Preparation of α-Fe2O3 hollow spheres, nanotubes, nanoplates and nanorings as highly efficient Cr(vi) adsorbents†
Abstract
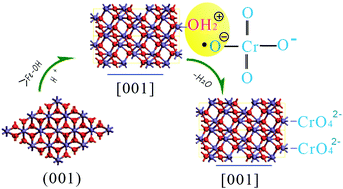
α-Fe2O3 nanoparticles with different morphologies, such as hollow spheres, nanotubes with a limited amount of the {0001} plane exposed, and nanoplates and nanorings with the {0001} plane predominantly exposed, have been synthesised by using NaH2PO4 and urea in a facile hydrothermal method. The mechanism of the morphology evolution from hollow sphere to nanoring has been investigated. It is proposed that the polymerisation of Fe3+/H2PO4− plays an important role in the formation of these morphologies. The adsorption of Cr(VI) from aqueous solution onto these α-Fe2O3 nanoparticles showed that the α-Fe2O3 with nanoring morphology has the highest removal efficiency, and the adsorption capacity reached 16.9 mg g−1. These results indicate that the adsorption mechanism of Cr(VI) onto hematite nanoparticles is a chemisorption process through doubly and triply coordinated hydroxyl groups on the outer surface of α-Fe2O3.

 Please wait while we load your content...
Please wait while we load your content...