Effective adsorption of phenolic pollutants from water using β-cyclodextrin polymer functionalized Fe3O4 magnetic nanoparticles†
Tao Gonga,
Yehong Zhoua,
Linlin Suna,
Wenting Liang*a,
Jun Yangb,
Shaomin Shuanga and
Chuan Dong*a
aInstitute of Environmental Sciences, Department of Chemistry, Shanxi University, Taiyuan 030006, China. E-mail: liangwt@sxu.edu.cn; dc@sxu.edu.cn
bDepartment of Mechanical and Materials Engineering, University of Western Ontario, London, Ontario N6G 0J3, Canada
First published on 9th August 2016
Abstract
β-Cyclodextrin polymer functionalized magnetic nanoparticles (CDP-MNPs) were prepared with a one-step co-precipitation method by anchoring a carboxymethyl-β-cyclodextrin polymer (CDP) onto the surface of Fe3O4 magnetic nanoparticles (MNPs). The CDP-MNPs possess the fascinating features of superparamagnetism and adsorption properties, which are favorable for the purpose of removing bisphenol A and resorcin. The maximum adsorption capacity for bisphenol A could reach values up to 74.63 mg g−1 at 25 °C, which is higher than those of previously reported magnetic adsorbents. Moreover, for the removal of resorcin by CDP-MNPs, the maximum uptake amounted to 114.91 mg g−1 under the same conditions. The adsorption kinetics and isotherm data fitted well with a pseudo-second-order kinetic model and the Langmuir isotherm, respectively. The CD polymer grafted onto the MNPs enhanced the adsorption capacity because of the host–guest interaction of cyclodextrin and the hydrogen bonding of the polymer network with the phenolic compounds. Additionally, a good recyclability of the CDP-MNPs was observed over three usage cycles, with only a slight decline in adsorption capability.
Introduction
Water pollutants from manufacturing operations and anthropogenic activities have become a global concern owing to their long-term potential risk to ecosystems and adverse effects on human health. Phenolic compounds, as typical organic water pollutants, have been considered as the primary pollutants in wastewater due to their high toxicity, high oxygen demand, and low biodegradability.1 Several approaches have been employed for the treatment of phenolic wastewater such as adsorption,2 membrane technology,3 advanced oxidation processes,4 photocatalytic degradation,5 solvent extraction6 and combinations of these processes.7 Among these, adsorption is the most versatile and commonly used technology owing to its high efficiency, easy handling and economy.8 Adsorption efficiency is strongly affected by the chemical properties and surface morphology of an adsorbent; therefore, several adsorbents have been investigated in the removal of phenolic pollutants, such as activated carbon, zeolite, diatomite, silica gel, polymers, nanomaterials, etc.9–12 However, the limited loading capacity or separation inconvenience of these ordinarily-used adsorbents strongly encouraged us to explore a suitable adsorbent with a high adsorption capacity, easy separation, and a low operating cost.In recent research, functional Fe3O4 magnetic nano-sized adsorbents have played an important role in the adsorption of organic or inorganic pollutants from aqueous solution.13–19 Fe3O4 nano-adsorbents have the natural properties of an extremely small size, a high surface-area-to volume ratio and the absence of internal diffusion resistance, which provide promising kinetics for adsorption. With the integrated characteristics of adsorption capacity and magnetite, Fe3O4 nano-adsorbents can be easily recovered or manipulated with an external magnetic field. The surfaces of these magnetic adsorbents are often modified by capping agents such as polymers, inorganic metals or oxides, and surfactants to make them stable, biocompatible, and suitable for further functionalizations and applications.20–23 Based on the structural diversity and favorable adsorption capability of polymers, various types of magnetic nano-adsorbents with tailored surface reactivity have been investigated recently for pollutant removal from wastewater by using natural or synthetic polymers such as polysaccharide, gum arabic, alginate, polyaniline etc.24–28 It would be interesting to introduce some useful functional polymers onto their surfaces to effectively enhance the adsorption capacity of Fe3O4 nano-adsorbents and provide some promising approaches to other desirable applications.
Interestingly, cyclodextrin (CD), a cyclic oligosaccharide consisting of 6–8 glucose units, is commonly used as a functional water-soluble unit to construct polymer materials by various approaches.29,30 CD and its derivatives contain a relatively hydrophobic cavity; therefore, it can be used as a molecular recognizer through host–guest interactions. A group of important contaminants such as polycyclic aromatic hydrocarbons (PAHs),31 phthalic acid esters (PAEs)32 and phenolic compounds33 could form inclusion complexes with CD and its derivatives. Based on these properties, CD polymer functionalized magnetic nanomaterials have been considerably explored to efficiently adsorb and eliminate contaminants from wastewater, as they improve the inclusion properties of β-CD and allow for the exploitation of the magnetic separation technology of the magnetic nanoparticles (MNPs). A. Z. Badruddoza's group synthesized magnetic CD polymer nanoparticles which could selectively remove heavy metal ions from water.34 Yuan et al. prepared CD polymer magnetic micelles for the adsorption of bisphenol A from water.35 Kang et al. designed a type of Fe3O4 nanoparticle modified by poly(glycidyl methacrylate) linked CD, which could be used as a catalyst and as an adsorbent for organic pollutants.36 However, most of the previously reported magnetic adsorbents have a low adsorption capacity, and the procedures of preparation via layer-by-layer modification are comparatively complicated and inefficient. In this work, carboxymethyl-β-cyclodextrin polymer (CDP) covered MNPs have been fabricated successfully by a one-step co-precipitation method which could be used in adsorption efficiently and repeatedly.
These CD polymer functionalized magnetic particles as nano-adsorbents for phenolic pollutants would facilitate site-specific removal and recycling under an externally applied magnetic field. Therefore, bisphenol A and resorcin, as model compounds of phenolic pollutants, have been used to explore the adsorption capacity of CDP-MNPs in aqueous solution. By means of a batch equilibrium method, the adsorption behavior of CDP-MNPs on bisphenol A and resorcin were explored from both equilibrium and kinetic viewpoints. Compared with previously reported magnetic adsorbents, the CDP-MNPs exhibited a much higher adsorption capacity for bisphenol A, amounting to 74.63 mg g−1. For resorcin, the maximum uptake was as high as 114.91 mg g−1, which therefore indicated another efficient example of CDP-MNPs as a magnetic nano-absorbent to remove this compound from water. Besides demonstrating the adsorption capacity, efforts were devoted to achieving recyclability of the CDP-MNPs.
Experimental section
Materials
Iron(II) chloride tetrahydrate (99%) and iron(III) chloride hexahydrate (98%) were procured from Tianjin Reagent Factory. β-CD (95%) was obtained from Guangdong Reagent Factory and purified by recrystallization from boiling water. Sodium chloroacetate (99%), epichlorohydrin (EPI; 99%) and cyanamide (98%) were purchased from Aladdin Reagent Co. Bisphenol A (99%) and resorcinol (99%) were supplied from the Tianjin Guangfu Fine Chemical Research Institute. All other chemicals were of analytical grade and were used as received without further purification. Doubly distilled water was used throughout this work.Preparation of CDP
CDP was synthesized via a cross-linking reaction using EPI as the cross-linking agent.37 Generally, a mixture of 4.50 g β-CD and 3.62 g of NaOH dissolved in 20 mL of doubly distilled water were treated with 8.73 g of sodium chloroacetate at 90 °C for 3 h with vigorous stirring. The reaction mixture was cooled to room temperature and its pH value was adjusted to 4–6 with HCl. A large amount of methanol was added and a white precipitate was produced. The addition of methanol was continued until no more precipitate was produced. The white precipitate was filtered and dried at 50 °C under vacuum for 12 h. The above-mentioned precipitate was further dissolved in 25 mL of 10% (w/v) NaOH, and 10 mL of EPI, the cross-linking agent, was dripped slowly into the solution at room temperature for 48 h under constant stirring. The product was precipitated by pouring excess ethanol solvent into the reaction mixture. After washing with ethanol several times, the obtained white gummy precipitate was filtered and dried at 50 °C under vacuum for 24 h to give CDP.The CD content was quantified by determining the reducing sugars of the polymer on the basis of concentrated H2SO4 acidolysis and phenol colorimetric analysis.29 The Fourier transform infrared (FTIR) spectrum of CDP was recorded using a Bruker Tensor II. Differential scanning calorimetry (DSC) was performed on TA Instruments Q50 under a flowing nitrogen atmosphere. The Brunauer–Emmett–Teller (BET) surface area, pore volume, and Barrett–Joyner–Halenda (BJH) pore size distribution of the materials were analyzed on a Micromeritics Tristar 3000 by recording nitrogen adsorption/desorption isotherms at 77 K.
Fabrication of CDP-MNPs
CDP-MNP superparamagnetic nanoparticles were fabricated by a one-step co-precipitation method according to the literature.34 Briefly, 0.86 g of FeCl2·4H2O, 2.36 g FeCl3·6H2O and 2.00 g of CDP were dissolved in 50 mL of doubly distilled water with vigorous stirring at 80 °C under a nitrogen environment. An adequate amount of NH4OH (25%) was added until its pH value was adjusted to 9–10. The reaction was continued for 1 h at 80 °C under constant stirring and a nitrogen environment. After cooling to room temperature, the resulting black nanoparticles were washed with doubly distilled water, separated with a permanent magnet repeatedly, and dried at 50 °C under vacuum for 8 h to give CDP-MNPs.Characterization and stability of CDP-MNPs
The size and morphology of the as-prepared CDP-MNPs were determined by transmission electron microscopy (TEM, JEOL JEM-2100). X-ray diffraction (XRD) measurements were performed on a Bruker D8 Advance diffractometer using Cu-Kα radiation in the 2θ range 10–80°. To detect whether CDP was capped onto the surface of the MNPs, FT-IR spectroscopy measurements were carried out on a Bruker Tensor 27, using KBr as the background, over the range of 4000–400 cm−1. DSC and thermogravimetric analysis (TGA) were performed on TA Instruments Q50 and Q20 under a flowing nitrogen atmosphere. For TGA measurements, the weight loss of the dried sample was monitored under a nitrogen environment from room temperature to 800 °C at a rate of 10 °C min−1. The zeta potentials were measured at different pH values using Malvern ZS90Puls. For the magnetization measurements, a vibrating sample magnetometer (VSM, Lakeshore Model 1600) was used at room temperature.In order to determine the stability and safety of the CDP-MNPs in a water environment, the content of iron ions released from the as-prepared CDP-MNPs in aqueous solution was determined by inductively coupled plasma mass spectrometry (ICP-MS) measurements (Agilent 7500A). The CDP-MNPs were dispersed in doubly distilled water or acidic water (adjusted to pH 5 using HNO3) to produce a sample solution with a concentration of 200 mg L−1. The working solutions were shaken in a thermostatic bath shaker operated at a speed of 180 rpm at 25 °C. After separating the CDP-MNPs solids using a permanent magnet, the residual solution was determined by ICP-MS to obtain the concentration of iron ions dissolved over time.
Adsorption and desorption studies
The adsorption of bisphenol A or resorcin onto CDP-MNPs was conducted using batch equilibrium technology in a mixed solution of ethanol and doubly distilled water (v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v = 3
v = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7) at the desired concentrations and appropriate pH values. In general, 0.01 g of CDP-MNPs was dispersed thoroughly in a 50 mL solution of bisphenol A or resorcin at various concentrations (5–30 mg L−1) and shaken in a thermostatic bath shaker operated at a speed of 180 rpm at 25 °C. After equilibrium was reached, the solid sample was separated using a permanent magnet. The concentration of bisphenol A or resorcin in the residual solution was determined by means of fluorescence spectrophotometry (bisphenol A: λex/λem = 274/307 nm, resorcin: λex/λem = 272/305 nm). Calibration curves were plotted between the fluorescence intensity and the concentration of the bisphenol A or resorcin solutions (Fig. S5†). The amount of bisphenol A or resorcin adsorbed onto the CDP-MNPs was calculated by a mass balance relationship (eqn (1)).
7) at the desired concentrations and appropriate pH values. In general, 0.01 g of CDP-MNPs was dispersed thoroughly in a 50 mL solution of bisphenol A or resorcin at various concentrations (5–30 mg L−1) and shaken in a thermostatic bath shaker operated at a speed of 180 rpm at 25 °C. After equilibrium was reached, the solid sample was separated using a permanent magnet. The concentration of bisphenol A or resorcin in the residual solution was determined by means of fluorescence spectrophotometry (bisphenol A: λex/λem = 274/307 nm, resorcin: λex/λem = 272/305 nm). Calibration curves were plotted between the fluorescence intensity and the concentration of the bisphenol A or resorcin solutions (Fig. S5†). The amount of bisphenol A or resorcin adsorbed onto the CDP-MNPs was calculated by a mass balance relationship (eqn (1)).| Qe = (C0 − Ce)V/m | (1) |
For the kinetic experiments, the initial bisphenol A or resorcin concentration was 15 mg L−1 and the pH value was adjusted to 5 using HCl or NaOH. A series of samples containing CDP-MNPs were agitated for a contact time varying in the range of 0–360 min under 180 rpm at 25 °C. At various time intervals, samples were collected after magnetic separation and the concentration of bisphenol A or resorcin was estimated as mentioned previously.
Desorption studies were conducted by employing a mixture of methanol and acetic acid (v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v = 9
v = 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as a desorption eluent at 25 °C. After sufficient adsorption, the bisphenol A- or resorcin-adsorbed CDP-MNPs were separated magnetically. Desorption was examined by adding the above solid sample to a copious amount of desorption eluent and shaking for 4 h at 180 rpm. Subsequently, the solid phase CDP-MNPs were collected by magnetic decantation and washed with ethanol several times. After drying at 50 °C under vacuum for 12 h, the CDP-MNPs were utilized for readsorption. The recyclability was checked by following the above adsorption–desorption–readsorption process for three cycles.
1) as a desorption eluent at 25 °C. After sufficient adsorption, the bisphenol A- or resorcin-adsorbed CDP-MNPs were separated magnetically. Desorption was examined by adding the above solid sample to a copious amount of desorption eluent and shaking for 4 h at 180 rpm. Subsequently, the solid phase CDP-MNPs were collected by magnetic decantation and washed with ethanol several times. After drying at 50 °C under vacuum for 12 h, the CDP-MNPs were utilized for readsorption. The recyclability was checked by following the above adsorption–desorption–readsorption process for three cycles.
All of the above syntheses, experiments and adsorption operations underwent several repetitions and the results obtained showed good reproducibility.
Results and discussion
Characterization of the CDP
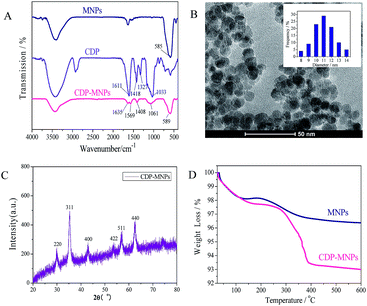
The as-prepared polymers were determined to possess the characteristic groups of the CD monomers via FTIR analysis (Fig. 1A). In the spectrum of CDP, peaks at 1418 cm−1 and 1327 cm−1 were assigned to the bending vibration of –OH. The presence of the intrinsic groups of CD suggested that the structure of the CD unit were essentially maintained in the polymers. Moreover, the strong adsorption bands at 1611 cm−1 and 1033 cm−1 were characteristic of the stretching vibrations of –COO− and the antisymmetric glycosidic vibrations of C–O–C, respectively. Thus, the carboxymethyl-β-cyclodextrin (CM-CD) polymers were considered as a copolymer of a CD unit and an EPI molecule, and the basic structural units were preserved in the polymer.Dried CDP exhibited the nature of a porous material, with a relatively uniform pore size of 4.29 nm, a BET surface area of 2.99 m2 g−1 and a pore volume of 3.22 × 10−3 cm g−1. These results were similar to the reported CD-based polymers which exhibited the nature of a mesoporous material with a relative pore size range of 2–50 nm.38,39 Moreover, the CD content of CDP was 36.5% (Text S1†), and the CDP displayed good solubility in water. Additionally, the polymers showed good thermostability, as the DSC curves show (Fig. S3†). The DSC curves gave two peaks at ca. 148 °C and 198 °C, which correspond to polymer melting and polymer decomposition, respectively.
Characterization and stability of the CDP-MNPs
In order to provide evidence for the successful assembly of the CDP onto the surface of the MNPs, the FTIR spectra of pristine CDP, MNPs and CDP-MNPs are depicted in Fig. 1A. For the sake of clarity, only the main adsorption bands are listed. For the spectrum of the CDP-MNPs, the significant bands of CDP were observed at 1635 cm−1 and 1061 cm−1 with a small shift and the representative adsorption of Fe–O bonds in MNPs is 585 cm−1 which was shifted to 589 cm−1 after surface encapsulation with CDP. Moreover, the adsorption peaks appeared at 1569 cm−1 and 1408 cm−1 due to the bands of COOM (M represents metal ions) groups, which suggested that the COOH groups of CDP reacted with the surface –OH groups of MNPs, leading to the formation of the iron carboxylate.34 Thus, it could be concluded that CDP had been grafted successfully onto the surface of Fe3O4 nanoparticles.The morphology and size distribution of the as-prepared CDP-MNPs were characterized by TEM images. From the TEM image in Fig. 1B, well-shaped spherical or ellipsoidal MNPs were observed, with average diameters around 11.0 ± 2 nm, which was similar to the uncoated MNPs prepared by the same process without added CDP (Fig. S2†). However, there was no detectable layer of CDP that could be observed on the MNPs. The results indicated that the binding process of the CDP could not enhance the size of the MNPs and result in agglomeration.
The XRD diffraction pattern of the CDP-MNPs is shown in Fig. 1C, which enables examination of the phase purity. Six characteristic peaks (2θ = 29.79, 35.34, 44.24, 53.58, 57.44, 62.66) were exhibited in the XRD pattern, which respectively correspond to the diffraction planes of (220), (311), (400), (422), (511) and (440) of iron oxide. It revealed that the prepared MNPs had a cubic spinel structure according to the standard XRD data cards of Fe3O4 crystal (JCPDS no. 85-1436).40 Moreover, the XRD pattern of the CDP-MNPs demonstrates that CDP grafting did not result in a crystalline phase change of Fe3O4. In addition, the crystal size of the CDP-MNPs could be evaluated by the following Scherrer's formula, according to the XRD pattern:
 | (2) |
The amount of CDP grafted onto the MNPs was evaluated from TGA analyses of naked MNPs and CDP-coated MNPs. As revealed in Fig. 1D, the TGA curve of the bare MNPs exhibits two significant weight losses of 1.9% and 1.6%, respectively. The first weight loss below 125 °C is ascribable to the loss of adsorbed water in the sample. The total mass loss over the full temperature range was estimated to be 3.5%, due to the loss of adsorbed water and the dehydration of the surface –OH groups. Similarly, the TGA curve of CDP-MNPs also displayed two steps of mass loss; the loss of residual water in the sample causes a weight loss of 2.3% below 200 °C. The considerable weight loss of 4.5% at 220–560 °C is contributed to by the loss and thermal decomposition of CDP, which was estimated to be 45 mg g−1, and the CD amount was 16.4 mg g−1. Additionally, as shown in the DSC curves of the CDP-MNPs in Fig. S4,† the polymer melting and polymer decomposition temperatures at ca. 153 °C and 199 °C were higher than for the CDP alone, which suggests that the thermostability of polymer was enhanced after binding with the MNPs.
Zeta potentials play a key role in determining the isoelectric point and the optimal pH where maximum adsorption can occur. Thus, the zeta potentials of the MNPs, CDP and CDP-MNPs (0.1 mg L−1 of each sample) were measured in 10−3 M NaCl aqueous solutions at different pH values, which were adjusted using NaOH or HCl. As shown in Fig. 2A, the pH value of the point of zero charge (pHPZC) for CDP was determined to be 3.4, which was lower than for the naked MNPs. This behavior is attributed to the presence of the highly negatively charged carboxylic groups (–COO−) from CM-CD used as the polymerization unit in the CDP. However, the MNPs were rich in hydroxy groups (–OH), which exhibited a higher point of zero charge (PZC) at pH 6.2. The pHPZC of CDP-MNPs was shifted to 3.9, apparently as a result of the ionization of the carboxylic acid, which further indicates that the grafting of CDP successfully coated onto the MNPs.
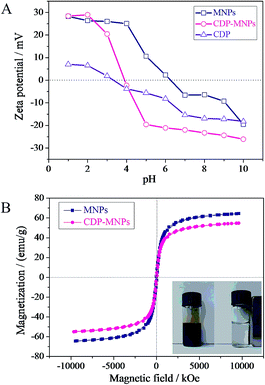 | ||
| Fig. 2 (A) Zeta potentials of CDP, MNPs and CDP-MNPs at different pH values. (B) Magnetization curves of MNPs and CDP-MNPs at room temperature. | ||
It is well known that the magnetic particle size is less than 20 nm and that the MNPs exhibit superparamagnetism.41 A magnetic hysteresis loop (Fig. 2B) was recorded using a vibrating sample magnetometer (VSM) at room temperature to evaluate the magnetic performance of the MNPs and CDP-MNPs. It could be found that the saturation magnetization value (Ms) of the CDP-MNPs was 54 emu g−1, slightly lower than that of bare MNPs (67 emu g−1), which can be attributed to the cladding of the non-magnetic materials (CDP layer) on the surface of the MNPs. Superparamagnetism means that when the external magnetic field was withdrawn, there was no residual magnetism of the MNPs. The reversible attraction of the CDP-MNPs towards magnets was also shown in the Fig. 2B inset. The CDP-MNPs in aqueous solution were homogeneous dark brown without a magnet. However, the CDP-MNPs started to accumulate towards the wall of the sample vial when placed in an external magnetic field. Finally, the CDP-MNPs were completely attracted towards the side of the sample vial close to magnets. Despite the slight decrease in the saturation magnetization value, the CDP-MNPs presented the features of no remanence, no coercivity and a superparamagnetic nature, which were beneficial to their dispersibility and magnetic separation in solution.
The content of the iron ions released from the CDP-MNPs in aqueous solution was determined by ICP-MS measurements, with the purpose of demonstrating the stability and safety of the CDP-MNPs in a water environment. As shown in Table S3,† the concentrations of dissolved iron ions in aqueous solution were all less than 31 μg L−1, even after a long storage time. These values were far below the maximum level of iron ions for drinking water quality (300 μg L−1) adopted by P. R. China (GB 5749-2006). This result indicated that only small quantities of iron ions were released from the magnetic nanocomposite; in other words, the CDP-MNPs were stable and friendly to the environment. The favorable stability of the CDP-MNPs also confirmed that the CD polymer covered the surface of the MNPs well, which effectively prevented the release iron ions.
Adsorption of bisphenol A or resorcin onto the CDP-MNPs
The adsorption capacity of the CDP-MNPs may be attributed to the strong hydrogen bond between the hydroxyl groups in the phenolic compound with the hydroxyl and carboxyl group of the CDP, the hydrophobic interaction of the aromatic ring with the void of the polymer network, and the inclusion behavior of the CD's cavity for bisphenol A and resorcin by host–guest interaction. The pKa1 of bisphenol A and resorcin is found to be 9.3 and 9.6, respectively;42 both of them exist in molecular form at pH 3.0–9.0. The variation in the uptake capacity with pH can be explained by the PZC of CDP-MNPs (pHPZC = 3.9). At pH values further from the PZC, more negatively charged carboxylate ions (COO−) remain in an ionic state, resulting in the partial breakage of hydrogen bonds and hence a decline in the adsorption capacity. Based on these results, a pH of 5.0 was chosen in subsequent experiments.
Adsorption kinetics
Fig. 4 illustrates the adsorption of bisphenol A or resorcin onto the CDP-MNPs as a function of contact time. It can be noted that the adsorption quantity increases with contact time and the process can be divided into three steps. In the first 50 min, both bisphenol A and resorcin exhibit a fast adsorption rate, which is mainly due to a large number of bisphenol A or resorcin molecules diffusing from solution to the surface of the CDP-MNPs by a high mass-transfer driving force. For bisphenol A, the adsorption rate gradually decreases from 50 min to 250 min and reaches an invariant rate after 250 min. In addition, the adsorption rate of resorcin gradually slows down from 50 to 175 min, owing to the increase in the internal diffusion resistance, and slightly changes after 175 min. These results suggest that the adsorption equilibrium was reached within 250 min and 175 min for bisphenol A and resorcin, respectively.With the intention of evaluating the adsorption effectiveness of the CDP-MNPs and to explore the adsorption mechanism, multiform kinetic studies were performed by Lagergren's pseudo-first-order kinetic model (eqn (3)), Ho's pseudo-second-order model (eqn (4)) and Weber's intra particle diffusion model (eqn (5)).
ln(Qe − Qt) = ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Qe − k1t Qe − k1t
| (3) |
 | (4) |
 | (5) |
The kinetic parameters of Lagergren's pseudo-first-order and Ho's pseudo-second-order equations were calculated by the slope and intercept of the linear fitting plots of ln(Qe − Qt) versus t (Fig. S6A†) and t/Qt versus t (Fig. S6B†), respectively. As summarized in Table 1, the Qe value of resorcin was larger than that of bisphenol A, which further indicated the excellent adsorptivity of the CDP-MNPs towards resorcin. The adsorption isotherm data of resorcin and bisphenol A on this adsorbent were better fitted to Ho's pseudo second-order model (R2 > 0.99), compared to Lagergren's pseudo-first-order model. Furthermore, the calculated Qe values based on Ho's pseudo-second-order model were much closer to the experimental data Qe. All the results demonstrated that the adsorption process of bisphenol A and resorcin agreed well with the Ho's pseudo-second-order kinetic model. Namely, chemisorption was involved in the whole process43 and the driving force might be due to the strong hydrogen bonds between the hydroxyl groups in the phenolic compound and CDP.
| Adsorbate | Qe,exp | Lagergren's pseudo-first-order kinetic | Ho's pseudo-second-order model | ||||
|---|---|---|---|---|---|---|---|
| k1 × 102 | Qe | R2 | k2 × 104 | Qe | R2 | ||
| Bisphenol A | 59.698 | 1.403 | 47.560 | 0.9854 | 4.091 | 60.789 | 0.9983 |
| Resorcin | 83.299 | 2.642 | 82.237 | 0.9826 | 4.202 | 83.957 | 0.9969 |
Weber's intraparticle diffusion model was used to identify the controlling mass-transfer steps in the bisphenol A or resorcin decontamination by CDP-MNPs. According to the plots of Qt versus t1/2 (Fig. S7†), piecewise linear regression of the data expressed that intra-particle diffusion was working along with other adsorption mechanisms to control the decontamination rate,44 which corresponded to the inference of Ho's pseudo-second-order model.
Adsorption isotherms
Isotherm studies can perceive the interaction between adsorbates and adsorbents and explore the sorption capacity of adsorbents, which are the crucial parameters for evaluating an expected adsorption system. Two famous adsorption isotherm models, the Langmuir (eqn (6)) and Freundlich models (eqn (7)), were used to fit the experimental data for bisphenol A and resorcin adsorbed onto CDP-MNPs at room temperature. As presented in Fig. 5, it could be observed that the adsorption capacity increased with the increase in the concentration of adsorbates and reached saturation as the bisphenol A and resorcin concentration exceeded 8.598 mg L−1 and 7.307 mg L−1, respectively. In accordance with the linear fitting plots of 1/Qe versus 1/Ce (Fig. S8A and S9A†) and ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Qe versus ln
Qe versus ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ce (Fig. S8B and S9B†), the comparison of the Langmuir and Freundlich isotherm parameters is listed in Table 2. The better linearity of the Langmuir plot suggested that the adsorption processes of bisphenol A and resorcin by CDP-MNPs were regarded as monolayer adsorption,45 which means that all adsorption sites located upon the surface of absorbents were distributed homogeneously and the adsorption force is equivalent. Additionally, it could be evaluated from the Langmuir equation that the Qm for resorcin and bisphenol A were 114.91 mg g−1 and 74.63 mg g−1, respectively.
Ce (Fig. S8B and S9B†), the comparison of the Langmuir and Freundlich isotherm parameters is listed in Table 2. The better linearity of the Langmuir plot suggested that the adsorption processes of bisphenol A and resorcin by CDP-MNPs were regarded as monolayer adsorption,45 which means that all adsorption sites located upon the surface of absorbents were distributed homogeneously and the adsorption force is equivalent. Additionally, it could be evaluated from the Langmuir equation that the Qm for resorcin and bisphenol A were 114.91 mg g−1 and 74.63 mg g−1, respectively.
 | (6) |
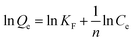 | (7) |
 | ||
| Fig. 5 Equilibrium isotherms for bisphenol A and resorcin adsorption by CDP-MNPs at pH 5.0 and 25 °C. | ||
| Adsorbate | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| KL/(L mg)−1 | Qm (mg g−1) | R2 | KF/(L mg)−1 | n | R2 | |
| Bisphenol A | 0.8156 | 74.63 | 0.9584 | 38.4362 | 4.1334 | 0.8058 |
| Resorcin | 0.3085 | 114.91 | 0.9865 | 32.6616 | 2.2482 | 0.9027 |
It is also important to compare the maximum adsorption capacity values obtained by the CDP-MNPs with the values given by other previous magnetic adsorbents, since it will suggest the effectiveness of the CDP-MNPs as a potential adsorbent for phenolic pollutant treatment. As shown in Table 3, the adsorption capacities for bisphenol A using the CDP-MNPs are comparable to those obtained when using other magnetic adsorbents. It is notable that most of the reported magnetic adsorbents have a general adsorption capacity lower than 45 mg g−1, and the CDP-MNPs ranks among the high adsorption capacity magnetisable adsorbents.
| Adsorbent | Phenolic pollutants | Qmax (mg g−1) | Reference |
|---|---|---|---|
| CDP-MNPs | Bisphenol A | 74.6 | This work |
| Molecular imprinted polymer (MIP)-MNPs | Bisphenol A | 16.3 | 24 |
| CD-poly(glycidyl methacrylate)-SiO2-MNPs | Bisphenol A | 22.8 | 26 |
| CD-poly(glycidyl methacrylate)-MNPs | Bisphenol A | 0.15 | 36 |
| POSS-PCL-β-CD/Fe3O4 micelles | Bisphenol A | 28.4 | 35 |
| MIP-magnetic carbon nanotube | Bisphenol A | 11.2 | 46 |
| Magnetic carbon nanotube | Bisphenol A | 45.3 | 47 |
| Chitosan/fly-ash-cenospheres/γ-Fe2O3 | Bisphenol A | 31.9 | 48 |
Comparing with other magnetic absorbents, the superior adsorption capacity of the CDP-MNPs for the phenolic pollutants may be due to two possible reasons. The prime reason is that the surface of the MNPs is functionalized by a cross-linked CD-polymer. For a cross-linked CD-polymer, three main adsorption mechanisms were proposed which involve (1) host–guest interactions in the CD cavities, (2) interactions in the pores of the polymeric network and (3) interactions on the surface (physical sorption).49 In general, physical sorption plays a small role in the interaction between the magnetic nano-adsorbent and the pollutant molecules and so it can be ignored. Therefore, we determined the binding constants (Kc) of the phenolic pollutants with CM-CD (Table S4 and Fig. S10†), and the large Kc indicates that the phenolic compounds could be included into the CD's cavity to form a host–guest complex. Thus, comparing with the magnetic adsorbents not modified by CD, the most important interaction is due to the inclusion ability. Moreover, secondary cavities could be formed in the polymer network by the cross-linking agent and the guest molecules could insert into them. This phenomenon further enhanced the adsorption capacity of the CDP-MNPs. The second reason is that the CDP-MNPs with a small diameter provide a higher specific surface area than other CD modified magnetic adsorbents reported in previous work. The high specific surface area leads to almost all adsorption active sites being available, which simultaneously enhanced the adsorption capacity of the CDP-MNPs.
Desorption and recyclability
From the viewpoints of practical applications, recyclability is a crucial factor for advanced adsorbents. To evaluate the reusability of the CDP-MNPs, the process of adsorption–desorption–readsorption was conducted for three cycles at 25 °C by introducing a mixture of methanol and acetic acid (v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v = 9
v = 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as desorption eluent. The adsorption efficiency in each cycle was analyzed and is exhibited in Fig. 6. Noticeably, the adsorption capacity of the CDP-MNPs declined with the increment of cycle numbers, which was probably due to the reduction of the adsorption sites caused by the agglomeration of adsorbents. After three cycles, the maximum adsorption amounts of bisphenol A and resorcin still maintained the adsorption capacity of 41% and 52%, respectively, indicating that CDP-MNPs could be reutilized to a certain degree.
1) as desorption eluent. The adsorption efficiency in each cycle was analyzed and is exhibited in Fig. 6. Noticeably, the adsorption capacity of the CDP-MNPs declined with the increment of cycle numbers, which was probably due to the reduction of the adsorption sites caused by the agglomeration of adsorbents. After three cycles, the maximum adsorption amounts of bisphenol A and resorcin still maintained the adsorption capacity of 41% and 52%, respectively, indicating that CDP-MNPs could be reutilized to a certain degree.
Conclusions
CDP-MNPs were readily prepared with a mean size around 11.0 ± 2 nm, and the coating amount of CDP was 45 mg g−1 as determined by thermo gravimetric analysis. The CDP-coated MNPs possess the fascinating features of superparamagnetism and adsorption properties, which are favorable for the purpose of removing bisphenol A and resorcin through the inclusion feature of CD and the hydrogen bonding of the polymer network. The maximum adsorption capacity for bisphenol A could reach values up to 74.63 mg g−1 at 25 °C, which is higher than that of previously reported magnetic adsorbents. Additionally, the maximum uptake for resorcin was up to 114.91 mg g−1 under the same conditions. Moreover, the CDP-MNPs could be regenerated by a mixture of methanol and acetic acid (v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v = 9
v = 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The remarkable adsorption capacity and reusability indicate that the CD-polymer functionalised Fe3O4 nanoparticles could be used as a promising adsorbent for the elimination of phenolic pollutants from wastewater by magnetic separation technology.
1). The remarkable adsorption capacity and reusability indicate that the CD-polymer functionalised Fe3O4 nanoparticles could be used as a promising adsorbent for the elimination of phenolic pollutants from wastewater by magnetic separation technology.
Acknowledgements
This work was supported by National Natural Science Foundation of China (No. 21575084, 21475080 and 21402110), Shanxi Scholarship Council of China, Scientific and Technological Innovation Programs of Higher Education Institutions in Shanxi (No. 2014108), Fund Program for the Scientific Activities of Selected Returned Overseas Professionals in Shanxi Province (No. 20551025) and Shanxi Provincial Hundreds of Scholars Program.References
- X. Zhu, S. Shi, J. Wei, F. Lv, H. Zhao, J. Kong, Q. He and J. Ni, Environ. Sci. Technol., 2007, 41, 6541–6546 CrossRef CAS PubMed.
- P. Bhowmick, D. Banerjee, S. Santra, D. Sen, B. Das and K. K. Chattopadhyay, RSC Adv., 2016, 6, 8913–8922 RSC.
- L. Yao, L. Z. Zhang, R. Wang, C. H. Lu and Z. L. Dong, Sep. Purif. Technol., 2013, 118, 162–169 CrossRef CAS.
- A. Babuponnusami and K. Muthukumar, Chem. Eng. J., 2012, 183, 1–9 CrossRef CAS.
- J. Kim and J. W. Kim, Environ. Sci. Technol., 2014, 48, 13384–13391 CrossRef CAS PubMed.
- C. Tubtimdee and A. Shotipruk, Sep. Purif. Technol., 2011, 77, 339–346 CrossRef CAS.
- X. Y. Cai, Q. Q. Liu, C. L. Xia, D. Shan, J. Du and J. W. Chen, Environ. Sci. Technol., 2015, 49, 9264–9272 CrossRef CAS PubMed.
- L. R. Rad, I. Haririan and F. Divsar, Spectrochim. Acta, Part A, 2015, 136, 423–428 CrossRef CAS PubMed.
- A. K. Hegazy, N. T. Abdel-Ghani and G. A. El-Chaghaby, Appl. Water Sci., 2013, 4, 273–281 CrossRef.
- N. Tsunoji, T. Ikeda, M. Sadakane and T. Sano, J. Mater. Chem. A, 2014, 2, 3372–3380 CAS.
- J. Pan, H. Yao, W. Guan, H. Ou, P. Huo, X. Wang, X. Zou and C. Li, Chem. Eng. J., 2011, 172, 847–855 CrossRef CAS.
- P. Bhowmick, D. Banerjee, S. Santra, D. Sen, B. Dasa and K. K. Chattopadhyay, RSC Adv., 2016, 6, 8913–8922 RSC.
- Y. R. Zhang, P. Su, J. Huang, Q. R. Wang and B. X. Zhao, Chem. Eng. J., 2015, 262, 313–318 CrossRef CAS.
- A. H. Lu, E. L. Salabas and F. Schuth, Angew. Chem., 2007, 46, 1222–1244 CrossRef CAS PubMed.
- R. Chalasani and S. Vasudevan, J. Mater. Chem., 2012, 22, 14925–14931 RSC.
- C. Cui, M. He, B. Chen and B. Hu, Anal. Methods, 2014, 6, 8577–8583 RSC.
- M. M. Lakouraj, F. Mojerlou and E. N. Zare, Carbohydr. Polym., 2014, 106, 34–41 CrossRef CAS PubMed.
- L. Wu, Y. Ye, F. Liu, C. Tan, H. Liu, S. Wang, J. Wang, W. Yi and W. Wu, Appl. Clay Sci., 2013, 83–84, 405–414 CrossRef CAS.
- D. H. Reddy and S. M. Lee, Adv. Colloid Interface Sci., 2013, 201–202, 68–93 CrossRef CAS PubMed.
- Y. H. Deng, D. W. Qi, C. H. Deng, X. M. Zhang and D. Y. Zhao, J. Am. Chem. Soc., 2008, 130, 28–29 CrossRef CAS PubMed.
- Y. Zhou, L. Sun, H. Wang, W. Liang, J. Yang, L. Wang and S. Shuang, Mater. Chem. Phys., 2016, 170, 83–89 CrossRef CAS.
- Q. Dai and A. Nelson, Chem. Soc. Rev., 2010, 39, 4057–4066 RSC.
- F. H. Chen, L. M. Zhang, Q. T. Chen, Y. Zhang and Z. J. Zhang, Chem. Commun., 2010, 46, 8633–8635 RSC.
- J. Liu, W. Wang, Y. Xie, Y. Huang, Y. Liu, X. Liu, R. Zhao, G. Liu and Y. Chen, J. Mater. Chem., 2011, 21, 9232–9238 RSC.
- S. Banerjee and D. H. Chen, J. Hazard. Mater., 2007, 147, 792–799 CrossRef CAS PubMed.
- N. Wang, L. Zhou, J. Guo, Q. Ye, J. Lin and J. Yuan, Appl. Surf. Sci., 2014, 316, 267–273 CrossRef.
- S. H. Huang and D. H. Chen, J. Hazard. Mater., 2009, 163, 174–179 CrossRef CAS PubMed.
- Q. Li, H. Yu, J. Song, X. Pan, J. Liu, Y. Wang and L. Tang, Appl. Surf. Sci., 2014, 316, 435–442 CrossRef.
- H. H. Liu, X. Cai, W. Yu and J. W. Chen, Water Res., 2011, 45, 3499–3511 CrossRef CAS PubMed.
- N. Morin-Crini and G. Crini, Prog. Polym. Sci., 2013, 38, 344–368 CrossRef CAS.
- E. Morillo, M. A. Sanchez-Trujillo, J. R. Moyano, J. Villaverde, M. E. Gomez-Pantoja and J. I. Perez-Martinez, PLoS One, 2012, 7, 44137 Search PubMed.
- J. Sun, H. He and S. Liu, J. Sep. Sci., 2014, 37, 1679–1686 CrossRef CAS PubMed.
- X. Xiong, X. Zhao and Z. Song, Anal. Biochem., 2014, 460, 54–60 CrossRef CAS PubMed.
- A. Z. Badruddoza, Z. Shawon, W. Tay, K. Hidajat and M. S. Uddin, Carbohydr. Polym., 2013, 91, 322–332 CrossRef CAS PubMed.
- W. Yuan, J. Shen, L. Li, X. Liu and H. Zou, Carbohydr. Polym., 2014, 113, 353–361 CrossRef CAS PubMed.
- Y. Kang, L. L. Zhou, X. Li and J. Y. Yuan, J. Mater. Chem., 2011, 21, 3704–3710 RSC.
- H. Yang, Y. Zhu, D. Chen, C. Li, S. Chen and Z. Ge, Biosens. Bioelectron., 2010, 26, 295–298 CrossRef CAS PubMed.
- R. Huq, L. Mercier and P. J. Kooyman, Chem. Mater., 2001, 13, 4512–4519 CrossRef CAS.
- C. Q. Liu, J. B. Lambert and L. Fu, J. Am. Chem. Soc., 2003, 125, 6452–6461 CrossRef CAS PubMed.
- Y. Ding, S. Z. Shen, H. Sun, K. Sun, F. Liu, Y. Qi and J. Yan, Mater. Sci. Eng., C, 2015, 48, 487–498 CrossRef CAS PubMed.
- D. Caruntu, G. Caruntu, Y. Chen, C. J. O'Connor, G. Goloverda and V. L. Kolesnichenko, Chem. Mater., 2004, 16, 5527–5534 CrossRef CAS.
- C. A. Staples, P. B. Dom, G. M. Klecka, S. T. O'Blook and L. R. Harris, Chemosphere, 1998, 36, 2149–2173 CrossRef CAS PubMed.
- G. M. Y. S. Ho, Process Biochem., 1999, 34, 451–465 CrossRef.
- H. Wang, Y. G. Liu, G. M. Zeng, X. J. Hu, X. Hu, T. T. Li, H. Y. Li, Y. Q. Wang and L. H. Jiang, Carbohydr. Polym., 2014, 113, 166–173 CrossRef CAS PubMed.
- J. A. García-Calzón and M. E. Díaz-García, Sens. Actuators, B, 2007, 123, 1180–1194 CrossRef.
- Z. Zhang, X. Chen, W. Rao, H. Chen and R. Cai, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2014, 965, 190–196 CrossRef CAS PubMed.
- S. Li, Y. Gong, Y. Yang, C. He, L. Hu, L. Zhu, L. Sun and D. Shu, Chem. Eng. J., 2015, 260, 231–239 CrossRef CAS.
- J. Pana, H. Yao, X. Li, B. Wang, P. Huo, W. Xu, H. Ou and Y. Yan, J. Hazard. Mater., 2011, 190, 276–284 CrossRef PubMed.
- N. Morin-Crini and G. Crini, Prog. Polym. Sci., 2013, 38, 344–368 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: CD content analyses, TEM image, differential scanning calorimetry, linear fitting plots of Lagergren's pseudo-first-order and Ho's pseudo-second-order equations and linear fitting plots of Langmuir and Freundlich adsorption isotherm models for bisphenol A and resorcin. See DOI: 10.1039/c6ra16383a |
| This journal is © The Royal Society of Chemistry 2016 |