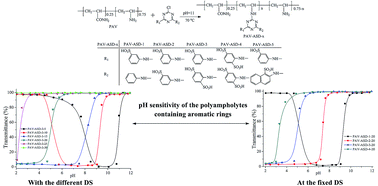
This work describes the synthesis and pH sensitivity of polyampholytes containing aromatic rings which were prepared from a poly(acrylamide-co-vinylamine) oligomer and aryl sulfonate derivatives. The structures of the products were characterized by Fourier transform infrared spectroscopy, liquid chromatography-mass spectrometry and nuclear magnetic resonance spectroscopy analysis. The effects of degree of substitution, concentration of the polyampholyte solution, the amount of sulfonate groups in the aryl sulfonate derivatives, and the aromatic ring on the pH sensitivity of the polyampholytes were discussed in detail. The results exhibited that the pH sensitivity of these polyampholytes was mainly affected by the molar ratio of cationic to anionic groups in the polyampholytes which can be controlled by changing both the degree of substitution and the amount of sulfonate groups in the aryl sulfonate derivatives at the fixed degree of substitution.