Novel magnetic nanoparticles with ionic liquid tags as a reusable catalyst in the synthesis of polyhydroquinolines†
Meysam Yariea,
Mohammad Ali Zolfigol*a,
Yadollah Bayatb,
Asiye Asgarib,
Diego A. Alonso *c and
Abbas Khoshnood*c
*c and
Abbas Khoshnood*c
aDepartment of Organic Chemistry, Faculty of Chemistry, Bu-Ali Sina University, Hamedan, Iran. E-mail: zolfi@basu.ac.ir; mzolfigol@yahoo.com; Fax: +98 8138257407; Fax: +98 8138380709; Tel: +98 8138282807
bFaculty of Chemistry and Chemical Engineering, Malek Ashtar University of Technology, Tehran, Iran
cOrganic Chemistry Department, Organic Synthesis Institute, Alicante University, Apdo. 99, 03080 Alicante, Spain. E-mail: diego.alonso@ua.es; abbas.khoshnood@ua.es; Tel: +34 965909841
First published on 17th August 2016
Abstract
In this study, we have introduced {Fe3O4@SiO2@(CH2)3Im}C(NO2)3 as a novel and heterogeneous reusable catalyst for the four component preparation of polyhydroquinoline derivatives under mild and eco-friendly reaction conditions. The structural confirmation of the novel heterogeneous reusable promoter was fully made using FT-IR, X-ray diffraction (XRD), field emission scanning electron microscopy (FESEM), energy-dispersive spectroscopy (EDS) elemental mapping analysis, high resolution transmission electron microscopy (HRTEM), thermogravimetry (TG), derivative thermal gravimetric (DTG), differential thermal (DTA) and vibrating sample magnetometer (VSM) analyses. The nanomagnetic heterogeneous catalyst was successfully applied for the synthesis of polyhydroquinoline derivatives via a four component condensation of a good range of aryl aldehydes, dimedone, ethyl acetoacetate or methyl acetoacetate as a β-ketoester, and ammonium acetate as a nitrogen source under solvent free conditions. Moreover, experimental evidence has demonstrated that {Fe3O4@SiO2@(CH2)3Im}C(NO2)3 could act as a recoverable nanomagnetic and reusable catalyst without any considerable drop in the yield and the reaction time for at least eight times.
Introduction
In recent times, the development and expansion of magnetic nanoparticles (MNPs) as versatile supports have been shown to be an influential branch in the field of green chemistry due to the environmentally benign nature of these compounds. For the easy separation of the magnetic materials from the reaction system, numerous attempts were focused on surface modifications in order to construct varied heterogeneous magnetically recoverable promoters for the organic functional transformation.1 Moreover, using nanomagnetic heterogeneous catalytic systems for the promotion of the reaction supports a high catalytic performance as in the case of the homogeneous catalysts; in addition, the facile and efficient recovery point of view is approved through applying a simple external magnet and these merits led to an increase in their potential applicability.2 The chemistry of MNPs has been extensively reviewed.1–3On the other hand, the capabilities of ionic liquids as reagents, catalysts, reaction media and proper solvents for different goals in the field of green chemistry have been well documented in the past few years.4–10 Despite the widespread applications of ionic liquids, investigations have disclosed that in some case, the ionic liquids are at a disadvantage due to their toxic nature.11,12 Therefore, ongoing studies are needed to overcome the defects connected with conventional, unsafe ionic liquids to replacing them with safer and more productive alternatives. Therefore, the combination of ionic liquids and magnetic nanoparticles (MNPs) as versatile supports for the immobilization of the ionic liquids offers several merits such as an easy synthetic pathway, a large surface area, a high thermal stability, a facile separation from the reaction mixture, and a low toxicity and price.13
The synthesis of complex compounds with high atom and step economy, improvement of the effectiveness and plainness in the synthetic pathway, providing easy and straightforward approach to library molecules and diversity-oriented synthesis (DOS) are the principal interest of the one pot multicomponent reactions (MCRs) and this beneficial technical protocol persuaded both academic and industrial chemists to access molecular diversity by this way.14–17
Recently, due to the varied pharmaceutical and biological applications connected to the polyhydroquinolines structural motif, major attention has been paid to the production of these versatile organic compounds. Substituted 1,4-dihydropyridines are renowned calcium channel modulators, and they can be applied as a treatment for cardiovascular diseases.18 Some biologically active species base on the 1,4-dihydropyridine moiety are illustrated in Fig. 1.19
Because of the therapeutic applications of the 1,4-dihydropyridine derivatives, synthetic chemists have explored several protocols for their synthesis using different reaction conditions and varied catalytic systems such as Yb(OTf)3,20 [pyridine-SO3H]Cl,21 [Dsim]HSO4,22 Triton X-100 in water,23 TiO2 NPs,24 SnO2 NPs,25 silica-bonded imidazolium-sulfonic acid chloride,26 [2-MPyH]OTf,27 SBA-15/SO3H,28 Ni-nanoparticles,29 IL-HSO4@SBA-15,30 Baker's yeast,31 L-proline,32 and [bmim]BF4.33 Although the reported methods offer several merits and provide improvements for the synthesis of the target molecules, many of these protocols need longer reaction times and use harsh reaction conditions, unsafe organic solvents, and drastic workups. Therefore, investigating a new, environmentally compatible catalytic system is still in great demand.
We performed this study in order to explore a new protocol for the production of polyhydroquinoline derivatives and continue our investigations on the knowledge-based design, construction and applications of ionic liquids,34 molten salts,35 nanocatalysts, magnetic nanoparticles (MNPs),36 magnetic nanoparticles with ionic liquid tags,37 and trinitromethane38 for organic functional group transformations. Specifically, we report on the design, synthesis and applicability of a novel nanomagnetically recoverable and reusable promoter catalyst, {Fe3O4@SiO2@(CH2)3Im}C(NO2)3, for the preparation of polyhydroquinolines through a four component condensation of several aryl aldehydes, dimedone, ethyl acetoacetate or methyl acetoacetate as a β-ketoester, and ammonium acetate as a nitrogen source under mild and solvent free conditions (Schemes 1 and 2).
 | ||
| Scheme 1 Synthesis of polyhydroquinoline derivatives in the presence of {Fe3O4@SiO2@(CH2)3Im}C(NO2)3. | ||
 | ||
| Scheme 2 Synthetic pathway for the preparation of {Fe3O4@SiO2@(CH2)3Im}C(NO2)3 as a heterogeneous core–shell catalyst. | ||
Characterization of the novel catalyst
The structural verification of the novel nanomagnetically recoverable and reusable promoter, {Fe3O4@SiO2@(CH2)3Im}C(NO2)3, was made using FT-IR, X-ray diffraction (XRD), field emission scanning electron microscopy (FESEM), elemental mapping, high resolution transmission electron microscopy (HRTEM), thermogravimetry (TG), and vibrating sample magnetometer (VSM) analysis.As depicted in Fig. 2, the FT-IR spectrum of the novel nanomagnetic heterogeneous core–shell catalyst in comparison with different synthetic pathway compounds from Fe3O4@SiO2 to {Fe3O4@SiO2@(CH2)3Im}C(NO2)3 was investigated in the range of 400–4000 cm−1. The overall differences in the FT-IR spectra can be used as proof for the preparation of the novel nanomagnetically recoverable and reusable promoter catalyst. According to the FT-IR spectrum, the two peaks at 1394 and 1592 cm−1 can be attributed to the nitro functional groups in the structure of the catalyst. Furthermore, the overlapping of the uncoated hydroxyl groups and N–H stretching mode in the imidazolium ring caused a broad peak in the region of about 2900–3700 cm−1.
 | ||
| Fig. 2 FT-IR spectrum of the novel nanomagnetic heterogeneous catalyst in comparison with different stepwise synthetic pathway materials. | ||
By comparison, for the investigation of the purity and the phase of the applied materials for the synthesis of the catalyst, the X-ray diffraction patterns (XRD) were obtained for the different stepwise prepared materials (Fig. 3). From the XRD patterns, it can be deduced that the addition of each layer to the surface of the Fe3O4 nanoparticles lead to a change compared with the previous stage, which is an indication of the synthesis of the novel nanomagnetic core–shell catalyst. The X-ray diffraction pattern of the prepared catalyst confirmed that it has a crystalline nature with diffraction lines at 2θ = 13.35°, 16.30° and 24.90°. In another study, according to the Scherrer equation,
D = Kλ/(β![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ) θ)
| (1) |
 | ||
| Fig. 3 XRD pattern of the new catalyst and comparison with the different stepwise synthesized intermediates. | ||
| Entry | 2θ | Peak width [FWHM] (degree) | Size [nm] | Inter planar distance [nm] |
|---|---|---|---|---|
| 1 | 13.35 | 0.68 | 11.76 | 0.662439 |
| 2 | 16.30 | 0.64 | 12.54 | 0.543152 |
| 3 | 24.90 | 0.80 | 10.17 | 0.357164 |
In another assay, for the investigation of the size and morphology of the novel catalyst, field emission scanning electron microscopy (FESEM) images were obtained (Fig. 4a–c). As the recorded FESEM images show, the size of the catalyst particles is in the nanometer scale.
 | ||
| Fig. 4 (a and b) Field emission scanning electron microscopy (FESEM) images of {Fe3O4@SiO2@(CH2)3Im}C(NO2)3 as a new nanomagnetic core–shell catalyst. Inset (c) expansion of image (b). | ||
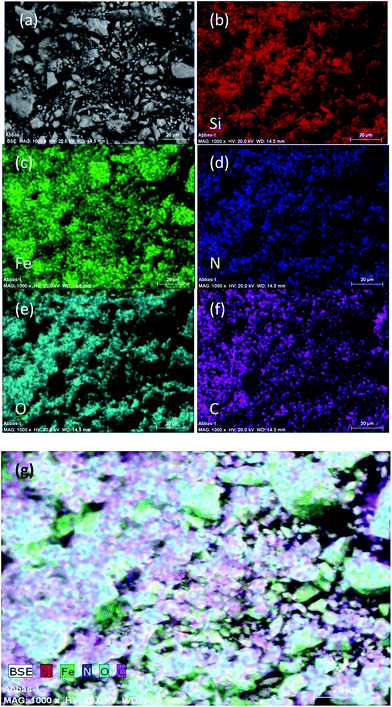
Moreover, in order to explore the elemental composition of the catalyst {Fe3O4@SiO2@(CH2)3Im}C(NO2)3, energy-dispersive spectroscopy (EDS) elemental mapping analysis was conducted (Fig. 5a–g). Examination of the SEM-EDS mapping images, which are shown in Fig. 5b–g, proved the presence of Si, Fe, N, O, and C in the catalyst. As shown in Fig. 5b–f, the elements were distributed well in the catalyst surface.
The data from energy dispersive X-ray (EDX) analysis of the new heterogeneous catalyst showed all the predicted elements in the structure of the catalyst, namely, iron, silicon, oxygen, carbon, and nitrogen, as indicated in Fig. 6. Moreover, SEM-coupled EDX data for the catalyst revealed C (0.53), N (0.27), Fe (93.56), O (4.64) and Si (0.99).
High resolution transmission electron microscopy (HRTEM) images of {Fe3O4@SiO2@(CH2)3Im}C(NO2)3 were recorded and illustrated to obtain structural information of the catalyst in more detail. As depicted in Fig. 8a and b, all the nanoparticles presented well-defined uniform spherical shapes, highly dispersed by the ionic liquid tags over the Fe3O4NPs after sonication in ethanol. As shown in the HRTEM images (Fig. 8a–c), amorphous silica coating layers over the crystalline nanoparticles of Fe3O4NPs are clearly seen at low or high magnification of the HRTEM. According to Fig. 8c, the thickness of the silica shell (@SiO2@(CH2)3Im}C(NO2)3) is around 2 to 3 nm with an interplanar crystalline spacing value of the Fe3O4NPs around 0.50 nm (Fig. 7). Moreover, it is worth mentioning that the images from both the FESEM and HRTEM analyses are in good agreement with the XRD extracted data.
 | ||
| Fig. 8 HRTEM images of the {Fe3O4@SiO2@(CH2)3Im}C(NO2)3 (a and b) and magnified HRTEM images of one nanoparticle (c). | ||
In order to investigate the thermal stability of the novel nanomagnetic catalyst, thermal gravimetric (TG), derivative thermal gravimetric (DTG), and differential thermal (DTA) analyses were conducted (Fig. 9). From these studies, it could be inferred that the main weight loss upon heating from ambient temperature to about 100 °C can be attributed to the evaporation of the physically adsorbed water and other organic solvents used during the catalyst preparation. Weight loss at around 210 °C is due to thermal decomposition of the ionic tag of the catalyst. Finally, decomposition of the catalyst occurred between 300 and 550 °C. Moreover, the study of the DTA analysis diagram revealed that it is downward and exothermic.
 | ||
| Fig. 9 Thermal gravimetric (TG), derivative thermal gravimetric (DTG), and differential thermal (DTA) analyses of the novel core–shell catalyst. | ||
In order to study the magnetic properties of the new catalyst, vibrating sample magnetometer (VSM) analysis of the catalyst was compared with that of nanomagnetic Fe3O4 (Fig. 10). By investigation of the obtained magnetization curves, it was observed that the saturation of {Fe3O4@SiO2@(CH2)3Im}C(NO2)3 as a catalyst reduced from 52.42 emu g−1 for Fe3O4 nanoparticles to 37.48 emu g−1. This decrease can be related to surface modification of the Fe3O4 nanoparticles during the preparation of the catalyst.
After characterizing the nanomagnetic catalyst, the applicability of the catalyst was explored for the synthesis of polyhydroquinoline derivatives through a four component condensation of several aryl aldehydes, dimedone, ethyl acetoacetate or methyl acetoacetate as β-ketoester, and ammonium acetate as a nitrogen source. In order to achieve the optimized reaction conditions, the condensation of 4-chlorobenzaldehyde, dimedone, ethylacetoacetate, and ammonium acetate was chosen as a test reaction. The resulting data for the optimization of temperatures, catalyst loadings, and solvents showed that the best results were acquired when the reaction was carried out under solvent-free conditions in the presence of 7 mg of {Fe3O4@SiO2@(CH2)3Im}C(NO2)3 as a catalyst at 80 °C (Table 2, entry 4). The data for the optimization of the reaction conditions are included in Table 2.
| Entry | Solvent | Load of catalyst (mg) | Temperature (°C) | Time (min) | Yieldb (%) |
|---|---|---|---|---|---|
| a Reaction conditions: 4-chlorobenzaldehyde (1 mmol, 0.144 g), dimedone (1 mmol, 0.140 g), ethyl acetoacetate (1 mmol, 0.130 g), and ammonium acetate (3 mmol, 0.231 g).b Isolated yield. | |||||
| 1 | — | 10 | 100 | 15 | 93 |
| 2 | — | 10 | 80 | 16 | 92 |
| 3 | — | 10 | 60 | 25 | 85 |
| 4 | — | 7 | 80 | 18 | 92 |
| 5 | — | 4 | 80 | 22 | 87 |
| 6 | — | — | 80 | 60 | 54 |
| 7 | EtOAc | 7 | Reflux | 80 | 55 |
| 8 | n-Hexane | 7 | Reflux | 70 | 35 |
| 9 | CH3CN | 7 | Reflux | 80 | 60 |
| 10 | EtOH | 7 | Reflux | 90 | 90 |
| 11 | H2O | 7 | Reflux | 90 | 78 |
After evaluation of the appropriate reaction conditions, we studied the scope and productivity of this process for the synthesis of biologically active target molecules in the presence of {Fe3O4@SiO2@(CH2)3Im}C(NO2)3 as a heterogeneous recoverable catalyst. Thus, polyhydroquinoline derivatives were prepared via a four component reaction of a wide range of aromatic aldehydes bearing electron-withdrawing and electron-releasing groups, ethyl acetoacetate or methyl acetoacetate, dimedone, and ammonium acetate under solvent free conditions. The results are displayed in Table 3.
| Product | X | Y | Time (min) | Yieldb (%) | Mp (°C) found [Lit]ref. |
|---|---|---|---|---|---|
| a Reaction conditions: aromatic aldehyde (1 mmol), dimedone (1 mmol, 0.140 g), ethyl acetoacetate or methyl acetoacetate (1 mmol), and ammonium acetate (3 mmol, 0.231 g).b Isolated yields. | |||||
| 1a | 4-Cl | OEt | 18 | 92 | 243–245 [242–244]36c |
| 1b | H | OEt | 19 | 91 | 224–226 [224–226]36c |
| 1c | 2,4-Cl2 | OEt | 16 | 93 | 244–246 [242–244]36c |
| 1d | 4-OMe | OEt | 18 | 90 | 255–257 [255–257]36c |
| 1e | 3-Br | OEt | 18 | 91 | 206–208 [229–231]26 |
| 1f | 2-Cl | OEt | 20 | 94 | 207–209 [208–209]39 |
| 1g | 4-Me | OEt | 17 | 90 | 263–265 [263–265]36c |
| 1h | 3,4-(OMe)2 | OEt | 16 | 92 | 205–207 [204–206]36c |
| 1i | 4-F | OEt | 18 | 90 | 193–195 [193–195]36c |
| 1j | 4-NO2 | OEt | 22 | 88 | 242–243 [241–242]36c |
| 1k | 2-OMe | OEt | 14 | 92 | 249–251 [248–250]41 |
| 1l | 3-OEt-4-OH | OEt | 15 | 95 | 195–196 [190–192]36c |
| 1m | 3-OH | OEt | 17 | 93 | 231–234 [230–232]36c |
| 1n | 4-Cl | OMe | 18 | 89 | 257–258 [219–222]40 |
| 1o | H | OMe | 17 | 90 | 259–261 [257–259]26 |
| 1p | 4-Br | OMe | 19 | 90 | 261–263 [263–264]42 |
| 1q | 2,4-Cl | OMe | 22 | 91 | 251–253 [250–252]42 |
| 1r | 4-OMe | OMe | 18 | 91 | 254–257 [256–259]26 |
| 1s | 4-OH | OMe | 17 | 92 | 297–298 [232–234]26 |
| 1t | 4-Me | OMe | 17 | 90 | 270–273 [283–285]23 |
One of the excellent merits of nanomagnetic heterogeneous core–shell catalysts is the possibility of recycling and the ease of separating them from the reaction mixture in a catalyzed system after completion of the reaction. In order to demonstrate the reusability of our catalyst, the reaction of benzaldehyde, dimedone, ethyl acetoacetate, and ammonium acetate was preferred as a model process. After each run, hot ethanol was added to the reaction mixture to dissolve the crude product. Then, the nanomagnetic catalyst was separated from the reaction mixture by applying an external magnet. The catalyst was reused for the next run after washing it with ethanol and drying. As depicted in Fig. 11, the catalytic activity of {Fe3O4@SiO2@(CH2)3Im}C(NO2)3 was conserved for eight consecutive runs without any discernible reduction.
Finally, the catalytic activity of {Fe3O4@SiO2@(CH2)3Im}C(NO2)3 was compared with some previously investigated recoverable catalysts (Table 4). As depicted, it can be deduced that the previously reported methods suffer from different drawbacks such as elevated temperatures, use of organic solvents and/or transition metal catalysts, activation by sonication irradiation, longer reaction times, or harsh reaction conditions.
| Entry | Reaction conditions | Time (min) | Yield (%) | Lit. |
|---|---|---|---|---|
| 1 | [TBA]2[W6O19] (7 mol%), solvent-free, 110 °C | 20 | 93 | 43 |
| 2 | Trifluoroethanol (TFE), 70 °C | 180 | 98 | 44 |
| 3 | Fe3O4-SA-PPCA (10 mg), EtOH, 50 °C | 120 | 97 | 45 |
| 4 | Mn@PMO-IL (1 mol%), solvent-free, 80 °C | 20 | 95 | 46 |
| 5 | Co3O4–CNTs (0.03 g), EtOH, 50 °C, sonication | 15 | 97 | 47 |
| 6 | Nafion-H®, PEG 400–water (60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40), 50 °C 40), 50 °C |
90 | 96 | 48 |
| 7 | (bzacen)MnCl (2.5 mol%), EtOH, reflux | 20 | 90 | 49 |
| 8 | Nano-Fe3O4 (5 mol%), solvent-free, 50 °C | 6 | 89 | 50 |
| 9 | Nanocat Fe–Ce (100 mg), EtOH, r.t. | 20 | 95 | 51 |
| 10 | Hf(NPf2)4 (1 mol%), C10F18, 60 °C | 180 | 95 | 52 |
| This work | {Fe3O4@SiO2@(CH2)3Im}C(NO2)3 (7 mg), solvent-free, 80 °C | 19 | 91 | — |
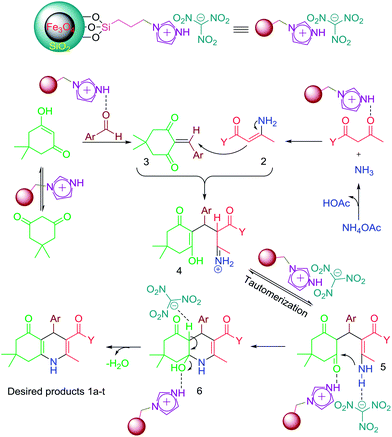
A suggested mechanistic pathway for the synthesis of polyhydroquinoline derivatives is portrayed in Fig. 12, where initially the in situ generated NH3 formed from ammonium acetate reacts with activated β-ketoester to yield the corresponding intermediate 2. On the other hand, in the presence of the novel nanomagnetic catalyst, dimedone is converted to its enol form, which performs a nucleophilic addition to the activated aryl aldehyde, providing the corresponding Knoevenagel adduct 3. Then, the reaction between these two intermediates generates intermediate 4. Tautomerization of 4 led to the formation of derivative 5, which suffers an intramolecular nucleophilic attack of the amino group to the activated carbonyl group providing intermediate 6. Finally, dehydration of this intermediate formed the desired target molecules 1a–t.
Conclusion
In this study, the design and synthesis of a novel and heterogeneous reusable catalyst, {Fe3O4@SiO2@(CH2)3Im}C(NO2)3, has been reported. The structure of this catalyst was fully characterized using FT-IR, X-ray diffraction (XRD), field emission scanning electron microscopy (FESEM), high resolution transmission electron microscopy (HRTEM), thermogravimetry (TG), and derivative thermal gravimetric (DTG), differential thermal (DTA), and vibrating sample magnetometer (VSM) analyses. The catalytic performance of {Fe3O4@SiO2@(CH2)3Im}C(NO2)3 was successfully explored in the four component preparation of polyhydroquinoline derivatives under mild and eco-friendly reaction conditions. The encouraging advantages of this study are the eco-friendly and mild reaction conditions, easy separation and reusability of the catalyst, short reaction times with high products yields, and an easy work-up.Experimental
General
Solvents and reagents were purchased from Sigma-Aldrich, Alfa Aesar, and Merck and were used without further purification. Melting points were obtained with an Amstrad/electrothermal apparatus. 1H NMR (400 MHz) and 1H NMR (300 MHz) spectra were obtained on a Bruker Avance 400 and Bruker Avance 300 NMR spectrometers, respectively, in proton coupled mode using deuterated DMSO as a solvent unless otherwise stated. 13C NMR (101 MHz) spectra were acquired on a Bruker Avance 400 NMR spectrometer in the proton decoupled mode at 20 °C in deuterated DMSO as a solvent unless otherwise stated. Chemical shifts are given in δ (parts per million) and the coupling constants (J) in hertz. 19F NMR (282 MHz) spectra were obtained on a Bruker Avance 300 NMR spectrometer in the proton coupled mode. Infrared spectra were acquired with an FT-IR 4100 LE (JASCO, Pike Miracle ATR) spectrometer. HRTEM was carried out on a high resolution transmission electron microscope JEOL JEM-2010 equipped with an X-ray detector OXFORD INCA Energy TEM 100 for microanalysis (EDS). The acquisition of the images was performed using a GATAN ORIUS SC600 digital camera mounted on-axis, integrated with the program GATAN Digital Micrograph 1.80.70 for GMS 1.8.0. Field emission scanning electron microscopy (FESEM) images were recorded on a Merlin VP Compact from Zeiss equipped with an EDS microanalysis system Quantax 400 from Bruker. The resolution was 0.8 nm at 15 kV and 1.6 nm at 1 kV. Field emission equipment is able to work at much reduced voltages (from 0.02 kV to 30 kV) allowing the observation of beam sensitive samples without damaging them and minimizing the charging effects. Scanning electron microscopy (SEM) studies were performed on a Hitachi S3000N, equipped with an X-ray detector Bruker XFlash 3001 for microanalysis (EDS) and mapping. The scanning microscope is able to work in the variable pressure mode for the observation of nonconductive specimens without coating with any conductive material. Low-resolution mass spectra (EI) were obtained at 70 eV with an Agilent 5973 Network spectrometer, with fragment ions m/z reported with relative intensities (%) in parentheses. HRMS analyses were carried out on an Agilent 7200 Q-TOF spectrometer. Magnetic measurements were performed using vibration sample magnetometry VSM (MDK Co. Kashan, Iran) analysis. TG-DTA analyses were carried out on a METTLER TOLEDO equipment (model TGA/SDTA851 and /SF/1100 TG-DTA). X-ray diffraction (XRD) analysis was performed using a Bruker D8-Advance apparatus with a Göbel mirror (non-planar samples) equipped with a high temperature chamber (up to 900 °C), a KRISTALLOFLEX K 760-80F X-ray generator (power: 3000 W, voltage: 20–60 kV and current: 5–80 mA), and a tube of RX with copper anode. Preparative thin-layer chromatography was carried on laboratory made TLC glass plates with silica gel 60 PF254 (Merck). Column chromatography was performed using silica gel 60 of 40–60 microns (hexane/EtOAc as eluent).Synthetic procedures
General procedure for the preparation of novel silica-coated magnetic nanoparticles with ionic liquid tags (Scheme 2)
First, the Fe3O4 nanoparticles were synthesized according to a previously reported method.53 Then, the surface of the Fe3O4 nanoparticles was modified with a layer of SiO2 by treating with tetraethyl orthosilicate (TEOS) to form compound B. In the next step, silanization of Fe3O4@SiO2 (B) by reaction with 3-chloropropyltrimethoxysilane in refluxing toluene afforded compound C. Subsequently, 6 mmol (0.408 g) of imidazole were added to compound C dispersed in toluene. The resulting mixture was refluxed for 12 h. Finally, trinitromethane (6 mmol, 0.906 g) was added to compound D in toluene and the reaction mixture was stirred for 12 h in refluxing toluene to form {Fe3O4@SiO2@(CH2)3Im}C(NO2)3 (E) as a reusable nanomagnetic core–shell catalyst with an ionic tag (Scheme 2).General procedure for the synthesis of polyhydroquinolines as target molecules (Scheme 1)
To a round bottom flask containing a mixture of aromatic aldehyde (1 mmol), dimedone (1 mmol, 0.14 g), ethyl acetoacetate or methyl acetoacetate as a β-ketoester (1 mmol), and ammonium acetate as a nitrogen source (3 mmol, 0.23 g), 7 mg of {Fe3O4@SiO2@(CH2)3Im}C(NO2)3 were added as a MNPs@IL catalyst. Then, the reaction mixture was stirred at 80 °C for adequate time under solvent-free conditions (Table 3). After completion of the reactions, as monitored by TLC (n-hexane/ethyl acetate), the reaction mixture was cooled to ambient temperature. Afterwards, in order to separate and recover the catalyst, hot ethanol was added to the mixture to dissolve the desired products and unreacted starting materials. The catalyst was insoluble in hot ethanol and easily separated from the reaction mixture by utilizing an external magnet. Finally, the target products were recrystallized from ethanol with high to excellent yields (Table 3).Spectral data
Ethyl 4-(4-chlorophenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate: (1a)36c
Melting point: 243–245 °C; FT-IR (KBr): ν(cm−1) = 3277, 3207, 3078, 2967, 1706, 1648, 1605, 1489, 1382, 1214; 1H NMR (400 MHz) δ 9.08 (br. s, 1H), 7.22 (s, 2H), 7.14 (s, 2H), 4.82 (s, 1H), 3.95 (m, 2H), 2.45 (d, J = 17.0, 1H), 2.35–2.25 (m with s at 2.27, 4H), 2.15 (d, J = 15.5, 1H), 1.96 (d, J = 15.5, 1H), 1.10 (br. s, 3H), 0.99 (s, 3H), 0.81 (s, 3H); 13C NMR (101 MHz) δ 194.7, 167.1, 150.0, 147.0, 145.8, 130.6, 129.7, 128.1, 110.1, 103.5, 59.5, 50.6, 36.0, 32.5, 29.5, 26.8, 18.7, 14.5; MS (EI) m/z (%): 375 (M+ + 2, 5), 373 (M+, 14), 262.1 (100), 234 (20); TOF-HRMS (EI): m/z (M)+ calcd for C21H24ClNO3 373.1445; found 373.1428.Ethyl 2,7,7-trimethyl-5-oxo-4-phenyl-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate: (1b)36c
Melting point: 224–226 °C; FT-IR (KBr): ν(cm−1) = 3289, 3216, 3081, 2962, 1701, 1614, 1485, 1382, 1212; 1H NMR (400 MHz) δ 9.03 (br. s, 1H), 7.15 (s, 4H), 7.05 (s, 1H), 4.85 (s, 1H), 3.96 (m, 2H), 2.41 (d, J = 17.0, 1H), 2.35–2.20 (m with s at 2.27, 4H), 2.15 (d, J = 16.0, 1H), 1.96 (d, J = 16.0, 1H), 1.11 (s, 3H), 1.00 (s, 3H), 0.83 (s, 3H); 13C NMR (101 MHz) δ 194.7, 167.3, 149.9, 148.1, 145.4, 128.1, 127.9, 126.1, 110.4, 104.1, 59.5, 50.7, 36.3, 32.6, 29.6, 26.9, 18.7, 14.6; MS (EI) m/z (%): 339 (M+, 13), 262 (100), 234 (20); TOF-HRMS (EI): m/z (M)+ calcd for C21H25NO3 339.1834; found 339.1863.Ethyl 4-(2,4-dichlorophenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate: (1c)36c
Melting point: 244–246 °C; FT-IR (KBr): ν(cm−1) = 3283, 3208, 3078, 2958, 1706, 1648, 1610, 1495; 1H NMR (400 MHz) δ 9.11 (s, 1H), 7.33 (s, 1H), 7.27 (s, 2H), 5.14 (s, 1H), 3.93 (m, 2H), 2.41 (d, J = 17.1, 1H), 2.30–2.20 (m with s at 2.24, 4H), 2.13 (d, J = 16.1, 1H), 1.91 (d, J = 16.0, 1H), 1.07 (t, J = 6.8, 3H), 0.99 (s, 3H), 0.83 (s, 3H); 13C NMR (101 MHz) δ 194.3, 167.0, 150.3, 145.9, 144.7, 133.3, 133.2, 131.2, 128.6, 127.3, 109.7, 103.2, 59.5, 50.6, 35.2, 32.4, 29.5, 26.8, 18.7, 14.5; MS (EI) m/z (%): 411 (M+ + 4, 1), 409 (M+ + 2, 5), 407 (M+, 9), 372 (14), 262 (100), 234 (19); TOF-HRMS (EI): m/z (M)+ calcd for C21H23Cl2NO3 407.1055; found 407.1062.Ethyl 4-(4-methoxyphenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate: (1d)36c
melting point: 255–257 °C; FT-IR (KBr): ν(cm−1) = 3277, 3202, 3078, 2957, 1701, 1649, 1606, 1496; 1H NMR (400 MHz) δ 8.99 (s, 1H), 7.04 (d, J = 8.2, 2H), 6.73 (d, J = 8.2, 2H), 4.78 (s, 1H), 3.96 (q, J = 6.9, 2H), 3.66 (s, 3H), 2.40 (d, J = 17.0, 1H), 2.35–2.20 (m with s at 2.26, 4H), 2.15 (d, J = 16.0, 1H), 1.96 (d, J = 16.0, 1H), 1.13 (t, J = 6.9, 3H), 1.00 (s, 3H), 0.84 (s, 3H); 13C NMR (101 MHz) δ 194.7, 167.4, 157.7, 149.6, 145.0, 140.5, 128.8, 113.5, 110.6, 104.4, 59.4, 55.3, 50.7, 35.4, 32.5, 29.6, 26.9, 18.7, 14.6; MS (EI) m/z (%): 369 (M+, 33), 340 (13), 296 (11), 262 (100), 234 (21); TOF-HRMS (EI): m/z (M)+ calcd for C22H27NO4 369.1940; found 369.1950.Ethyl 4-(3-bromophenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate: (1e)26
Melting point: 206–208 °C; FT-IR (KBr): ν(cm−1) = 3273, 3073, 2958, 1702, 1604, 1489, 1380, 1211, 1071, 769; 1H NMR (400 MHz) δ 9.12 (s, 1H), 7.32–7.22 (m, 2H), 7.20–7.08 (m, 2H), 4.82 (s, 1H), 4.05–3.90 (m, 2H), 2.42 (d, J = 17.2, 1H), 2.38–2.25 (m with s at 2.29, 4H), 2.17 (d, J = 16.2, 1H), 1.99 (d, J = 15.8, 1H), 1.12 (t, J = 6.8, 3H), 1.00 (s, 3H), 0.85 (s, 3H); 13C NMR (101 MHz) δ 194.7, 167.0, 150.7, 150.3, 146.0, 130.8, 130.5, 129.0, 127.0, 121.5, 109.9, 103.4, 59.6, 50.6, 36.5, 32.6, 29.5, 26.8, 18.8, 14.5; MS (EI) m/z (%): 419 (M+ + 2, 6), 417 (M+, 6), 262 (100), 234 (23); TOF-HRMS (EI): m/z (M)+ calcd for C21H24BrNO3 417.0940; found 417.0933.Ethyl 4-(2-chlorophenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate: (1f)39
Melting point: 207–209 °C; FT-IR (KBr): ν(cm−1) = 3277, 3210, 3073, 2957, 1701, 1649, 1606, 1496, 1380; 1H NMR (400 MHz) δ 9.07 (s, 1H), 7.28 (d, J = 6.8, 1H), 7.24–7.12 (m, 2H), 7.06 (t, J = 7.0, 1H), 5.18 (s, 1H), 3.98–3.87 (m, 2H), 2.41 (d, J = 17.0, 1H), 2.32–2.20 (m with s at 2.24, 4H), 2.13 (d, J = 16.1, 1H), 1.91 (d, J = 16.1, 1H), 1.07 (t, J = 7.1, 3H), 1.00 (s, 3H), 0.84 (s, 3H); 13C NMR (101 MHz) δ 194.3, 167.2, 150.1, 145.6, 145.4, 132.4, 131.9, 129.4, 127.7, 127.1, 110.0, 103.7, 59.4, 50.7, 35.4, 32.4, 29.6, 26.8, 18.6, 14.5; MS (EI) m/z (%): 375 (M+ + 2, 7), 373 (M+, 22), 338 (39), 262 (100), 234 (48); TOF-HRMS (EI): m/z (M)+ calcd for C21H24ClNO3 373.1445; found 373.1457.Ethyl 2,7,7-trimethyl-5-oxo-4-(p-tolyl)-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate: (1g)36c
Melting point: 263–265 °C; FT-IR (KBr): ν(cm−1) = 3276, 3208, 3078, 2958, 1702, 1648, 1606, 1493, 1282; 1H NMR (400 MHz) δ 8.99 (s, 1H), 7.15–6.90 (m, 4H), 4.79 (s, 1H), 3.95 (m, 2H), 2.39 (d, J = 17.1, 1H), 2.36–2.10 (m with 2 s at 2.25 and 2.18, 8H), 1.95 (d, J = 16.0, 1H), 1.12 (s, 3H), 0.99 (s, 3H), 0.83 (s, 3H); 13C NMR (101 MHz) δ 194.7, 167.3, 149.8, 145.2 (2c), 135.0, 128.7, 127.8, 110.5, 104.2, 59.4, 50.7, 35.8, 32.6, 29.6, 26.9, 21.0, 18.7, 14.6; MS (EI) m/z (%): 353 (M+, 18), 262 (100), 234 (18); TOF-HRMS (EI): m/z (M)+ calcd for C22H27NO3 353.1991; found 353.2005.Ethyl 4-(3,4-dimethoxyphenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate: (1h)36c
Melting point: 205–207 °C; FT-IR (KBr): ν(cm−1) = 3280, 3213, 3081, 2941, 1696, 1605, 1514; 1H NMR (300 MHz, EtOH-d6) δ 6.80 (d, J = 1.2 Hz, 1H), 6.72–6.53 (m, 2H), 4.83 (s, 1H), 3.94 (q, J = 7.1, 2H), 3.66 (s, 3H), 3.63 (s, 3H), 2.35 (d, J = 17.0 Hz, 1H), 2.29–2.19 (m with s at 2.26, 4H), 2.13 (d, J = 16.4 Hz, 1H), 1.98 (d, J = 16.4 Hz, 1H), 1.09 (t, J = 7.1 Hz, 3H), 0.97 (s, 3H), 0.82 (s, 3H); 13C NMR (101 MHz) δ 194.7, 167.3, 149.7, 148.3, 147.3, 144.9, 140.8, 119.6, 112.0, 111.8, 110.4, 104.2, 59.4, 55.7, 55.6, 50.6, 35.5, 32.5, 29.6, 26.8, 18.6, 14.6; MS (EI) m/z (%): 399 (M+, 33), 370 (10), 262 (100), 234 (21); TOF-HRMS (EI): m/z (M)+ calcd for C23H29NO5 399.2046; found 399.2056.Ethyl 4-(4-fluorophenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate: (1i)36c
Melting point: 193–195 °C; FT-IR (KBr): ν(cm−1) = 3291, 3219, 3078, 2959, 1702, 1607, 1487, 1382, 1212, 853, 531; 1H NMR (400 MHz) δ 9.06 (s, 1H), 7.14 (m, 2H), 6.99 (m, 2H), 4.83 (s, 1H), 3.96 (m, 2H), 2.40 (d, J = 17.1, 1H), 2.34–2.20 (m with s at 2.28, 4H), 2.15 (d, J = 15.8, 1H), 1.96 (d, J = 15.9, 1H), 1.10 (s, 3H), 0.99 (s, 3H), 0.82 (s, 3H); 13C NMR (101 MHz) δ 194.7, 167.2, 160.8 (d, J = 241.2), 149.9, 145.6, 144.3, 129.6 (d, J = 8.0), 114.8 (d, J = 21.0), 110.4, 103.9, 59.5, 50.6, 35.7, 32.6, 29.5, 26.9, 18.7, 14.6; MS (EI) m/z (%): 357 (M+, 21), 262 (100), 234 (22); 19F NMR (282 MHz, DMSO) δ −115.39; TOF-HRMS (EI): m/z (M)+ calcd for C21H24FNO3 357.1740; found 357.1746.Ethyl 2,7,7-trimethyl-4-(4-nitrophenyl)-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate: (1j)36c
Melting point: 242–243 °C; FT-IR (KBr): ν(cm−1) = 3274, 3189, 3076, 2966, 1703, 1649, 1609, 1493, 1349, 1215; 1H NMR (400 MHz) δ 9.22 (s, 1H), 8.09 (d, J = 8.5, 2H), 7.41 (d, J = 8.5, 2H), 4.96 (s, 1H), 3.96 (q, J = 6.9, 2H), 2.43 (d, J = 17.1, 1H), 2.35–2.25 (m with s at 2.31, 4H), 2.18 (d, J = 16.2, 1H), 1.97 (d, J = 16.2, 1H), 1.10 (t, J = 7.0, 3H), 1.00 (s, 3H), 0.81 (s, 3H); 13C NMR (101 MHz) δ 194.7, 166.8, 155.4, 150.5, 146.6, 146.1, 129.2, 123.6, 109.5, 102.8, 59.7, 50.5, 37.1, 32.6, 29.4, 26.9, 18.8, 14.5; MS (EI) m/z (%): 384 (M+, 15), 262 (100), 234 (22); TOF-HRMS (EI): m/z (M)+ calcd for C21H24N2O5 384.1685; found 384.1692.Ethyl 4-(2-methoxyphenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate: (1k)41
Melting point: 249–251 °C; FT-IR (KBr): ν(cm−1) = 3284, 3078, 2964, 1689, 1623, 1611, 1488, 1361, 1215, 751; 1H NMR (300 MHz, EtOH-d6) δ 7.15 (d, J = 7.0, 1H), 6.93 (t, J = 7.4, 1H), 6.79–6.56 (m, 2H), 5.06 (s, 1H), 3.87 (q, J = 7.0, 2H), 3.65 (s, 3H), 2.32 (d, J = 17.0, 1H), 2.26–2.15 (m with s at 2.18, 4H), 2.14–1.97 (m, 1H), 1.91 (d, J = 16.4, 1H), 1.10–0.90 (m, 6H), 0.80 (s, 3H); 13C NMR (101 MHz) δ 193.8, 167.3, 157.1, 149.9, 144.1, 134.9, 130.5, 126.9, 119.4, 111.0, 108.6, 102.9, 58.7, 55.1, 50.4, 32.8, 32.0, 29.3, 26.2, 18.0, 14.1; MS (EI) m/z (%): 369 (M+, 61), 340 (37), 262 (100), 234 (31); TOF-HRMS (EI): m/z (M)+ calcd for C22H27NO4 369.1940; found 369.1946.Ethyl 4-(3-ethoxy-4-hydroxyphenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate: (1l)36c
Melting point: 195–196 °C; FT-IR (KBr): ν(cm−1) = 3443, 3282, 3200, 3079, 2959, 1657, 1616, 1493, 1381; 1H NMR (300 MHz, EtOH-d6) δ 6.74 (s, 1H), 6.52 (s, 2H), 4.79 (s, 1H), 4.02–3.81 (m, 4H), 2.33 (d, J = 17.0, 1H), 2.28–2.18 (m with s at 2.25, 4H), 2.12 (d, J = 16.5, 1H), 1.98 (d, J = 16.4, 1H), 1.27 (t, J = 7.0, 3H), 1.08 (t, J = 7.1, 3H), 0.96 (s, 3H), 0.81 (s, 3H); 13C NMR (101 MHz) δ 194.8, 167.4, 149.6, 146.2, 145.3, 144.8, 139.4, 120.1, 115.4, 113.9, 110.6, 104.5, 64.2, 59.4, 50.7, 35.4, 32.5, 29.6, 26.8, 18.7, 15.2, 14.6; MS (EI) m/z (%): 399 (M+, 30), 370 (11), 262 (100), 234 (19); TOF-HRMS (EI): m/z (M)+ calcd for C23H29NO5 399.2046; found 399.2048.Ethyl 4-(3-hydroxyphenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate: (1m)36c
Melting point: 231–234 °C; FT-IR (KBr): ν(cm−1) = 3463, 3291, 2961, 1669, 1620, 1487, 1381, 1277, 1216; 1H NMR (400 MHz) δ 9.05 (s, 1H), 9.00 (s, 1H), 6.94 (t, J = 7.7, 1H), 6.59–6.56 (m, 2H), 6.45 (d, J = 7.3, 1H), 4.78 (s, 1H), 3.98 (q, J = 7.0, 2H), 2.40 (d, J = 17.0, 1H), 2.32–2.25 (m with s at 2.27, 4H), 2.16 (d, J = 16.1, 1H), 1.99 (d, J = 16.1, 1H), 1.14 (t, J = 7.1, 3H), 1.00 (s, 3H), 0.87 (s, 3H); 13C NMR (101 MHz) δ 194.7, 167.4, 157.3, 149.9, 149.4, 145.1, 128.9, 118.6, 115.0, 113.1, 110.4, 104.1, 59.5, 50.8, 36.1, 32.5, 29.6, 27.0, 18.7, 14.6; MS (EI) m/z (%): 355 (M+, 13), 262 (100), 234 (20); TOF-HRMS (EI): m/z (M)+ calcd for C21H25NO4 355.1784; found 355.1792.Methyl 4-(4-chlorophenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate: (1n)40
Melting point: 257–258 °C; FT-IR (KBr): ν(cm−1) = 3288, 3198, 3076, 1682, 1606, 1492, 1226; 1H NMR (400 MHz) δ 9.12 (s, 1H), 7.23 (br. d, J = 6.6, 2H), 7.13 (br. d, J = 6.4, 2H), 4.83 (s, 1H), 3.51 (s, 3H), 2.40 (d, J = 17.0, 1H), 2.35–2.20 (m with s at 2.28, 4H), 2.16 (d, J = 16.0, 1H), 1.96 (d, J = 15.7, 1H), 0.99 (s, 3H), 0.81 (s, 3H); 13C NMR (101 MHz) δ 194.7, 167.6, 150.0, 146.8, 146.1, 130.7, 129.6, 128.2, 110.1, 103.2, 51.1, 50.6, 35.8, 32.6, 29.5, 26.8, 18.8; MS (EI) m/z (%): 361 (M+ + 2, 4), 359 (M+, 11), 248 (100); TOF-HRMS (EI): m/z (M)+ calcd for C20H22ClNO3 359.1288; found 359.1275.Methyl 2,7,7-trimethyl-5-oxo-4-phenyl-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate: (1o)26
Melting point: 259–261 °C; FT-IR (KBr): ν(cm−1) = 3278, 3202, 3079, 2986, 1709, 1607, 1789, 1309; 1H NMR (300 MHz, EtOH-d6) δ 7.13 (d, J = 7.3, 2H), 7.02 (t, J = 7.4, 2H), 6.92 (t, J = 7.1, 1H), 4.89 (s, 1H), 3.53 (s, 3H), 2.34 (d, J = 17.0, 1H), 2.28–2.18 (m with s at 2.26, 4H), 2.12 (d, J = 16.5, 1H), 1.97 (d, J = 16.5, 1H), 0.96 (s, 3H), 0.79 (s, 3H); 13C NMR (101 MHz) δ 194.7, 167.7, 149.9, 147.9, 145.7, 128.2, 127.7, 126.1, 110.4, 103.6, 51.1, 50.7, 36.0, 32.6, 29.6, 26.8, 18.7; MS (EI) m/z (%): 325 (M+, 11), 248 (100); TOF-HRMS (EI): m/z (M)+ calcd for C20H23NO3 325.1678; found 325.1673.Methyl 4-(4-bromophenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate: (1p)42
Melting point: 261–263 °C; FT-IR (KBr): ν(cm−1) = 3291, 3202, 3078, 2958, 1683, 1606, 1492, 1382, 1332, 1227; 1H NMR (300 MHz, EtOH-d6) δ 7.17 (d, J = 8.3, 2H), 7.05 (d, J = 8.3, 2H), 4.85 (s, 1H), 3.47 (s, 3H), 2.34 (d, J = 17.0, 1H), 2.30–2.19 (m with s at 2.27, 4H), 2.12 (d, J = 16.4, 1H), 1.98 (d, J = 16.6, 1H), 0.96 (s, 3H), 0.79 (s, 3H); 13C NMR (101 MHz) δ 194.7, 167.5, 150.1, 147.2, 146.1, 131.1, 130.0, 119.2, 110.0, 103.1, 51.2, 50.6, 35.6, 32.6, 29.5, 26.9, 18.8; MS (EI) m/z (%): 405 (M+ + 2, 7), 403 (M+, 7), 248 (100); TOF-HRMS (EI): m/z (M)+ calcd for C20H22BrNO3 403.0783; found 403.0674.Methyl 4-(2,4-dichlorophenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate: (1q)42
Melting point: 251–253 °C; FT-IR (KBr): ν(cm−1) = 3416, 3263, 1378, 2957, 1710, 1650, 1588, 1493, 1216; 1H NMR (300 MHz, EtOH-d6) δ 7.21 (d, J = 8.3, 1H), 7.09 (d, J = 1.8, 1H), 7.01 (dd, J = 8.3, 1.7, 1H), 5.19 (s, 1H), 3.43 (s, 3H), 2.35 (d, J = 17.0, 1H), 2.30–2.15 (m with s at 2.21, 4H), 2.10 (d, J = 16.4, 1H), 1.93 (d, J = 16.3, 1H), 0.97 (s, 3H), 0.83 (s, 3H); 13C NMR (101 MHz) δ 194.4, 167.5, 150.3, 146.0, 144.9, 133.2, 132.9, 131.2, 128.6, 127.4, 109.9, 100.5, 50.9, 50.6, 35.0, 32.5, 29.5, 26.8, 18.6; MS (EI) m/z (%): 397 (M+ + 4, 1), 395 (M+ + 2, 5), 393 (M+, 7), 358 (15), 248 (100); TOF-HRMS (EI): m/z (M)+ calcd for C20H21Cl2NO3 393.0898; found 393.0910.Methyl 4-(4-methoxyphenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate: (1r)26
Melting point: 254–257 °C; FT-IR (KBr): ν(cm−1) = 3188, 3067, 2961, 1705, 1649, 1605, 1498; 11H NMR (300 MHz, EtOH-d6) δ 7.03 (d, J = 8.1, 2H), 6.58 (d, J = 8.1, 2H), 4.82 (s, 1H), 3.59 (s, 3H), 3.46 (s, 3H), 2.33 (d, J = 17.0, 1H), 2.28–2.16 (m with s at 2.25, 4H), 2.12 (d, J = 16.5, 1H), 1.97 (d, J = 16.5, 1H), 0.96 (s, 3H), 0.80 (s, 3H); 13C NMR (101 MHz) δ 194.7, 167.8, 157.7, 149.6, 145.4, 140.3, 128.6, 113.6, 110.7, 104.0, 55.3, 51.1, 50.7, 35.2, 32.6, 29.6, 26.9, 18.7; MS (EI) m/z (%): 355.2 (M+, 26), 248.1 (100); TOF-HRMS (EI): m/z (M)+ calcd for C21H25NO4 355.1784; found 355.1788.Methyl 4-(4-hydroxyphenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate: (1s)26
Melting point: 297–298 °C; FT-IR (KBr): ν(cm−1) = 3400, 3276, 3199, 2960, 1687, 1611, 1487, 1380, 1222, 1168, 768; 1H NMR (300 MHz, EtOH-d6) δ 6.93 (d, J = 8.1 Hz, 2H), 6.49 (d, J = 8.1 Hz, 2H), 4.79 (s, 1H), 3.47 (s, 3H), 2.33 (d, J = 17.0 Hz, 1H), 2.22 (m with s at 2.25, 4H), 2.11 (d, J = 16.5 Hz, 1H), 1.97 (d, J = 16.5 Hz, 1H), 0.96 (s, 3H), 0.80 (s, 3H); 13C NMR (101 MHz) δ 194.3, 167.5, 155.2, 149.1, 144.7, 138.2, 128.1, 114.5, 110.4, 103.7, 50.6, 50.3, 34.5, 32.1, 29.1, 26.4, 18.2; MS (EI) m/z (%): 341 (M+, 23), 248 (100); TOF-HRMS (EI): m/z (M)+ calcd for C20H23NO4 341.1627; found 341.1628.Methyl 2,7,7-trimethyl-5-oxo-4-(p-tolyl)-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate: (1t)23
Melting point: 270–273 °C; FT-IR (KBr): ν(cm−1) = 3191, 3072, 2958, 1686, 1644, 1503, 1491, 1227; 1H NMR (300 MHz, EtOH-d6) δ 7.01 (d, J = 7.8, 2H), 6.83 (d, J = 7.6, 2H), 4.84 (s, 1H), 3.47 (s, 3H), 2.33 (d, J = 16.7, 1H), 2.27–2.19 (m with s at 2.25, 4H), 2.15–1.90 (m with s at 2.11, 5H), 0.96 (s, 3H), 0.80 (s, 3H); 13C NMR (101 MHz) δ 194.7, 167.8, 149.7, 145.5, 145.0, 135.0, 128.8, 127.6, 110.5, 103.8, 51.0, 50.7, 35.6, 32.5, 29.6, 26.9, 21.0, 18.7; MS (EI) m/z (%): 339 (M+, 15), 248 (100); TOF-HRMS (EI): m/z (M)+ calcd for C21H25NO3 339.1834; found 339.1834.Acknowledgements
We thank the Bu-Ali Sina University, the Iran National Science Foundation (INSF) (Allameh Tabataba'i's Award, Grant Number BN093), the University of Alicante (VIGROB-173), and the Spanish Ministerio de Economía y Competitividad (CTQ2015-66624-P) for financial support to our research groups.References
- T. Cheng, D. Zhang, H. Li and G. Liu, Green Chem., 2014, 16, 3401 RSC.
- (a) D. Zhang, C. Zhou, Z. Sun, L. Z. Wu, C. H. Tung and T. Zhang, Nanoscale, 2012, 4, 6244 RSC; (b) B. Karimi, F. Mansouri and H. M. Mirzaei, ChemCatChem, 2015, 7, 1736 CrossRef CAS.
- (a) S. Ganesh Babu and R. Karvembu, Catal. Surv. Asia, 2013, 17, 156 CrossRef; (b) D. Zhang, C. Zhou, Z. Sun, L. Z. Wu, C. H. Tung and T. Zhang, Nanoscale, 2012, 4, 6244 RSC; (c) V. Polshettiwar, R. Luque, A. Fihri, H. Zhu, M. Bouhrara and J. M. Basset, Chem. Rev., 2011, 111, 3036 CrossRef CAS PubMed; (d) S. Shylesh, V. Schunemann and W. R. Thiel, Angew. Chem., Int. Ed., 2010, 49, 3428 CrossRef CAS PubMed; (e) S. Laurent, D. Forge, M. Port, A. Roch, C. Robic, L. V. Elst and R. N. Muller, Chem. Rev., 2008, 108, 2064–2110 CrossRef CAS PubMed; (f) A. H. Lu, E. L. Salabas and F. Schìth, Angew. Chem., Int. Ed., 2007, 46, 1222 CrossRef CAS PubMed; (g) R. Hudson, Y. Feng, R. S. Varma and A. Moores, Green Chem., 2014, 16, 4493 RSC; (h) M. Mokhtary, J. Iran. Chem. Soc., 2016, 13, 1827 CrossRef CAS.
- M. Vafaeezadeh and H. Alinezhad, J. Mol. Liq., 2016, 218, 95 CrossRef CAS.
- Y. Gua and G. Li, Adv. Synth. Catal., 2009, 351, 817 CrossRef.
- C. Yue, D. Fang, L. Liu and T. F. Yi, J. Mol. Liq., 2011, 163, 99 CrossRef CAS.
- T. L. Greaves and C. J. Drummond, Chem. Rev., 2008, 108, 206 CrossRef CAS PubMed.
- J. P. Hallett and T. Welton, Chem. Rev., 2011, 111, 3508 CrossRef CAS PubMed.
- M. Smiglak, J. M. Pringle, X. Lu, L. Han, S. Zhang, H. Gao, D. R. MacFarlane and R. D. Rogers, Chem. Commun., 2014, 50, 9228 RSC.
- (a) Q. Zhang, S. Zhang and Y. Deng, Green Chem., 2011, 13, 2619 RSC; (b) F. Dong, F. Zhenghao and L. Zuliang, Catal. Commun., 2009, 10, 1267 CrossRef CAS; (c) E. S. Putilova, G. V. Kryshtal, G. M. Zhdankina, N. A. Troitskii and S. G. Zlotin, Russ. J. Org. Chem., 2005, 41, 512 CrossRef CAS.
- K. S. Egorova and V. P. Ananikov, ChemSusChem, 2014, 7, 336 CrossRef CAS PubMed.
- T. P. T. Pham, C. W. Cho and Y. S. Yun, Water Res., 2010, 44, 352 CrossRef CAS PubMed.
- Z. Zarnegar and J. Safari, J. Nanopart. Res., 2014, 16, 1 CrossRef CAS.
- Multicomponent Reactions, ed. J. Zhu and H. Bienayme, Wiley-VCH, Weinheim, 2005 Search PubMed.
- A. Domling and I. Ugi, Angew. Chem., Int. Ed., 2000, 39, 3169 CrossRef.
- J. E. Biggs-Houck, A. Younai and J. T. Shaw, Curr. Opin. Chem. Biol., 2010, 14, 371 CrossRef CAS PubMed.
- R. P. Gore and A. P. Rajput, Drug Invent. Today, 2013, 5, 148 CrossRef CAS.
- A. Heydari, S. Khaksar, M. Tajbakhsh and R. Bijanzadeh, J. Fluorine Chem., 2009, 130, 609 CrossRef CAS.
- (a) P. P. Ghosh, P. Mukherjee and A. R. Das, RSC Adv., 2013, 3, 8220 RSC; (b) B. H. Rotstein, S. H. Liang, V. V. Belov, E. Livni, D. B. Levine, A. A. Bonab, M. I. Papisov, R. H. Perlis and N. Vasdev, Molecules, 2015, 20, 9550 CrossRef CAS PubMed; (c) A. Kumar and S. Sharma, Green Chem., 2017, 2011, 13 Search PubMed; (d) C. O. Okoro, M. A. Ogunwale and T. Siddiquee, Appl. Sci., 2012, 2, 368 CrossRef CAS.
- C. G. Evans, U. K. Jinwal, L. N. Makley, C. A. Dickey and J. E. Gestwicki, Chem. Commun., 2011, 47, 529 RSC.
- D. Zhang, L. Z. Wu, L. Zhou, X. Han, Q. Z. Yang, L. P. Zhang and C. H. Tung, J. Am. Chem. Soc., 2004, 126, 3440 CrossRef CAS PubMed.
- A. Khazaei, M. A. Zolfigol, A. R. Moosavi-Zare, J. Afsar, A. Zare, V. Khakyzadeh and M. H. Beyzavi, Chin. J. Catal., 2013, 34, 1936 CrossRef CAS.
- M. R. Poor Heravi, S. Mehranfar and N. Shabani, C. R. Chim., 2014, 17, 141 CrossRef CAS.
- M. Tajbakhsh, E. Alaee, H. Alinezhad, M. Khanian, F. Jahani, S. Khaksar, P. Rezaee and M. Tajbakhsh, Chin. J. Catal., 2012, 33, 1517 CrossRef CAS.
- S. M. Vahdat, F. Chekin, M. Hatami, M. Khavarpour, S. Baghery and Z. Roshan-Kouhi, Chin. J. Catal., 2013, 34, 758 CrossRef CAS.
- A. R. Moosavi-Zare, M. A. Zolfigol, M. Zarei, A. Zare and J. Afsar, Appl. Catal., A, 2015, 505, 224 CrossRef CAS.
- M. Tajbakhsh, A. Alinezhad, M. Norouzi, S. Baghery and M. Akbari, J. Mol. Liq., 2013, 177, 44 CrossRef CAS.
- S. Rostamnia, H. Xin, X. Liu and K. Lamei, J. Mol. Catal. A: Chem., 2013, 374, 85 CrossRef.
- S. B. Sapkal, K. F. Shelke, B. B. Shingate and M. S. Shingare, Tetrahedron Lett., 2009, 50, 1754 CrossRef CAS.
- S. Rostamnia, A. Hassankhani, H. Golchin, H. Behnam Gholipour and H. Xin, J. Mol. Catal. A: Chem., 2014, 395, 463 CrossRef CAS.
- A. Kumar and R. A. Maurya, Tetrahedron Lett., 2007, 48, 3887 CrossRef CAS.
- N. N. Karade, V. H. Budhewar, S. V. Shinde and W. N. Jadhav, Lett. Org. Chem., 2007, 4, 16 CrossRef CAS.
- S. J. Ji, Z. Q. Jiang, J. Lu and T. P. Loh, Synlett, 2004, 831 CrossRef CAS.
- E. Kianpour, S. Azizian, M. Yarie, M. A. Zolfigol and M. Bayat, Chem. Eng. J., 2016, 295, 500 CrossRef CAS , and references cited therein.
- (a) M. A. Zolfigol, N. Mansouri and S. Baghery, Synlett, 2016, 27, 1511 CrossRef CAS; (b) M. A. Zolfigol, M. Yarie and S. Baghery, Synlett, 2016, 27, 1418 CrossRef CAS; (c) M. A. Zolfigol, N. Bahraminezhad and S. Baghery, J. Mol. Liq., 2016, 218, 558 CrossRef CAS; (d) M. A. Zolfigol, S. Baghery, A. R. Moosavi-Zare and S. M. Vahdat, J. Mol. Catal. A: Chem., 2015, 409, 216 CrossRef CAS , and references cited therein.
- (a) T. Azadbakht, M. A. Zolfigol, R. A. V. Khakyzadeh and D. M. Perrin, New J. Chem., 2015, 39, 439 RSC; (b) M. A. Zolfigol, T. Azadbakht, V. Khakyzadeh, R. Nejatyami and D. M. Perrin, RSC Adv., 2014, 4, 40036 RSC; (c) M. A. Zolfigol, V. Khakyzadeh, A. R. Moosavi-Zare, A. Rostami, A. Zare, N. Iranpoor, M. H. Beyzavie and R. Luque, Green Chem., 2013, 15, 2132 RSC; (d) N. Koukabi, E. Kolvari, A. Khazaei, M. A. Zolfigol, B. Shirmardi-Shaghasemi and H. R. Khavasi, Chem. Commun., 2011, 47, 9230 RSC; (e) M. A. Zolfigol, R. Ayazi-Nasrabadi, S. Baghery, V. Khakyzadeh and S. Azizian, J. Mol. Catal. A: Chem., 2016, 418, 54 CrossRef; (f) M. Daraei, M. A. Zolfigol, F. Derakhshan-Panah, M. Shiri, H. G. Kruger and M. Mokhlesi, J. Iran. Chem. Soc., 2015, 12, 855 CrossRef CAS; (g) M. Safaiee, M. A. Zolfigol, M. Tavasoli and M. Mokhlesi, J. Iran. Chem. Soc., 2014, 11, 1593 CrossRef CAS; (h) D. Azarifar, S. M. Khatami, M. A. Zolfigol and R. Nejat-Yami, J. Iran. Chem. Soc., 2014, 11, 1223 CrossRef CAS.
- (a) M. A. Zolfigol and M. Yarie, RSC Adv., 2015, 5, 103617 RSC; (b) M. A. Zolfigol, M. Kiafar, M. Yarie, A. Taherpour and M. Saeidirad, RSC Adv., 2016, 6, 50100 RSC.
- (a) M. A. Zolfigol, S. Baghery, A. R. Moosavi-Zare, S. M. Vahdat, H. Alinezhad and M. Norouzi, RSC Adv., 2014, 4, 57662 RSC; (b) M. A. Zolfigol, F. Afsharnaderee, S. Baghery, S. Salehzadeh and F. Maleki, RSC Adv., 2015, 5, 75555 RSC.
- A. Khazaei, A. R. Moosavi-Zare, H. Afshar-Hezarkhania and V. Khakyzadeh, RSC Adv., 2014, 4, 32142 RSC.
- M. Nasr-Esfahani, D. Elhamifar, T. Amadeh and B. Karimi, RSC Adv., 2015, 5, 13087 RSC.
- O. Goli-Jolodar, F. Shirini and M. Seddighi, RSC Adv., 2016, 6, 26026 RSC.
- C. X. Yu, D. Q. Shi, Q. Y. Zhuang and S. J. Tu, Chin. J. Org. Chem., 2006, 26, 263 CAS.
- A. Davoodnia, M. Khashi and N. Tavakoli-Hoseini, Chin. J. Catal., 2013, 34, 1173 CrossRef CAS.
- A. Heydari, S. Khaksar, M. Tajbakhsh and H. R. Bijanzadeh, J. Fluorine Chem., 2009, 130, 609 CrossRef CAS.
- A. Ghorbani-Choghamarani and G. Azadi, RSC Adv., 2015, 5, 9752 RSC.
- M. Nasr-Esfahani, D. Elhamifar, T. Amadeha and B. Karimi, RSC Adv., 2015, 5, 13087 RSC.
- Z. Zarnegar, J. Safari and Z. M. Kafroudi, New J. Chem., 2015, 39, 1445 RSC.
- M. Kidwai, R. Chauhan, D. Bhatnagar, A. K. Singh, B. Mishra and S. Dey, Monatsh. Chem., 2012, 143, 1675 CrossRef CAS.
- E. Mosaddegh and A. Hassankhani, Arabian J. Chem., 2012, 5, 315 CrossRef CAS.
- A. Khazaei, A. R. Moosavi-Zare, H. Afshar-Hezarkhania and V. Khakyzadeh, RSC Adv., 2014, 4, 32142 RSC.
- M. B. Gawande, V. D. B. Bonifácio, R. S. Varma, I. D. Nogueira, N. Bundaleski, C. A. A. Ghumman, O. M. N. D. Teodorod and P. S. Branco, Green Chem., 2013, 15, 1226 RSC.
- M. Hong, C. Cai and W. B. Yi, J. Fluorine Chem., 2010, 131, 111 CrossRef CAS.
- S. Qu, H. Yang, D. Ren, S. Kan, G. Zou, D. Li and M. Li, J. Colloid Interface Sci., 1999, 215, 190 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra16459e |
| This journal is © The Royal Society of Chemistry 2016 |