Detergent-free decellularization of bovine costal cartilage for chondrogenic differentiation of human adipose mesenchymal stem cells in vitro†
Evren Erten a,
Tugba Sezgin Arslan
a,
Tugba Sezgin Arslan a,
Burak Derkusb and
Yavuz Emre Arslan
a,
Burak Derkusb and
Yavuz Emre Arslan *a
*a
aRegenerative Biomaterials Laboratory, Department of Bioengineering, Engineering Faculty, Canakkale Onsekiz Mart University, Canakkale 17100, Turkey. E-mail: yavuzea@gmail.com; Fax: +90-286-218-0018; Fax: +90-286-218-0541
bBioelectrochemistry Laboratory, Department of Chemistry, Ankara University, Tandogan, Ankara 06100, Turkey
First published on 12th September 2016
Abstract
In this study, we report a novel, detergent-free decellularization protocol for the preparation of intact cartilage ECM-based scaffolds (CEbS) during an effective decalcification process. On treatment with 10 mM Na2EDTA, the amount of calcium lost was around 55% ± 5% (percent ± S.D.%) (n = 3) and nearly 84% of the nuclear material was removed; however, the most effective removal was observed on treatment with 10 mM Na2EDTA combined with 0.5% Triton X-100 for 48 hours. Notably, our proposed method decreased the GAG content by only 5% compared to untreated CEbS (380.37 ± 16.02 μg mg−1 dry weight). There was no significant difference in hydroxyproline content between the untreated (13.04 ± 1.51 μg mg−1 dry weight) sample and our proposed method (12.95 ± 1.55 μg mg−1 dry weight). The scaffold morphology and cell attachment were evaluated using SEM micrographs, and the cells that were inoculated with detergent-free decellularized CEbS for 14, 21 and 28 days covered the scaffold area, including the porous cavities. Microscopic observations showed that the cell density increased day by day and there was no cytotoxic evidence for the scaffolds, which is a desirable environment for cells. The histochemical and immunohistochemical assessments are supported by glycosaminoglycan and hydroxyproline assays. The proposed detergent-free decellularization technique could be a promising method for cartilage tissue regeneration.
1 Introduction
Tissue engineering is an interdisciplinary field that offers promising, therapeutic approaches to treat injuries and it also provides an emerging medical market.1,2 Main strategies in tissue engineering include using cells, scaffolds and signaling factors alone, or together, depending on the tissue problem. Various tissues have been previously studied, such as bladder,3 heart,4 skin5 and blood vessels,6 with tissue engineering techniques, which are also available for cartilage tissue engineering.7 Cartilage is mainly avascular tissue, composed of a relatively small number of chondrocytes that are embedded in a dense extracellular matrix (ECM) with sparse distribution. This ECM is significantly established by collagen type II and proteoglycans, which impart their own mechanical properties to the cartilage tissue.8,9Many people suffer from musculoskeletal conditions because of aging and sports related injuries;10 other complications of cartilage may be developmental or tumor related.11,12 Injuries to this tissue cause pain and decreased mobility, thus affecting the quality of life. Articular cartilage has limited self-repair capacity that decreases with aging. Conventional therapeutic strategies, such as microfracture or autologous chondrocyte transplantation are limited.7 Since one of the most important problems is donor shortage, allografts also have limitations, just like autografts. Therefore, it is observed that sources of human origin are not sufficient to solve the problem.13 These limitations make processed cartilage tissue a good candidate for regenerative medicine applications.
Biological scaffolds derived from whole decellularized tissues are used successfully for research and clinical purposes. The decellularization process provides acellular ECM, inducing the stem cells with the chemical signals they contain within their own structure, and supporting the repopulated cells with their native and mechanically durable construction, and thus, enable the creation of promising regenerative biomaterials to be used in regenerative medicine. The procedures of harvesting ECM, and other conditions like sterilization and species of origin affecting ECM quality, host responses of the organism after the transplantation. The ECM quality may be considered with its biological, mechanical and biochemical properties as biocompatible behavior for the organism to be treated.14 At the same time, the decellularized ECM quality should support stem cells for the differentiation of the applied tissue. The scaffolds used in tissue engineering must be non-immunogenic and with the technique of decellularization, this problem can be surmountable.4 The biological origin of scaffolds may be allogeneic or xenogeneic. To prepare decellularized ECM, Gilbert et al. have applied various decellularization methods in tissue engineering, depending on the applied tissue.14 Synthetic materials, like silicone, are also available to be used for cartilage related injuries, but these materials may cause infections and complications.15 This problem and the limitations of synthetic materials make ECM based matrices more attractive, compared to the synthetic materials.16
Decellularized ECM is one of the best choices to establish a scaffold for tissue engineering, due to the following reasons. ECM supports the growth and migration of cells residing on a tissue, and it provides tissue specific functions, such as structural and mechanical properties, and elasticity or rigidity. Moreover, ECM may provide cell regulations for their own activities and it may show the properties of the reservoir for growth factors, and promote cells for their bioactivities. ECM also provides a flexible physical environment during morphogenesis or the developmental process, by which it helps to maintain homeostasis, an important issue for wound healing.17 Previous studies show that the removal of cells from cartilage ECM decreases the amount of glycosaminoglycans (GAG), which are critical for soft connective tissues.18 There are a number of studies on the decellularization of cartilage, yet they report similar problems.16,19,20 Alternatively, Xu et al. showed that Triton X-100 treatment maintained the ECM structure more than sodium dodecyl sulfate (SDS) treatment; however it undesirably decreased the GAG amount as well. Nevertheless, Triton X-100 maintained the GAG amount more than other protocols.21
Gilbert et al. reviewed several decellularization techniques and reported that almost all detergent based procedures disrupt native tissue structure. Enzymatic treatments can also be used for decellularization but like other protocols, this treatment reduces vital components and the method may cause immune response. A good decellularization process should remove cellular and nuclear material, but vital components and ECM structure must remain and the process should not cause adverse effects. Additionally, duration and decellularization agent rate are among the important issues; this rate was also discussed by Elder et al.14,21,22
In this study, we aimed to establish a quick and effective decellularization process, and construct ECM-based scaffolds from bovine costal cartilage, maintaining fundamental cartilage ECM contents, such as GAGs, hydroxyproline and collagen type II. To compare the proposed decellularization protocol, Triton X-100 detergent was used with different concentrations (0.1% and 0.5% (v/v)) and with different time points (24 and 48 hours). DNA content analysis was performed to determine the cellular remnants. Hydroxyproline and GAG contents were also assessed with calorimetric methods to evaluate the alterations of the extracellular matrix composition. Chemical and physical characterizations were carried out by means of ATR-FTIR and thermal analysis. SEM imaging was performed to characterize scaffold morphology, with and without cells, and histological and immunohistochemical examinations were carried out with hematoxylin eosin, safranin O, anti-collagen type II and anti-aggrecan to investigate the chondrogenic differentiation of human adipose mesenchymal stem cells.
2 Materials and methods
2.1 Materials
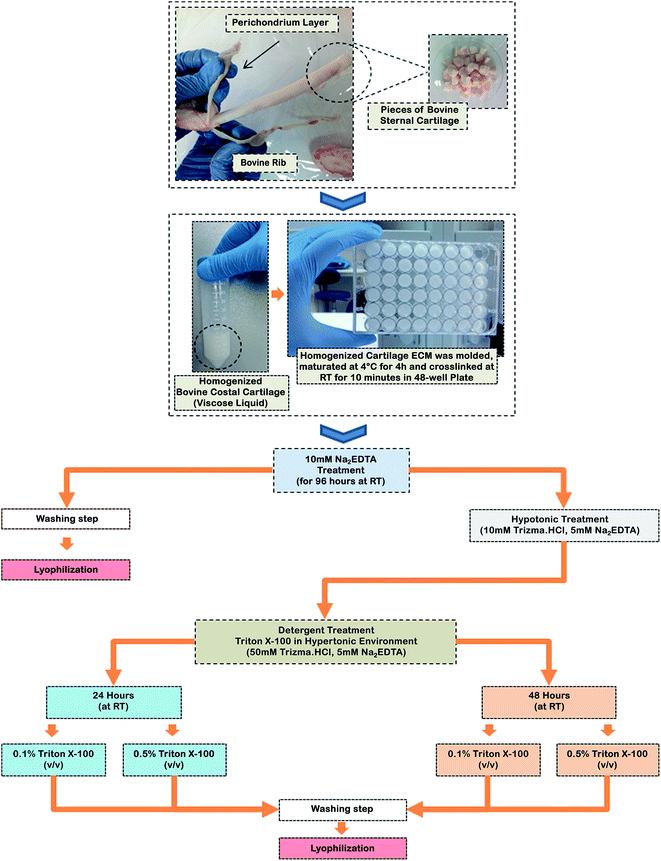
Bovine costal cartilage (BCC) was obtained within 4–6 hours following the slaughtering of the healthy animals (8–10 months old) at a local slaughter house located in Canakkale, Turkey. The native cartilage was obtained and cut into 1 × 1 cm cubic-like dimensions and then promptly stored at −86 °C until use. All chemicals were purchased from Sigma-Aldrich, unless otherwise noted.2.2 Homogenization of BCC
BCC, also known as sternal cartilage, was harvested from anterior ends of the ribs attached to the sternum. The perichondrium layer of BCC was mechanically separated using surgical equipment. The cartilage was then washed with tap water and cut into small pieces of about 0.5 × 0.5 cm cubic-like dimensions, with surgical equipment, to be used in the homogenization process. Small native cartilage, weighing about 5 grams in the wet form, was then rinsed with Milli-Q water (Merck-Millipore, Germany) to avoid possible artifacts. Cartilage was added to 0.01 M HCl solution and the solution was homogenized for two minutes at 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm (IKA, T-18 Basic Ultra TURRAX, Germany), followed by incubation for 2 minutes in an ice bath to prevent over-heating of the cartilage–HCl solution. This step was repeated two times. After homogenization, the solution turned into gel form and the final volume was adjusted to 20 ml with 0.01 M HCl.
000 rpm (IKA, T-18 Basic Ultra TURRAX, Germany), followed by incubation for 2 minutes in an ice bath to prevent over-heating of the cartilage–HCl solution. This step was repeated two times. After homogenization, the solution turned into gel form and the final volume was adjusted to 20 ml with 0.01 M HCl.
2.3 Preparation of cartilage ECM-based scaffolds
To prepare cartilage ECM-based scaffolds (CEbS), 200 μL of the gel solution was transferred into each well of a 48-well plate. The plate was left to maturate at 4 °C for four hours, and was subsequently stored overnight at −86 °C, then the homogenized-cartilage matrix was lyophilized for 24 hours (Telstar, LyoQuest, Spain). The lyophilized scaffolds were crosslinked using N-hydroxysuccinimide (NHS)/N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDC) for ten minutes, as described previously by Buttofoco et al.23 Following the washing step, the crosslinked CEbS were stored overnight at −86 °C, then lyophilized for 24 hours. Crosslinked and non-crosslinked CEbS were prepared; the dimensions were h = 2 mm and Ø = 1 cm (Fig. 1A). For physicochemical characterization of the scaffolds, FT-IR measurements were carried out with a Shimadzu IRAffinity-1 model infrared spectrometer (Japan) and TGA thermograms were obtained using Shimadzu DTA60 (Japan) for prepared scaffolds.2.4 Detergent-free decellularization of CEbS
The decellularization protocol was conducted using 10 mM ethylenediaminetetraacetic acid disodium salt dihydrate (Na2EDTA, pH 7.2–7.4), for 96 hours, as represented in Scheme 1. The Na2EDTA solution was used as a decalcification agent and refreshed every 48 hours. After the washing step, crosslinked-CEbS were stored overnight at −86 °C and lyophilized for 24 hours. Calcium loss was estimated using eqn (1), as given below:| Initial weight − final weight/initial weight × 100 = dry weight% | (1) |
Samples were then treated with Triton X-100 at different concentrations [0.1% and 0.5% (v/v)] to understand the effect of detergent treatments on the decellularization process after the decalcification step. Briefly, decalcified-CEbS were treated with a hypotonic solution (10 mM Trizma, HCl, 5 mM Na2EDTA and pH 8.0) for 24 hours. Then 0.1% and 0.5% (v/v) of Triton X-100, prepared in hypertonic solution (50 mM Trizma. HCl, 5 mM Na2EDTA and pH 8.0), were applied for 24 and 48 h, respectively. Decellularized and crosslinked CEbS were rinsed with Milli-Q water and stored overnight at −86 °C, then were lyophilized for 24 hours. Decalcified CEbS were used in all cell culture experiments and were defined as detergent-free decellularized CEbS.
2.5 Evaluating the DNA content of decellularized CEbS
The nuclear contents of decalcified and decalcified/detergent treated CEbS were assessed using a genomic DNA purification kit (Thermo Scientific, GeneJET) by following the manufacturer's instructions. The ratio of adsorptions at 260 nm and 320 nm was measured using a microplate reader (Thermo-Scientific, Multiskan™ GO with μdrop™ plate). Calculations were performed using the equation given below.| DNA conc. (μg ml−1) = (A260 − A320) × 50 × (10 mm per 0.51 mm) | (2) |
2.6 Glycosaminoglycan assay
The quantity of sulfated GAG levels in decalcified and decalcified/detergent treated CEbS was determined as previously described by Farndale et al.24 and Hoemann et al.25 Briefly, all samples were digested with papain solution (100 mM Na2HPO4, 10 mM Na2EDTA, 10 mM L-cysteine and 0.125 mg mL−1 papain in Milli-Q water, pH 7.5) at 65 °C overnight, with gentle shaking by a block heater (Jeiotech, Korea). Afterwards, the papain reagent was deactivated at 100 °C for ten minutes. The extracts were then treated with 1,9-dimethylmethylene blue (DMMB) dye reagent. Finally, absorbance was immediately measured at 525 nm (Shimadzu UV mini-1240 UV-Vis Spectrophotometer, Japan). Sulfated GAG contents of the samples were calculated according to the equation given below:| μg GAG per mg dry weight = [(OD525/slope) × (dilution factor × total volume)]/cartilage weight (mg) | (3) |
2.7 Hydroxyproline assay
The total collagen amount of decalcified and decalcified/detergent treated CEbS was determined using a commercially available hydroxyproline assay kit, adhering to manufacturer's instructions (Sigma-Aldrich, Germany). To ensure accurate determination, all samples were spiked with 0.4 μg of the hydroxyproline standard. Absorbance at 560 nm was measured with the microplate reader. The hydroxyproline concentration was calculated using eqn (4):| Hydroxyproline amount in sample = A560 (sample)/A560 (spiked control) − A560 (sample) × 0.4 μg | (4) |
2.8 Scaffold porosity
Liquid displacement analysis was performed to evaluate the porosity of detergent-free decellularized CEbS, according to Kim et al.26 Briefly, a dry scaffold was placed into a known volume (V1) of hexane in a graduated cylinder for 5 minutes and then the total volume was recorded as V2. Subsequently, the hexane-impregnated scaffold was removed from the cylinder and the remaining volume in the graduated cylinder was recorded as V3. The porosity (%) of the scaffold was calculated using the eqn (5) as follows:| Porosity (%) = (V1 − V3)/(V2 − V3) × 100 | (5) |
2.9 Mechanical testing
A uniaxial confined compression test was applied to native cartilage tissue and CEbS (n = 3), in order to assess the mechanical properties (Zwick/Roell Z250 Universal Instron) using a 100 N load cell. The test speed was set to 5 mm min−1, whereas the preload was set to 5 mN. The test was aborted when a maximum load of 100 N was reached and the linear modulus was calculated using testXpert software.2.10 Cell culture study
Human adipose mesenchymal stem cells (hAMSCs) and expansion medium were purchased from Merck-Millipore (Human Adipose Mesenchymal Stem Cell Kit, Cat. no. SCC038, Lot no. QVP1303200, USA). hAMSCs were expanded and observed with an inverted phase-contrast microscope (Zeiss, PrimoVert, Germany). Passages 2 and 5 were used for all experiments. To validate the multipotency capacity of purchased hAMSCs, multilineage differentiation studies were performed. Briefly, hAMSCs were cultured with osteogenesis, chondrogenesis and adipogenesis differentiation kits (Gibco, StemPro®, USA) according to the manufacturers' procedures. Osteogenic (alizarin red S staining) and adipogenic (oil red O staining) differentiations were characterized as histological, and observed with an inverted phase-contrast microscope. For chondrogenic differentiation, 3–5 micron thick sections of chondrogenic pellet were stained with safranin O and alcian blue 8gx, and observed with a light microscope (Zeiss, Axio Scope A1, Germany). Standard histological staining was performed for all studies.The detergent-free decellularized CEbS were sterilized under ultraviolet light (254 nm) for two hours and rinsed with sterile phosphate buffered saline (pH 7.2–7.4). The cells (4.5 × 105) were seeded on each detergent-free decellularized CEbS. Then, cells were cultured in DMEM low glycose supplemented with 10% Fetal Bovine Serum (FBS), 1% L-glutamine and 1% penicillin–streptomycin (all from Biological Industries, USA). The medium was replaced every 2–3 days. The cell-scaffold constructs were incubated at 37 °C, 5% CO2 and 95% relative humidity conditions (Panasonic, Japan) for 14, 21 and 28 days.
The viability of cells on the scaffolds was confirmed by a cell growth determination kit, MTT based (Sigma-Aldrich, Germany). The assay was performed as described by the manufacturer's procedure. Cell viability was assessed by colorimetric measurements at a wavelength of 570 nm on days 14, 21 and 28.
2.11 Scanning electron microscopy
Field emission scanning electron microscopy (FE-SEM JFM 7100F EDS, JEOL, Japan) was used to obtain information about the surface morphology and cellular behaviors of untreated, detergent-free decellularized and recellularized CEbS. Specimens were fixed in 2.5% glutaraldehyde, prepared in PBS (pH 7.2–7.4) and immersed in an ethanol series (50%, 70%, 80%, 90%, 95%, and 100%) for dehydration. They were then allowed to dry at room temperature. Dried samples were then sputter-coated with Pd–Au and SEM micrographs were achieved at 10 kV and different magnifications in a high vacuum.2.12 Histological analysis
Cellular behaviors such as attachment, proliferation and differentiation on detergent-free decellularized CEbS were assessed by qualitative histological analysis. Tissue samples were fixed in 10% neutral buffered formalin for at least 48 hours. Detergent-free decellularized CEbS were used as the control group. Briefly, specimens were immersed in a series of ethanol and xylene solutions, then embedded in paraffin. Sectioned at a thickness of 3–5 μm, the sections were deparaffinized, rehydrated then stained with hematoxylin & eosin (H & E) and safranin O by following routine staining protocols. Immunohistochemical evaluation of the scaffolds was performed by Ultra Vision Quanto Detection System (Thermo, USA) according to the manufacturer's protocol. Primer antibodies, aggrecan (Anti-Aggrecan, MMP Cleaved, clone AF-28, Ascites Free Cat. # MAB19310-C, Merck) and collagen type II (Collagen II Ab-2 Clone 2B1.5 Cat #MS-235-P0, Thermo) were applied to tissue sections and incubated for an hour in humidified box. Then, slides were rinsed with PBS and secondary antibody applied for 20 min. Finally, all sections were observed with light microscope.2.13 Statistical analysis
In order to analyze the mean differences, one-way analysis of variance (ANOVA) and Tukey's test were conducted using Origin Pro 8SR0 (v8.0724, OriginLab Corporation, MA, USA). P-value less than 0.05 (p < 0.05) was considered as significant. The mean and standard deviation (±SD) of the variables were calculated using Excel, Microsoft Office Professional Plus 2016.3. Results and discussion
The articular cartilage contained about 20–50% calcium27 and it was considered that this component might be an obstacle for effective decellularization. By treating with 10 mM Na2EDTA, the lost calcium amount was found to be around 55 ± 5% (percent ± S.D.%; n = 3). The calculation of lost calcium amount was conducted using eqn (1). The homogenization process was performed and the bovine costal cartilage was successfully prepared as desired, with the dimensions of the mold (Scheme 1). Homogenization with 0.01 M HCl and preparation of the scaffold changed the nature of cartilage tissue, which prevented the penetration of solution into the deep tissue areas, due to its dense structure.28,29 It also provided an important opportunity to prepare scaffolds with desired dimensions and porous structure, together with vital ECM components, and the post crosslink process also indicated the same morphology as the pre-crosslink process (Fig. 1A).The success of the decellularization protocols was examined with DNA, hydroxyproline and GAG content analyses. The DNA quantification results showed that 10 mM Na2EDTA, 10 mM Na2EDTA combined with 0.1% Triton X-100 (24 h), 10 mM Na2EDTA combined with 0.5% Triton X-100 (24 h), 10 mM Na2EDTA combined with 0.1% Triton X-100 (48 h), and 10 mM Na2EDTA combined with 0.5% Triton X-100 (48 h), reduced the dsDNA content by nearly 84 ± 0.9%, 83 ± 0.8%, 87 ± 0.3%, 87 ± 1.9%, 90 ± 0.5%, respectively (Fig. 1B). About 84% of the nuclear material was removed by the 10 mM Na2EDTA protocol; however, the most effective removal was observed with 10 mM Na2EDTA combined with 0.5% Triton X-100 (48 h) (Fig. 1B); thus showing the power of this method, which is also in agreement with literature studies.16,21 Removing the cellular content from native tissue greatly reduces several risks, such as viral transmissions, potential immune responses and tissue compatibility problems after clinical application.14,28,30 As a result, an effective one step decellularization was performed during the decalcification process.
To characterize the ECM composition of the decellularized CEbS, the sulfated GAG content of the decellularized scaffolds were investigated. Sulfated GAGs were extracted from untreated, decalcified and decalcified/detergent treated CEbS, and then the amount of sulfated GAGs was ascertained using DMMB assay. The GAG contents for 10 mM Na2EDTA, 10 mM Na2EDTA combined with 0.1% Triton X-100 (24 h), 10 mM Na2EDTA combined with 0.5% Triton X-100 (24 h), 10 mM Na2EDTA combined with 0.1% Triton X-100 (48 h), and 10 mM Na2EDTA combined with 0.5% Triton X-100 (48 h) were found to be 360.94 ± 9.97 μg mg−1, 336.44 ± 12.00 μg mg−1, 328.07 ± 3.39 μg mg−1, 303.69 ± 10.44 μg mg−1, 300.39 ± 16.02 μg mg−1 dry weight, respectively (Fig. 1C). It is noteworthy that our proposed method (10 mM Na2EDTA) decreased the GAG content by only 5%, compared to untreated CEbS (380.37 ± 16.02 μg mg−1), which makes the proposed method superior for cartilage tissue engineering. It was found that compared to other procedures, the 10 mM Na2EDTA combined with 0.5% Triton X-100 (48 h) procedure reduced the sulfated GAG content of ECM up to 21%, which is significant, according to the ANOVA test (p < 0.05). Calculations were performed according to the standard curve (Fig. 1E). The results depicted that all detergent based decellularization protocols were harmful for the sulfated GAGs (p < 0.05), but our protocol was not (p > 0.05). As mentioned above, 0.5% Triton X-100 for 48 hours might be more effective in terms of dsDNA removal but this application destroyed GAG content more than other applications. Destruction of a huge amount of GAG may be the biggest problem of cartilage tissue engineering applications, and studies in literature clearly support this thesis.16,22,31 There are also some studies reporting a near complete removal of sulfated GAG content after cartilage tissue decellularization.19 After all, the composed study may promisingly be a novel decellularization protocol without destroying the GAGs and the other biochemical components of native tissues.20
Collagen, one of the most abundant proteins embedded within ECM, is of great significance, enabling mechanical durability, as well as guiding migration, proliferation and differentiation. The hydroxyproline assay was performed to determine the collagen content of untreated, decalcified and decalcified/detergent treated CEbS. The results show that there were no significant differences for hydroxyproline content between untreated (13.04 ± 1.51 μg mg−1 dry weight) and decellularized CEbS using the protocols mentioned before. The hydroxyproline contents were found for 10 mM Na2EDTA, 10 mM Na2EDTA and 0.1% Triton X-100 (24 h), 10 mM Na2EDTA combined with 0.5% Triton X-100 (24 h), 10 mM Na2EDTA combined with 0.1% Triton X-100 (48 h), 10 mM Na2EDTA combined with 0.5% Triton X-100 (48 h) as 12.95 ± 1.55 μg mg−1, 13.33 ± 1.06 μg mg−1, 13.48 ± 1.37 μg mg−1, 11.76 ± 1.00 μg mg−1, 11.06 ± 0.41 μg mg−1 dry weight, respectively (Fig. 1D). Maximum hydroxyproline removal was observed with the 0.5% Triton X-100 (48 h) procedure, which was about 15%. When the data were examined, it was clearly seen that there were no statistical differences between the positive control and other protocols. Similar results have been presented previously.32,33 However, increasing the duration and concentration of Triton X-100 caused collagen reducing effects (Fig. 1D). To avoid collagen loss, the method presented in this study may be efficiently used instead of detergent treatment for cartilage tissue decellularization for regenerative medicine applications.
For further characterization of the scaffolds, FT-IR spectra were obtained and are presented in Fig. 2A. There are two important points in the FT-IR spectra of untreated and detergent-free decellularized CEbS. First of all, the phosphate peak at 1026 cm−1 decreased following the decellularization step. This reduction occurs due to a decrease of calcium in cartilage tissue, and calcium loss is a consequence of decellularization. Decrease in the phosphate peak intensity is evidence of the successful decalcification of cartilage tissue. The second remarkable point is the increase in peak intensities of amide I and II bands at 1635 and 1546 cm−1, respectively. This increase is an outcome of NHS/EDC crosslinking and it proves that the crosslinking process was successfully conducted. Thermal stability is an important feature for materials used in biomedical applications. Thermal treatment during the process of manufacturing and long-term usage at body temperature can lead to damage in the thermally unstable biomaterials. Thermal gravimetric analysis was performed in this study to show the success of the decalcification method, in addition to the thermal properties of the scaffolds. TGA curves of the untreated and decalcified scaffolds were obtained at a heating rate of 10 °C min−1, up to 800 °C, under a dry nitrogen flow. TGA curves indicate the success of decalcification in terms of thermal degradation (Fig. 2B). The weight loss between 50–210 °C corresponds to water desorption and the destruction of glycosidic bonds, while degradation between 180 and 350 °C is attributed to protein chain breakage and peptide bond rupture in cartilage tissue. The gap between the weight loss of the control and decalcified/crosslinked scaffolds, approximately 60% and 99%, is a result of the decalcification event and due to the loss of calcium ions in the cartilage tissue. TGA results support the FT-IR results and they prove the fact that the cartilage tissue is properly decalcified. It was shown that the linear modulus decreased significantly from 11.3 MPa in native cartilage tissue, to 83 kPa in processed tissue (Fig. 2C). Consequently, the matrix stiffness was reduced by nearly 93% and it can be said that the decellularization process was carried out properly, in terms of mechanical behavior.31
Human adipose mesenchymal stem cells were induced for osteogenic, chondrogenic and adipogenic differentiation to validate their multipotency before cell culture experiments. The cells were differentiated successfully and each cell type showed its own specific properties after histological staining (Fig. 3). As seen in Fig. 3B–F, the cells successfully showed adipogenic, osteogenic, and chondrogenic character on day 14, 21, and 28, respectively, following histochemical staining with oil red O for adipogenesis, alizarin red S for osteogenesis, safranin O and alcian blue 8gx for chondrogenesis.
Scaffold morphology and cell attachment were evaluated by SEM micrographs (Fig. 4). SEM images show that untreated and detergent-free decellularized CEbS have a clear difference, due to the removal of cellular debris or various ingredients consisting of the cartilage tissue (indicated with yellow arrows, Fig. 4A and B). What is more, the detergent-free, decellularized CEbS shows a smooth surface that indicates the success of decalcification and decellularization. While the image at the bottom left of Fig. 4A, taken at 50× magnification, shows the sparse, porous and compact structure of native tissue, the image at the top right of Fig. 4B shows the dense and porous structure of the scaffold at 50× magnification.
 | ||
| Fig. 4 SEM micrographs: (A) untreated, (B) detergent-free decellularized CEbS (5000× and 50×). Recellularized scaffold on (C) day 14, (D) day 21, and (E) day 28 (2500× and 50×). | ||
Detergent-free decellularized CEbS also showed inter-connected networks (chambers and channels; approx. pore size = 39.98 ± 15.83 μm) (n = 10) morphology enabling efficient cell attachment, due to its porous structure (Fig. 4C and S1†). Fig. S1 can be found in the ESI section.† In close agreement with SEM analysis, the porosity of the detergent-free decellularized CEbS was determined to be 96.06 ± 0.65% porous (n = 3) by liquid displacement analysis. The picture on the bottom left taken at 50× magnification shows an overview of recellularized CEbS. The cells that were inoculated with detergent-free decellularized CEbS for 14, 21 and 28 days covered the scaffold area, including porous cavities (Fig. 4C–E).
After the cell harvesting on the 14th 21st and 28th days, the MTT test revealed 82%, 93% and 100% cell proliferation, respectively (Fig. 5A). Microscopic observations showed that cell density increased day by day (Fig. 5B–D), and there was no cytotoxic evidence for the scaffolds, which is a desirable environment for cells. As can be seen in Fig. 5A, days 14, 21 and 28 did not show any significant difference, which means that proliferation slowed and the differentiation started. Cartilage ECM based scaffolds, which mimicked native ECM and maintained vital ECM components, induced the stem cells to chondrogenic lineage differentiation.
Lastly, histological assessment was performed to characterize the decellularization, alteration of the ECM components, recellularization and chondrogenic differentiation on days 14, 21, and 28 (Fig. 6). H & E staining demonstrates that cellular remnants were reduced by the decellularization process, which supports the DNA content analysis results. However, the scaffolds were covered by the cells day by day (14, 21, 28 days); the cell viability on the scaffolds also supported this phenomenon.14
Cells are undesirable materials in decellularized scaffolds. These materials are the main factors of the immunogenicity.21 The method proposed has reduced nuclear materials up to 84% and hence, immunogenicity is also lowered. Host immune response and tissue remodelling play a significant role in terms of successful applications.34 Wiles et al. reported that decellularized matrices may have antigenic or immunogenic effects. However, these dose dependent effects may contribute to the graft remodelling and improve the material outcomes.35 In addition, Wang et al. showed that the crosslinking process diminished the host immune response by reducing lymphocyte proliferation, and decreased Th1 and Th2 cytokines release.36 These results obtained in other studies give some clues about the issue and efficiency of the method proposed. Further research, including in vivo experiments related to immunogenicity, will be performed in forthcoming studies.
The existence of sulfated GAGs are required to produce functional scaffolds for cartilage tissue engineering.37 Sutherland et al. mentioned the effects of GAGs in ECM on chondroinductive properties.16 Histological safranin O staining showed that sulfated GAGs remained and the intensity of GAG was increased from day 14 to day 28. Immunohistochemically, newly synthesized tissue in decellularized CEbS stained very intensely for GAG by anti-aggrecan on days 14, 21 and 28. The histochemical and immunohistochemical assessments are supported by GAG assay. Collagen type II is one of the major components that are specific to articular cartilage ECM.38 Decellularized CEbS maintain collagen type II content, which was shown by a pale stained acellular scaffold. As seen on days 14, 21 and 28, the collagen type II staining is remarkably different from the decellularized scaffold, when the cells were stained with collagen type II antibody.
4. Conclusion
Intact extracellular matrix based scaffold fabrication using decellularization techniques is a promising approach to tissue engineering and regenerative medicine. Having unique mechanical and biological properties, the intact extracellular matrices are the major candidates for repairing or regenerating damaged or missing tissues. Furthermore, they can naturally act as a niche for stem cells or other cell types. Harsh decellularization protocols having low penetration, like strong detergent and high pH treatments, are used for cartilage tissues to remove cellular content from the extracellular matrix; however, these protocols usually destroy vital components of ECM, and the desired attributes of the matrix are severely damaged, thus, cartilage tissue becomes a challenging issue for tissue engineering. Decellularization during the decalcification process maintained vital ECM components. In conclusion, the prepared scaffolds successfully directed stem cells into the chondrogenic differentiation and it is believed that revealing novel, detergent-free decellularization methods would be valuable for regenerative medicine applications based on cartilage tissue engineering.Conflict of interest
The authors declare that they have no conflict of interest.Acknowledgements
This study was financially supported by the Scientific and Technological Research Council of Turkey (Project ID. 114S851) and Çanakkale Onsekiz Mart University, Scientific Research Projects Coordination Unit (Project ID. FYL-2015-631). We would also thank to Çanakkale Onsekiz Mart University, Science and Technology Application & Research Center for collaborating with analyses. The authors would like to thank Dr Selin Marangoz, for comments and language proofreading.References
- R. S. Langer and J. P. Vacanti, Sci. Am., 1999, 280, 86–89 CrossRef CAS PubMed.
- R. Langer and J. P. Vacanti, Science, 1993, 260, 920–926 CAS.
- F. Chen, J. J. Yoo and A. Atala, Urology, 1999, 54, 407–410 CrossRef CAS PubMed.
- A. Bader, T. Schilling, O. E. Teebken, G. Brandes, T. Herden, G. Steinhoff and A. Haverich, Eur. J. Cardio. Thorac. Surg., 1998, 14, 279–284 CrossRef CAS PubMed.
- R.-N. Chen, H.-O. Ho, Y.-T. Tsai and M.-T. Sheu, Biomaterials, 2004, 25, 2679–2686 CrossRef CAS PubMed.
- B. S. Conklin, E. R. Richter, K. L. Kreutziger, D. S. Zhong and C. Chen, Med. Eng. Phys., 2002, 24, 173–183 CrossRef CAS PubMed.
- S. Marlovits, P. Zeller, P. Singer, C. Resinger and V. Vécsei, Eur. J. Radiol., 2006, 57, 24–31 CrossRef PubMed.
- L. E. Fitzpatrick and T. C. McDevitt, Biomater. Sci., 2015, 3, 12–24 RSC.
- R. L. Mauck and J. A. Burdick, Tissue Eng., 2011, 60, 493–520 Search PubMed.
- C. T. Laurencin, A. M. Ambrosio, M. D. Borden and J. A. Cooper, Annu. Rev. Biomed. Eng., 1999, 1, 19–46 CrossRef CAS PubMed.
- A. I. Edelstein, R. L. Linn, M. K. Fritsch and M. Sagan, Am. J. Orthop., 2015, 44, E355–E357 Search PubMed.
- H. Tomo, Y. Ito, M. Aono and K. Takaoka, J. Shoulder Elbow. Surg., 2005, 14, 103–106 CrossRef PubMed.
- L. Zhang, J. Hu and A. A. Kyriacos, CRC Crit. Rev. Bioeng., 2009, 37, 1–57 Search PubMed.
- T. W. Gilbert, T. L. Sellaro and S. F. Badylak, Biomaterials, 2006, 27, 3675–3683 CAS.
- M. F. Griffin, Y. Premakumar, A. M. Seifalian, M. Szarko and P. E. M. Butler, J. Mater. Sci.: Mater. Med., 2016, 27, 1–6 CrossRef CAS PubMed.
- A. J. Sutherland, E. C. Beck, S. C. Dennis, G. L. Converse, R. A. Hopkins, C. J. Berkland and M. S. Detamore, PLoS One, 2015, 10, e0121966 Search PubMed.
- B. P. Chan and K. W. Leong, European Spine Journal, 2008, 17, 467–479 CrossRef PubMed.
- P. Somers, F. de Somer, M. Cornelissen, H. Thierens and G. Van Nooten, Artif. Cells, Blood Substitutes, Biotechnol., 2012, 40, 151–162 CrossRef CAS PubMed.
- L. Luo, R. Eswaramoorthy, K. J. Mulhall and D. J. Kelly, J. Mech. Behav. Biomed. Mater., 2015, 55, 21–31 CrossRef PubMed.
- E. Kheir, T. Stapleton, D. Shaw, Z. Jin, J. Fisher and E. Ingham, J. Biomed. Mater. Res., Part A, 2011, 99, 283–294 CrossRef PubMed.
- H. Xu, B. Xu, Q. Yang, X. Li, X. Ma, Q. Xia, Y. Zhang, C. Zhang, Y. Wu and Y. Zhang, PLoS One, 2014, 9, 1–13 Search PubMed.
- B. D. Elder, S. V. Eleswarapu and K. A. Athanasiou, Biomaterials, 2009, 30, 3749–3756 CrossRef CAS PubMed.
- L. Buttafoco, N. G. Kolkman, P. Engbers-Buijtenhuijs, A. A. Poot, P. J. Dijkstra, I. Vermes and J. Feijen, Biomaterials, 2006, 27, 724–734 CrossRef CAS PubMed.
- R. W. Farndale, D. J. Buttle and A. J. Barrett, Biochim. Biophys. Acta, 1986, 883, 173–177 CrossRef CAS.
- C. D. Hoemann, J. Sun, V. Chrzanowski and M. D. Buschmann, Anal. Biochem., 2002, 300, 1–10 CrossRef CAS PubMed.
- U. J. Kim, J. Park, H. Joo Kim, M. Wada and D. L. Kaplan, Biomaterials, 2005, 26, 2775–2785 CrossRef CAS PubMed.
- S. Camarero-Espinosa, B. Rothen-Rutishauser, E. J. Foster and C. Weder, Biomater. Sci., 2016, 4, 734–767 RSC.
- Q. Yang, J. Peng, Q. Guo, J. Huang, L. Zhang, J. Yao, F. Yang, S. Wang, W. Xu, A. Wang and S. Lu, Biomaterials, 2008, 29, 2378–2387 CrossRef CAS PubMed.
- C. R. Rowland, L. A. Colucci and F. Guilak, Biomaterials, 2016, 91, 57–72 CrossRef CAS PubMed.
- S. F. Badylak, Transplant Immunol., 2004, 12, 367–377 CrossRef CAS PubMed.
- S. Schwarz, L. Koerber, A. F. Elsaesser, E. Goldberg-Bockhorn, A. M. Seitz, L. Dürselen, A. Ignatius, P. Walther, R. Breiter and N. Rotter, Tissue Eng., Part A, 2012, 18, 120720085805006 Search PubMed.
- R. Nair, A. V. Ngangan and T. C. McDevitt, J. Biomater. Sci., Polym. Ed., 2008, 19, 801–819 CrossRef CAS PubMed.
- Q. Wu, J. Bao, Y.-J. Zhou, Y. Wang, Z.-G. Du, Y. Shi, L. Li and H. Bu, BioMed Res. Int., 2015, 2015, 1–9 Search PubMed.
- S. F. Badylak and T. W. Gilbert, Semin. Immunol., 2008, 20, 109–116 CrossRef CAS PubMed.
- K. Wiles, J. M. Fishman, P. De Coppi and M. A. Birchall, Tissue Eng., Part B, 2016, 22, 208–219 CrossRef PubMed.
- Y. Wang, J. Bao, X. Wu, Q. Wu, Y. Li, Y. Zhou, L. Li and H. Bu, Sci. Rep., 2016, 6, 24779 CrossRef CAS PubMed.
- E. Schuh, S. Hofmann, K. Stok, H. Notbohm, R. Müller and N. Rotter, J. Biomed. Mater. Res., Part A, 2012, 100, 38–47 CrossRef PubMed.
- N. Naveena, J. Venugopal, R. Rajeswari, S. Sundarrajan, R. Sridhar, M. Shayanti, S. Narayanan and S. Ramakrishna, J. Mater. Chem., 2012, 22, 5239–5253 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra16647d |
| This journal is © The Royal Society of Chemistry 2016 |