this analysis proved the successful photodeposition of palladium and silver ions on the titanium surface. Signals of C seen in the spectrum were due to the carbon film on the gold grid used to support the sample.
The morphologies of the Fe3O4@SiO2@TiO2, Fe3O4@SiO2@TiO2/Pd and Fe3O4@SiO2@TiO2/Pd–Ag powder samples were evaluated using scanning electron microscopy (SEM) as illustrated in Fig. 6. The images reveal that for Fe3O4@SiO2@TiO2, synthesized using the co-precipitation method and calcined at 400 °C, the particles are almost of uniform size with an average size of about 24 nm (Fig. 6a). When the surface of the Fe3O4@SiO2@TiO2 composite was coated with palladium and silver, the roughness and size of the particles increased (Fig. 6b and c). As seen from the SEM images, the nanocatalysts are aggregated which is due to calcination at high temperature, another reason for this is related to the lack of sonication before imaging while sonication before dye degradation was performed.
 | ||
| Fig. 6 SEM images of (a) Fe3O4@SiO2@TiO2, (b) Fe3O4@SiO2@TiO2/Pd and (c) Fe3O4@SiO2@TiO2/Pd–Ag nanocomposites. | ||
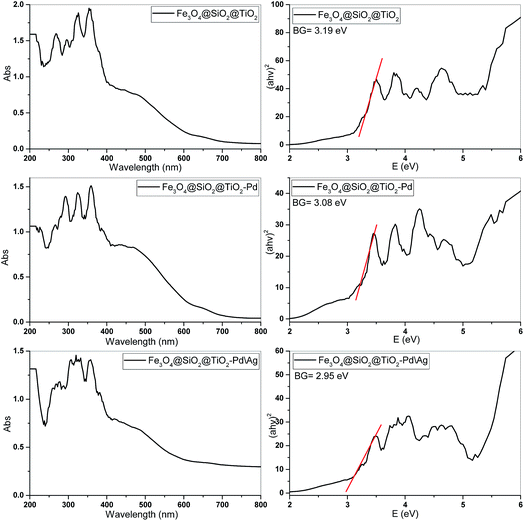
Light absorption by the material and the migration of the light-induced electrons and holes are the key factors controlling a photocatalytic reaction, and these features are related to the electronic structure characteristics of the material. The UV-Vis diffuse reflectance spectrum and the plot obtained by the transformation based on the Kubelka–Munk function versus the energy of light for the Fe3O4@SiO2@TiO2, Fe3O4@SiO2@TiO2/Pd and Fe3O4@SiO2@TiO2/Pd–Ag powder samples have been illustrated in Fig. 7.
Calculation of the band gap was performed using a Tauc plot. The Tauc plot is a method that is widely used for the determination of band gaps. The estimated band gap values (Eg) were 3.19, 3.08 and 2.95 eV for the Fe3O4@SiO2@TiO2, Fe3O4@SiO2@TiO2/Pd and Fe3O4@SiO2@TiO2/Pd–Ag samples, respectively. As shown, in addition to the UV ranges, all of the nanoparticles have photoresponsive properties in the visible region (approximately λ = 500 nm). The absorption band in the UV region (320–350 nm) corresponds to a band-to-band transition from the Ti(3d) level of titanium (conduction band) to the O(2p) level of oxygen (valence band). However, TiO2 coated on the Fe3O4@SiO2 support has a visible light response, ascribed to typical d–d transitions.
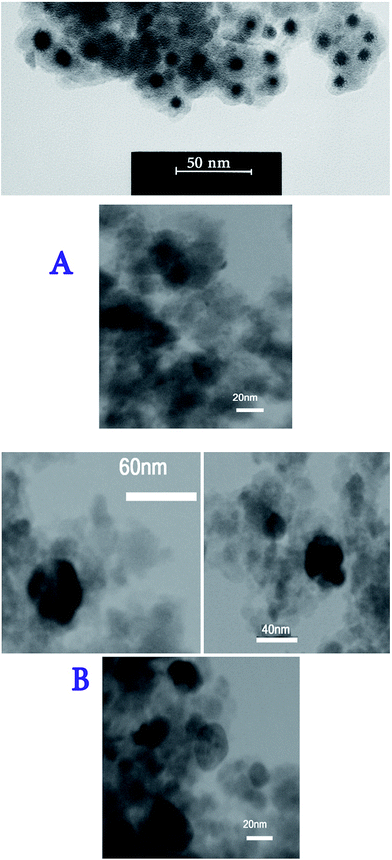
Transmission electron microscopy (TEM) was used to observe the core–shell structures of Fe3O4@SiO2@TiO2/Pd and Fe3O4@SiO2@TiO2/Pd–Ag NPs. Fig. 8 shows typical TEM images of the core–shell NPs. The TEM images demonstrate that the NPs have a core–shell structure with light contrast silica and TiO2 shells and dark contrast cores of Fe3O4, implying that the Fe3O4 magnetic NPs were successfully coated with silica shells.
Furthermore, TEM analysis of the nanoparticles indicated that both Fe3O4@SiO2@TiO2/Pd and Fe3O4@SiO2@TiO2/Pd–Ag nanocomposites were approximately spherical in nature with sizes ranging from 60 to 80 nm, with an average of 65 nm, and the average size of the Fe3O4 cores was about 40 nm. Moreover, the images showed that the coating was almost homogeneous and there are a lot of small particles that are related to the Pd and Ag nanoparticles.
FT-IR spectra were recorded separately at different stages of the preparation (Fig. 9). A significant feature in the FT-IR spectrum was the appearance of a stretching vibration of Fe–O at 590 cm−1. After coating the magnetic nanoparticles with a silica layer, wide bands at 1035 and 423 cm−1, related to Si–O stretching, appeared for the Fe3O4@SiO2 nanoparticles. For all samples, the absorption band at 1620–1630 cm−1 and the absorption band at approximately 3430 cm−1 were due to the stretching of Si–O–H and the vibrations of residual OH on the surface of the nanocomposites, and are also related to the vibrations of hydroxyl groups from adsorbed water or water from KBr used for spectroscopy. Since the silica coating has a large number of OH groups, the intensity and width of the OH bond peaks has increased for the Fe3O4@SiO2 sample. These surface groups are a benefit and allow the nanocores to be easily modified in the future. After modification of Fe3O4@SiO2 with TNBT to obtain Fe3O4@SiO2@TiO2, the band at 1035 cm−1 disappeared and the broad bands at 500–600 cm−1 appeared in the spectrum, and were assigned to the stretching of the Ti–O bond and the bending of the O–Ti–O bond which showed the successful formation of the Fe3O4@SiO2@TiO2 composite.
In addition, a stretching vibration at a wave number of 950 cm−1 of Ti–O–Si was detected which ascertains that TiO2 is strongly attached to the surface of the silica layer by covalent bonds. In the FT-IR spectrum for the immobilized palladium and silver components, the intensity of the hydroxyl characteristic peaks will diminish because of the localized silver and palladium species on the support surface.
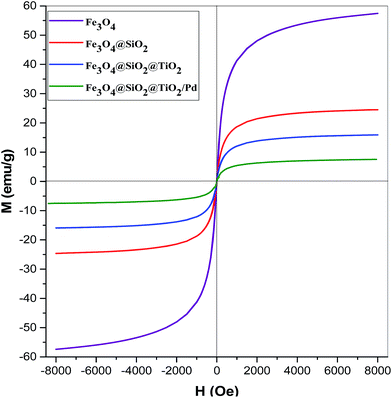
The magnetic properties of the magnetic nanocatalysts were characterized using a vibrating sample magnetometer (VSM). Fig. 10 shows the typical room temperature magnetization curves of bare Fe3O4 MNPs, silica-coated Fe3O4 MNPs and the Fe3O4@SiO2@TiO2 and Fe3O4@SiO2@TiO2/Pd samples. As shown in this figure, the saturation magnetizations (Ms) of the samples are 57.4, 24.5, 15.9 and 7.5 emu g−1, respectively. The results of the VSM analysis prove that the immobilized palladium and palladium/silver catalysts show a typical superparamagnetic behaviour. However, the saturation magnetization of the Fe3O4 nanoparticles is 57.4 emu g−1, which is significantly smaller than that of bulk magnetite. The reduction in the measured saturation magnetization is due to the ultra-small size of the Fe3O4 particles and the added mass of some of the nonmagnetic layers on them.
Photocatalytic degradation of rhodamine B by Fe3O4@SiO2@TiO2, Fe3O4@SiO2@TiO2/Pd and Fe3O4@SiO2@TiO2/Pd–Ag nanoparticles was performed under ultraviolet light irradiation. Changes in dye concentration were followed by UV absorption spectroscopy. The degradation of rhodamine B over time t (DP(t)) was calculated using the following equation:
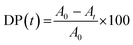 | (1) |
To further understand the photodegradation process and the enhanced photocatalytic mechanism with Pd NPs, a proposed reaction mechanism is depicted in Fig. 11. When the Pd NPs come into contact with the TiO2 surface in the absence of UV light, the electrons transfer from palladium to TiO2 until the two systems achieve equilibrium (Ef). This phenomenon occurs because the Fermi energy level of Pd is higher than that of TiO2.29 When the photocatalysts were exposed to UV irradiation with a photon that has higher energy than the band gap of TiO2, electrons and holes are formed in the conduction band (CB) and valence band (VB) of TiO2, respectively. Since palladium nanoparticles have the ability to accumulate a large quantity of electrons, the transfer of photoexcited electrons from TiO2 to the Pd NPs causes the overall Fermi level (Ef) of the composites to shift to a more negative potential (E′′f).
The new Fermi level (E′′f) of the CB of TiO2 has a lower energy level, so the electrons in the CB can transfer from TiO2 to the Pd NPs. This transfer causes charge separation and results from a Schottky barrier being formed at the metal–semiconductor interface and leads to a decrease in the recombination probability of photo electrons with their counterparts.30
To confirm the catalysts effect, first the mixture was stirred in dark conditions. No dye degradation was observed after 40 minutes without using UV light irradiation or nanopowder photocatalysts, so from this result we can say that the contribution of self-degradation is insignificant. For Fe3O4@SiO2@TiO2/Pd and Fe3O4@SiO2@TiO2/Pd–Ag nanocatalysts, as time passed, the dye was decomposed and the color of the solution became brighter, until at t = 40 min a rather transparent solution was obtained (Fig. 12).
 | ||
| Fig. 12 Rhodamine B after 40 minutes photodegradation in the presence of Fe3O4@SiO2@TiO2/Pd nanocomposite. | ||
Results for the change in the concentration of the dye after 40 min under UV irradiation are depicted in Fig. 13. The results confirm that most dye degradation processes occur in the initial minutes. Over time, the degradation rate decreases because of a decrement in the dye molecules surrounding the catalyst nanoparticles. Calculations of the photocatalytic activity were performed according to eqn (1). The rhodamine B degradation after 40 minutes by the Fe3O4@SiO2@TiO2/Pd–Ag catalyst was obtained at 96.1%, while it was about 84% for Fe3O4@SiO2@TiO2/Pd and 63% for Fe3O4@SiO2@TiO2.
All of the reaction conditions for all samples were kept the same, so the higher catalytic activity of Fe3O4@SiO2@TiO2/Pd–Ag versus Fe3O4@SiO2@TiO2/Pd can be attributed to the synergistic effect between Ag and Pd, because the Pd catalyst surface was modified by the addition of a second metal. Also previous research has demonstrated that the presence of Ag NPs enhances the photocatalytic activity of TiO2 films due to the effect of localized surface plasmon resonance.31
The as prepared nanopowder photocatalysts presented good photocatalytic activity. Here, palladium and silver act as electron acceptors and collecting the photo-induced electrons leads to a decrease in the recombination probability of the photoholes with their counterparts. This phenomenon will increase the fraction of photoholes available for the oxidizing interfacial charge-transfer reactions and will make active sites for dye degradation.32 The magnetic properties of the catalysts allow fast separation of the catalysts from the reaction media, and simplify the catalyst separation and reuse of them in successive reactions which is an important issue from economical points of view. The catalyst recycling was carried out under the same previous reaction conditions.
After each 40 minute time period, the catalyst was removed easily using an external magnet, washed with ethanol, water and acetone, successively, and reused in the next catalytic cycle with fresh reactants. The results are shown in Fig. 14. The Fe3O4@SiO2@TiO2/Pd and Fe3O4@SiO2@TiO2/Pd–Ag catalysts were reused for five and six consecutive cycles, respectively. For the Fe3O4@SiO2@TiO2/Pd–Ag sample after six cycles, the degradation percentage was decreased to 84%, also this factor for the Fe3O4@SiO2@TiO2/Pd sample after 5 cycles was reduced to 77%. The decrease in catalytic activity after recycling relates to palladium and silver leaching.
4. Conclusion
In summary, herein we described an effective procedure to synthesize magnetically separable photocatalysts. Fe3O4@SiO2@TiO2, Fe3O4@SiO2@TiO2/Pd and Fe3O4@SiO2@TiO2/Pd–Ag nanocomposites were synthesized using a combination of co-precipitation, sol–gel and photodeposition methods and characterized using XRD, SEM, VSM, EDS, DRS and IR techniques.VSM analysis showed superparamagnetic properties for all samples and scanning electron microscopy confirmed the nanosize of the composites. The photocatalytic activity of the synthesized nanopowders was studied. Rhodamine B was degraded by the catalysts under similar conditions. The results showed that Fe3O4@SiO2@TiO2/Pd–Ag has the best photocatalytic activity of the catalysts studied. Previous investigations proved that heterogeneous photocatalytic processes consists of diffusion, adsorption and reaction steps and suitable distribution of the pores will improve the diffusion of reactants, which increases the rate of photocatalytic reaction. In this study, the enhanced photocatalytic activity may be attributed to a synergic effect between Ag and Pd. The high photocatalytic activity, efficient magnetic separation, good chemical stability, and relatively low cost means that the multifunctional Fe3O4@SiO2@TiO2/Pd–Ag photocatalyst has promising applications in large-scale photocatalytic reactions in industry.
Acknowledgements
The authors are grateful to the council of the Iran National Science Foundation and University of Kashan for supporting this work with Grant No. 159271/879.References
- B. M. Choudary, M. L. Kantam, K. V. S. Ranganath, K. Mahendar and B. Sreedhar, J. Am. Chem. Soc., 2004, 126, 3396–3397 CrossRef CAS PubMed.
- R. Abu-Reziq, H. Alper, D. Wang and M. L. Post, J. Am. Chem. Soc., 2006, 128, 5279–5282 CrossRef CAS PubMed.
- Q. Yang, S. Ma, J. Li, F. Xiao and H. Xiong, Chem. Commun., 2006, 2495–2497, 10.1039/b605733k.
- B. Feng, R. Y. Hong, L. S. Wang, L. Guo, H. Z. Li, J. Ding, Y. Zheng and D. G. Wei, Colloids Surf., A, 2008, 328, 52–59 CrossRef CAS.
- K. Mertins, I. Iovel, J. Kischel, A. Zapf and M. Beller, Angew. Chem., Int. Ed., 2005, 44, 238–242 CrossRef CAS PubMed.
- R. Tatumi, T. Akita and H. Fujihara, Chem. Commun., 2006, 3349–3351, 10.1039/b605390b.
- D. Astruc, F. Lu and J. R. Aranzaes, Angew. Chem., Int. Ed. Engl., 2005, 44, 7852–7872 CrossRef CAS PubMed.
- N. T. S. Phan and C. W. Jones, J. Mol. Catal. A: Chem., 2006, 253, 123–131 CrossRef CAS.
- J. Liu, Y.-Q. Li and W.-J. Zheng, Monatsh. Chem. Chem. Mon., 2009, 140, 1425–1429 CrossRef CAS.
- J. J. E. Hardy, S. Hubert, D. J. Macquarrie and A. J. Wilson, Green Chem., 2004, 6, 53 RSC.
- D. Guin, B. Baruwati and S. V. Manorama, Org. Lett., 2007, 9, 1419–1421 CrossRef CAS PubMed.
- N. T. S. Phan, C. S. Gill, J. V. Nguyen, Z. J. Zhang and C. W. Jones, Angew. Chem., Int. Ed., 2006, 45, 2209–2212 CrossRef CAS PubMed.
- Z. Zi, Y. Sun, X. Zhu, Z. Yang, J. Dai and W. Song, J. Magn. Magn. Mater., 2009, 321, 1251–1255 CrossRef CAS.
- H. Khojasteh, V. Mirkhani, M. Moghadam, S. Tangestaninejad and I. Mohammadpoor-Baltork, J. Nano Struct., 2015, 5, 271–280 Search PubMed.
- P. Sathishkumar, N. Pugazhenthiran, R. V. Mangalaraja, A. M. Asiri and S. Anandan, J. Hazard. Mater., 2013, 252–253, 171–179 CrossRef CAS PubMed.
- H. Zhao, Y. Dong, P. Jiang, G. Wang, J. Zhang and K. Li, Catal. Sci. Technol., 2014, 4, 494–501 CAS.
- S. Shylesh, V. Schünemann and W. R. Thiel, Angew. Chem., Int. Ed., 2010, 49, 3428–3459 CrossRef CAS PubMed.
- V. Polshettiwar, B. Baruwati and R. S. Varma, Green Chem., 2009, 11, 127–131 RSC.
- N. T. S. Phan and H. V. Le, J. Mol. Catal. A: Chem., 2011, 334, 130–138 CrossRef CAS.
- M. J. Jin and D. H. Lee, Angew. Chem., Int. Ed. Engl., 2010, 49, 1119–1122 CrossRef CAS PubMed.
- T.-J. Yoon, W. Lee, Y.-S. Oh and J.-K. Lee, New J. Chem., 2003, 27, 227–229 RSC.
- X. Li, D. Liu, S. Song and H. Zhang, Cryst. Growth Des., 2014, 14, 5506–5511 CAS.
- C. Xue, Q. Zhang, J. Li, X. Chou, W. Zhang, H. Ye, Z. Cui and P. J. Dobson, J. Nanomater., 2013, 2013, 8 Search PubMed.
- Y. Chi, Q. Yuan, Y. Li, L. Zhao, N. Li, X. Li and W. Yan, J. Hazard. Mater., 2013, 262, 404–411 CrossRef CAS PubMed.
- F. Niu, L. Zhang, S.-Z. Luo and W.-G. Song, Chem. Commun., 2010, 46, 1109–1111 RSC.
- K. S. Lee, M. H. Woo, H. S. Kim, E. Y. Lee and I. S. Lee, Chem. Commun., 2009, 3780–3782, 10.1039/b905220h.
- Y. Jiang, C. Guo, H. Xia, I. Mahmood, C. Liu and H. Liu, J. Mol. Catal. B: Enzym., 2009, 58, 103–109 CrossRef CAS.
- S. Shylesh, L. Wang and W. R. Thiel, Adv. Synth. Catal., 2010, 352, 425–432 CrossRef CAS.
- N. Zhang, S. Liu, X. Fu and Y.-J. Xu, J. Phys. Chem. C, 2011, 115, 9136–9145 CAS.
- P. Wang, T.-F. Xie, H.-Y. Li, L. Peng, Y. Zhang, T.-S. Wu, S. Pang, Y.-F. Zhao and D.-J. Wang, Chem.–Eur. J., 2009, 15, 4366–4372 CrossRef CAS PubMed.
- J.-H. Oh, H. Lee, D. Kim and T.-Y. Seong, Surf. Coat. Technol., 2011, 206, 185–189 CrossRef CAS.
- A. Gautam, A. Kshirsagar, R. Biswas, S. Banerjee and P. K. Khanna, RSC Adv., 2016, 6, 2746–2759 RSC.
| This journal is © The Royal Society of Chemistry 2016 |