Biosynthesized Vitis vinifera seed gold nanoparticles induce apoptotic cell death in A431 skin cancer cells
J. Grace Nirmalaa,
S. Akilab,
M. S. A. Muthukumar Nadara,
R. T. Narendhirakannan*a and
Suvro Chatterjeeb
aDepartment of Biotechnology, School of Biotechnology and Health Sciences, Karunya University, Karunya Institute of Technology and Sciences, Karunya Nagar, Coimbatore – 641 114, Tamil Nadu, India. E-mail: bionaren_phd@yahoo.co.in; Fax: +91 422 2615615; Tel: +91 422 2614300
bLife Sciences Division, AU-KBC Research Centre, Madras Institute of Technology, Anna University, Chromepet, Chennai (Madras) – 600 044, India
First published on 15th August 2016
Abstract
Biologically synthesized gold nanoparticles provide many useful characteristics and can be used as carriers to deliver drugs because they are non-toxic and inert. In the present study, phytochemicals present in Vitis vinifera seeds were utilized as reducing agents for the synthesis of gold nanoparticles. The morphology, particle size and properties of V. vinifera gold nanoparticles (AuNPs) were characterized using transmission electron microscopy, X-ray diffraction, dynamic light scattering, Fourier transform infrared spectroscopy and zeta potential. The nanoparticles were then incubated for 24 h with human epidermoid skin cancer cells (A431) and were evaluated for their antiproliferative activities and induction of apoptosis. Spherical gold nanoparticles of ∼50 ± 5 nm were synthesized at room temperature within ten min and were capped with phytochemicals from V. vinifera seeds. At the inhibitory concentration (IC50) of V. vinifera seed AuNPs (24.2 μM), they imparted cytotoxic effects, increased the level of reactive oxygen species, and induced apoptosis and apoptotic morphological changes in A431 cells significantly (p < 0.01); these effects are associated with interference in mitochondrial membrane potential. This reduction in mitochondrial membrane potential probably initiated the apoptotic cascade in the nanoparticle-treated cells. Thus, gold nanoparticles synthesized utilizing Vitis vinifera seed phytochemicals may selectively target cancer cells, and the phytochemicals that are occluded within the nanoparticles may serve as anticancer agents, providing greater efficacy in killing cancer cells.
1. Introduction
Skin carcinogenesis represents a growing major public health problem, and almost one-third of cancer among all new cancers diagnosed in the world annually originates in the skin.1 Oxidative stress can lead to DNA damage in the skin; uncontrolled release of reactive oxygen species can cause skin cancer and is involved in the pathogenesis of a number of human skin disorders.2,3 Enhanced therapeutic efficacy with minimal side effects is the primary goal of cancer treatment.4 Chemoprevention with distinct molecular mechanisms of dietary agents has received much interest as a means to achieve potency with reduced toxicity and higher efficacy.5 Defects of the apoptosis pathway are associated with the development of cancer; therefore, anticancer drugs are intended to specifically target signaling molecules of survival pathways and cell death.6,7Nanoparticles are usually several hundred nanometers in size and about 100 to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 times smaller than human cells.8 Metal nanoparticles, a principally new class of compounds, possess substantial biological activity and are used for the treatment of diseases, especially cancer.9 Delivery of the drug to cancer cells is achieved using nanoparticles as the delivery vehicle,10–12 and gold nanoparticles seem to have strong potential as drug delivery carriers. Gold nanoparticles exhibit physicochemical properties and have been utilized in many applications, such as diagnosing, imaging and therapeutic purposes. Specific control of size and shape, ease of synthesis and surface chemistry provide multi-functionality to gold nanoparticles and facilitate attachment of a diverse class of drugs and compounds to the surface.13–15
000 times smaller than human cells.8 Metal nanoparticles, a principally new class of compounds, possess substantial biological activity and are used for the treatment of diseases, especially cancer.9 Delivery of the drug to cancer cells is achieved using nanoparticles as the delivery vehicle,10–12 and gold nanoparticles seem to have strong potential as drug delivery carriers. Gold nanoparticles exhibit physicochemical properties and have been utilized in many applications, such as diagnosing, imaging and therapeutic purposes. Specific control of size and shape, ease of synthesis and surface chemistry provide multi-functionality to gold nanoparticles and facilitate attachment of a diverse class of drugs and compounds to the surface.13–15
Chithrani et al.16 demonstrated that spherical shaped gold nanoparticles had superior internalization compared to other nanoparticles and exhibited increased uptake over rod-shaped nanoparticles in the cells. Previous studies reported that biologically synthesized gold nanoparticles can be used as carriers in drug-delivery systems and that the functionalized gold nanoparticle core is essentially nontoxic and inert.17,18 Previous studies also showed that nanoparticles encapsulated with other biological agents have potential for cancer control.19
More than 60% of anticancer agents currently used are derived from natural sources.20 Phytochemicals exhibit anticancer activity by induction of apoptosis and antiproliferative activity in cancer cells.21 Vitis vinifera L., commonly known as grapes, are one of the most widely consumed fruits in the world; they possess antioxidant-rich phytochemicals, and ∼60 to 70% of grape polyphenols exist in the seeds.22 Grape seeds contain gallic acid, epicatechin, (+)-catechin, proanthocyanidins, dimeric procyanidin and flavan-3-ol.23,24 Grape seed extracts exhibit cytotoxicity towards breast,25 lung,26 skin,27 colon28 and prostate cancers29 while enhancing the viability and growth of normal cells.30 Various research studies have demonstrated the anti-proliferative, apoptotic and anticancer activities of grape seed extracts on A431 cell lines.27,31 The cytotoxicity of grape seed extracts against A431 cell lines was studied, and the IC50 concentration was found to be 480 μg mL−1.31
The phytochemicals present in Vitis vinifera seeds can be utilized as reducing agents for the reduction of gold metal ions to gold nanoparticles. These polyphenols are potent antioxidants and function as capping agents on the gold nanoparticles.32 The present study aimed to evaluate the anti-carcinogenic and antiproliferative effects of gold nanoparticles synthesized biologically using Vitis vinifera seed extracts. This is the first study to determine the cytotoxic and anticancer activity of Vitis vinifera seed gold nanoparticles. A431 human epidermoid carcinoma cells (skin cancer) were used as an in vitro model to study the anti-carcinogenic effects and induction of apoptosis. The nanoparticles were also tested on normal human epidermal keratinocytes (HaCaT) to check cell viability. We further presumed that the catechins, phenolic acids, flavonoids and proanthocyanidins along with a host of polyphenols of the grape seeds might act as capping agents on the gold nanoparticles, thus paving a novel path for the synthesis of stable gold nanoparticles.
2. Materials and methods
Hydrogen tetrachloroaurate(III) trihydrate [HAuCl4·3H2O (99.9%)] used in the experiments was purchased from Sigma-Aldrich Chemicals, Bangalore, India. Freshly prepared triple distilled water was used throughout the experimental studies. Phosphate buffered saline (PBS), Dulbecco's Modified Eagle's Medium (DMEM), Fetal Bovine Serum (FBS), trypsin–EDTA (0.25%, 0.02%) solution, penicillin and streptomycin were purchased from Gibco, Life Technologies, India. MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) and DMSO (dimethyl sulfoxide) were obtained from Himedia, India. The Annexin V-FITC Apoptosis Detection kit was purchased from Sigma, USA. Rhodamine 123, 2′,7′-dichlorodihydro fluorescein diacetate (DCFH-DA), ethidium bromide and acridine orange were purchased from Sigma-Aldrich, India. All other solvents and chemicals were of analytical grade.2.1 Preparation of Vitis vinifera seed extract
The seeds of Vitis vinifera were removed from the pulp manually, separated and washed with double distilled water. The seeds were then shade dried, pulverized in a mechanical grinder and passed through a 40-mesh sieve. Then, 2 g of the powdered material was extracted with 100 mL of double distilled water in a 250 mL Erlenmeyer flask and stirred using a magnetic stirrer for 10 minutes. The mixture was then boiled for 5 min at 60 °C. The extract obtained was passed through a Whatman filter and then stored at 4 °C for further procedures.2.2 Synthesis of gold nanoparticles
Gold nanoparticles were synthesized using Vitis vinifera seed extracts with a slight modification to the method of Newman and Maccrehan.33 To synthesize the gold nanoparticles, 10 mL of the Vitis vinifera seed aqueous extracts were added to 90 mL of 1 mM hydrogen tetrachloroaurate(III) trihydrate solution, and the mixture was incubated in the dark. The solution was maintained for another 1 h to complete the reaction. Accordingly, the formed gold nanoparticles were separated by centrifugation at 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 20 min and the collected pellet was then resuspended in double distilled deionized water for further investigations.
000 rpm for 20 min and the collected pellet was then resuspended in double distilled deionized water for further investigations.
2.3 Characterization of synthesized gold nanoparticles
UV-visible spectra were recorded on a Perkin-Elmer Lambda-45 spectrophotometer, USA, with compartments provided with double beams to fit both the reference and testing solutions, using 1 cm path length quartz cuvettes. The formation and growth of the gold nanoparticles synthesized using Vitis vinifera seed extracts were examined using UV-visible spectroscopy, and the absorption spectra were recorded in the range of 450 to 600 nm. The surface plasmon resonance (SPR) band at ∼540 nm confirms the formation of gold nanoparticles.34 The morphology and size of the nanoparticles were elucidated using a JEOL 1400 Transmission Electron Microscope (TEM) (JEOL Ltd, Tokyo, Japan). The samples for TEM were prepared by placing 10 μL of gold nanoparticle solution on a carbon coated mesh copper grid and allowing it to stand for ten minutes; the remaining solution was carefully wiped off and the grid was allowed to dry for another 15 min.35 Energy dispersive X-ray spectroscopy (EDX) analysis (INCA, Oxford Instruments, UK) was performed to confirm the presence of elemental gold and other elements of the gold nanoparticles.The crystallinity and chemical composition of the AuNPs were studied with the help of X-ray diffraction (XRD, Shimadzu 6000, Japan) operating at 40 kV, and a current of 30 mA with Cu Kα radiation (λ = 1.54 Å) was used. The particle size was also calculated using the obtained 2θ and d values using the formula
| d = 0.9 × λ/Δ × cos(θ) |
Graphs of the particle size distribution and the average particle size were obtained using a particle size analyzer (Zetasizer Nano-ZS90, Malvern Instruments Ltd, Worcestershire, UK) by measuring about 250 particles.36 The surface charge and stability of the synthesized gold nanoparticles were analysed using a zeta potential analyser (Zetasizer Nano-ZS90, Malvern Instruments Ltd, Worcestershire, UK). Fourier transform infrared spectroscopic analysis (FTIR) was performed for both the grape seed powdered sample and the lyophilized synthesized AuNPs. FTIR spectral analyses were measured by a Spectrum RX1 instrument (Perkin Elmer, USA) functioning in transmission mode (400 to 4000 cm−1) at a resolution of 4 cm−1.37,38
2.4 Cell culture
The A431 cancer cell line (skin carcinoma, human) and HaCaT cell line (normal, human immortalized keratinocyte cells) were obtained from the National Centre for Cell Science (NCCS), Pune, India. The A431 skin cancer and HaCaT cell lines were maintained as monolayers in tissue culture Petri dishes in DMEM medium supplemented with 10% heat-inactivated fetal bovine serum (FBS), 100 IU mL−1 penicillin, 100 μg mL−1 streptomycin and 2 mM L-glutamine. The cultured cells were passaged twice each week, seeding at a density of about 2 × 103 cells per mL, and the medium was replaced every 2 days. The cultures were maintained in a humidified atmosphere with 5% CO2 at 37 °C. Cell viability was determined by the trypan blue dye exclusion method. In all experiments, 70% to 85% confluent cultures were used.2.5 Cell cytotoxicity assay
The cell cytotoxicity was measured by MTT (3-(4,5-dimethylthiazol-2-yl)-diphenyl tetrazolium bromide) assays.39 A431 cells and HaCaT cells (5 × 103 cells per well) were plated in 96-well plates with 100 μL of medium and incubated for 24 h. After 24 h, the cells reached confluency and were incubated in the presence or absence of a series of increasing concentrations of Vitis vinifera seed biosynthesized AuNPs dissolved in 0.1% DMSO for 24 h at 37 °C to a final volume of 100 μL per well. 5-Fluorouracil was used a standard drug at increasing concentrations to test the cytotoxicity on the A431 cells. At the end of the treatment, 20 μL of MTT (5 mg mL−1) dissolved in PBS was added to each well by diluting with the medium and was incubated with the plates wrapped in aluminium foil for 4 h at 37 °C. The purple formazan crystals formed by the live cells were dissolved in 100 μL of dimethyl sulfoxide (DMSO, Sigma), replaced by media, and the optical density was measured at 570 nm on a microplate reader (BIO-RAD microplate reader-550, Japan). The inhibitory concentration (IC50), i.e. the drug concentration inhibiting 50% of cell growth, was elucidated from the graph.2.6 Morphological changes in A431 cells
A431 cells were grown in 35 mm sterile cell culture Petri dishes at 37 °C in a CO2 incubator and were exposed to Vitis vinifera seed gold nanoparticles and fluorouracil at their IC50 concentrations. The morphologies of the A431 cells were visualized using an inverted phase contrast microscope (Nikon TS100F, Japan). Untreated cells were retained as a control, and the morphological changes were visualized by the extent of cell roundedness.402.7 Assessment of reactive oxygen species by DCFH-DA
Cells were incubated with grape seed AuNPs at the IC50 concentration for 24 h and with H2O2 for 30 min to induce free radical damage to the cells with or without N-acetyl cysteine (NAC). NAC was used as an antioxidant at a concentration of 100 μM mL−1 and was also tested along with the test compounds. PBS was used as a negative control, and H2O2 was used as a positive control. Fluorouracil was used as a standard drug at the IC50 concentration of 23.43 μM with or without NAC. A determined amount of cell suspension (150 μL) was grown in a 24-well plate for 24 h. The test compounds (10 μL) were added to the 24-well plate after the growth of the cells and the plate was incubated for 24 h. H2O2 (20 μL) was used to induce free radical damage in the A431 cells; then, after incubation, DCFH-DA (10 μL, 5 mM) was added to all the wells. The intensity of the fluorescence (DCF) was measured using an inverted fluorescent microscope (Olympus IX71, USA).41 The images were analyzed using ImageJ software, National Institutes of Health, USA.2.8 Annexin V-FITC apoptosis assay (TUNEL)
The percentage of apoptotic cells was determined using an Annexin V-FITC apoptosis detection kit (Sigma-Aldrich, USA) according to the manufacturer's instructions. An equal amount of cells per well were grown overnight in a 24-well culture plate for 24 h and then treated with IC50 concentrations of Vitis vinifera seed AuNPs; the cells were then grown for another 24 h. Staurosporine was used as a positive control.42 Then, the cells were viewed under a fluorescence microscope (Olympus IX71, USA). Images were analyzed using ImageJ software, National Institutes of Health, USA.2.9 Mitochondrial membrane potential by rhodamine 123
Exponentially grown A431 cells (2 × 105 cells per mL per well) seeded in 24 well plates for 24 h were treated with Vitis vinifera seed AuNPs at the IC50 concentration for 24 h. Rhodamine 123 (10 μg mL−1) was added to the cells in cell medium and incubated for 30 min at 37 °C. Cells were detached from the plate after washing with ice-cold PBS, and the cells were analyzed by fluorescence microscope (Olympus IX71, USA). Data is expressed in percentage of cells stained with rhodamine 123.432.10 Apoptotic morphological changes by acridine orange (AO) and ethidium bromide (EtBr)
Morphological analysis of apoptosis by the AO/EtBr dual staining procedure was performed.44 To determine the apoptotic morphological changes, cells were seeded in 24-well plates for 24 h and then treated with IC50 concentrations of Vitis vinifera seed AuNPs for 24 h. After harvesting by trypsinization, cells were washed with 1× PBS once or twice. Twenty-five microliters of the cell suspension were then mixed with 1 μL of the dyes, containing 100 mg mL−1 of acridine orange and 100 mg mL−1 of ethidium bromide in 1× PBS. After staining, cells were visualised immediately under a fluorescence microscope (Olympus IX71, USA). Approximately 200 cells were counted, and quantitative estimations were carried out using ImageJ software, National Institutes of Health, USA.2.11 Statistical analysis
Results are expressed as mean ± SD for each experiment performed. All in vitro data were obtained from at least three independent experiments. Comparisons were made between the control and the treated groups by one-way ANOVA test (SPSS 20) (SPSS Inc., Chicago, IL, USA) followed by Duncan's test. p values less than 0.05 (p < 0.05) were considered to be statistically significant.3. Results
In the present study, the synthesis of gold nanoparticles using Vitis vinifera seeds was found to be beneficial; the phytochemicals acted as both reducing and capping agents during the process, resulting in gold nanoparticles that are more biocompatible for therapeutic applications. This study aimed to synthesize gold nanoparticles using the direct interaction of hydrogen tetrachloroaurate(III) trihydrate with Vitis vinifera seed extract without the intervention of any toxic chemicals as reducing agents.3.1 Visible spectroscopy analysis
The reduction of tetrachloro aurate solution was visually obvious from the color change of yellow to deep purple-red within a completed time of ten minutes, indicating the formation of gold nanoparticles from Au3+ after the addition of Vitis vinifera seed extract. Fig. 1 shows the UV-vis absorption spectra of gold nanoparticles synthesized using Vitis vinifera seed extract. The formation of gold nanoparticles from a 1 mM solution of tetrachloroauric acid was confirmed using UV-Vis spectral analysis. Surface plasmon resonance spectra for the AuNPs were obtained at 540 nm after the addition of Vitis vinifera seed extract, resulting in a dark purple-red color. A rapid increase in the synthesis of the nanoparticles was observed with increasing reaction time.3.2 Transmission electron microscopy (TEM) and energy dispersive X-ray analysis (EDX)
The size and shape of the bioreduced Vitis vinifera seed AuNPs were revealed by TEM analysis, and the TEM images confirmed the formation of AuNPs. Fig. 2 shows TEM images of the seed gold nanoparticles; they are predominantly spherical in morphology, with sizes ranging from ~20 nm to 30 nm. The Vitis vinifera seed AuNPs were found to be in a nearly monodispersed form. There were relatively few nanoparticles with distinct anisotropic morphologies, such as hexagonal shapes. TEM was used to determine the core size and morphology of the gold nanoparticles generated using Vitis vinifera seed AuNPs in aqueous solution.The presence of elemental gold (Au) is evident in the graph (Fig. 3) by energy dispersive X-ray analysis (EDX), which indicates the reduction of gold ions to gold. The presence of an elemental gold signal (2 keV) along with weak carbon and oxygen signals was confirmed for the Vitis vinifera seed AuNPs; this may have originated from the biomolecules bound to the surface of the gold nanoparticles. The carbon peak is highly characteristic of the presence of an organic matrix. The EDX profile showed a strong gold signal along with weak oxygen and carbon signals; the AuNPs are surrounded by the capping material from the seeds of Vitis vinifera as a thin layer, which remains stable in solution even after synthesis.
3.3 X-ray diffraction analysis
The phase formation and the crystalline nature of the AuNPs synthesized using grape seed extract were analyzed by X-ray diffraction (XRD) (Fig. 4). For Vitis vinifera seed gold nanoparticles, the Bragg reflections corresponding to the (111), (200), (220) and (311) sets of the lattice planes of Au are located at 2θ = 38.28°, 44.43° and 22.10°, respectively; this proved the face-centered cubic crystalline formation of the gold nanoparticles and indicated that the sample is composed of crystalline gold (ICDD no. 04-0784). The average crystal size, calculated using the X-ray diffractograms, suggested the presence of gold nanoparticles in the range of 41 nm to 65 nm for the Vitis vinifera seed nanoparticles. Gold nanoparticles in XRD patterns exhibited several different size-dependent features, leading to anomalous peak positions, heights and widths. XRD analysis was mainly used to study the crystalline nature of the gold nanoparticles.3.4 Particle size distribution
The particle size distribution histogram of the grape seed AuNPs after 30 days was determined by dynamic light scattering (DLS) (Fig. 5); the hydrodynamic sizes of the wide spectrum of particles were 50.1 ± 5.1 nm for the Vitis vinifera seed AuNPs with sizes ranging from 40 to 60 nm, respectively. The DLS method was employed to calculate the sizes of gold nanoparticles coated with phytochemicals of Vitis vinifera seeds. The size distribution histogram of the gold nanoparticles confirmed that the particles are well distributed in solution.3.5 Fourier transform infrared spectroscopy (FTIR) analysis
FTIR analysis was used to determine the functional groups of the extract on the synthesized gold nanoparticles; the band intensities in different regions of the spectra were analyzed and are shown in Fig. 6. The FTIR spectra of the grape seed extracts and synthesized nanoparticles were compared to analyze the changes before and after the bioreduction with peak assignment. FTIR analysis was performed to identify the molecules that were capped onto the gold nanoparticles as well as to identify the molecules involved in the reduction of the Au+ ions. The FTIR spectrum of the grape seeds showed the presence of a strong peak at ∼3724.5 cm−1; this is characteristic of phenols and alcohols with hydrogen bonded O–H stretches. The sharp peak at ∼2926 cm−1 represents the C–H stretch of alkane groups and the O–H stretching vibration band characteristic of carboxylic acid groups. The bands at 1745.5 cm−1 were assigned to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibrations of aldehyde, ketone, and carboxylic acid groups. The absorption peak at around 1512.1 cm−1 can be assigned to the absorption peaks of the C
O stretching vibrations of aldehyde, ketone, and carboxylic acid groups. The absorption peak at around 1512.1 cm−1 can be assigned to the absorption peaks of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C vibrations of the aromatic rings and the NO2 asymmetrical stretch of nitro compounds. This showed that biomolecules in the grape seeds, such as flavonoids or other polyphenols, containing abundant C
C vibrations of the aromatic rings and the NO2 asymmetrical stretch of nitro compounds. This showed that biomolecules in the grape seeds, such as flavonoids or other polyphenols, containing abundant C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O or N–H groups may play a significant role in the reduction and stability of gold nanoparticles during the synthesis process.
O or N–H groups may play a significant role in the reduction and stability of gold nanoparticles during the synthesis process.
Gold nanoparticles may be coated and stabilized by functional groups present within grape seeds, such as phytochemicals or proteins, through the interactions of free amine groups. After the bioreduction of AuNPs, the FTIR spectrum of the nanoparticles showed a sharp peak at 3282.8 cm−1, which represents the hydrogen-bonded O–H stretch of alcohol and phenol groups. The peak at 1656.5 cm−1 is characteristic of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretch of alkene groups, and the band at 1521.8 cm−1 represents the characteristic C
C stretch of alkene groups, and the band at 1521.8 cm−1 represents the characteristic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretch of aromatic rings. Other bands at 1234.4 cm−1 and 1105.2 cm−1 are related to the C–O stretch of alcohols, ethers, and carboxylic acids and the C–N stretch of amine groups, respectively.
C stretch of aromatic rings. Other bands at 1234.4 cm−1 and 1105.2 cm−1 are related to the C–O stretch of alcohols, ethers, and carboxylic acids and the C–N stretch of amine groups, respectively.
3.6 Zeta potential analysis
The zeta potential values (ζ) of the Vitis vinifera seed nanoparticles provided vital evidence of the stability and surface charge of the nanoparticle distribution. The negative zeta potential of −15 ± 1 mV for Vitis vinifera seed AuNPs specifies that the particles repel each other; thus, there is less tendency for the particles to aggregate (Fig. 7). The zeta potential measured for the samples after 30 days showed negligible variation.3.7 Cytotoxic effect of Vitis vinifera seed AuNPs
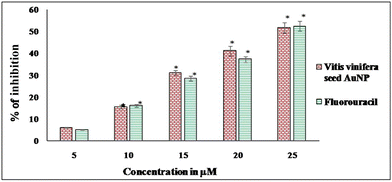
In Fig. 8, the percentage growth inhibition can be seen to increase with increasing concentration of Vitis vinifera seed bioconjugated AuNPs, and the percentage of inhibition is plotted against the Vitis vinifera seed AuNPs and fluorouracil concentration. The A431 cells were exposed to seed AuNPs with sizes of 50 ± 5 nm at concentrations of 5, 10, 15, 20, 25 μM for 24 h in the logarithmic growth phase, and the cytotoxicity was measured by MTT assay. The IC50 value was recorded to be 24.2 μM for Vitis vinifera seed AuNPs. The IC50 value was recorded to be 23.43 μM for fluorouracil, which was used as a standard drug. The percentage inhibition of cell growth by the gold nanoparticles was calculated based on the difference in inhibition between the respective controls and AuNP-treated cell lines; 100% cell proliferation was maintained as a control.3.8 Effect of Vitis vinifera seed AuNPs on cell viability
HaCaT cells were treated with increasing concentrations of Vitis vinifera seed AuNPs for 24 h to verify the cell viability. Fig. 9 clearly illustrates that the seed gold nanoparticles at concentrations of 10, 20, 30, 40, and 50 μM did not show any cytotoxicity to the normal immortalized keratinocyte cell line (HaCaT). It was observed that even at higher concentrations, viz., 50 μM of grape seed AuNPs, 91.9% of the cells remained viable after incubation for 24 h.3.9 Effect of Vitis vinifera seed AuNPs on the morphology of A431 cells
A431 cells incubated with the IC50 concentrations of V. vinifera seed gold nanoparticles were observed under a phase contrast microscope to detect any morphological changes that occurred, and the images are presented in Fig. 10. In the control group, no changes in the morphology of the cells were found, while the cells treated with the nanoparticles showed significant morphological changes when observed under a phase contrast microscope, including roundedness (a characteristic of stressed cells), irregular shapes, cytoplasmic vacuolation and enlarged cells due to AuNP-induced stress. Apoptotic cells were clearly visible among the seed gold nanoparticles treated cells. Morphological changes such as roundedness, irregular cell shapes and stressed cells were evident in nearly all the fluorouracil drug treated cells; this result is comparable with the V. vinifera seed gold nanoparticle-treated cells.3.10 Effect of Vitis vinifera seed AuNPs on the measurement of reactive oxygen species (ROS)
The effect of Vitis vinifera seed gold nanoparticles on the generation of ROS such as H2O2, O2− and peroxynitrite was examined using 2′,7′-dichlorodihydro fluorescein diacetate (DCFH-DA), which shows enhanced fluorescence when oxidative stress is generated intracellularly. A431 cells were exposed to seed gold nanoparticles, resulting in a significant increase (p < 0.01) in ROS generation at the IC50 concentration (Fig. 11 and 12). The intensity of ROS production was more significantly increased (p < 0.001) when the cells were incubated with V. vinifera seed gold nanoparticles compared to the control group. Hydrogen peroxide treated cells showed more ROS production, with a fluorescence pixel intensity of 131.5, while the control group showed 10.1. Fluorouracil was used as a standard drug to compare with the test compounds; it showed 73.62, whereas V. vinifera seed nanoparticles showed an intensity of 75.6.The fluorescence intensity increased continuously with intracellular ROS content until the end of 24 h. This increase in ROS and subsequent fluorescence was abrogated significantly in cells treated with N-acetyl cysteine, suggesting the role of ROS in gold nanoparticle-mediated cell death. NAC inhibited ROS generation, which was evident from the fluorescence intensities. To determine whether apoptosis was related to ROS, the cancer cells were incubated with NAC with gold nanoparticles. NAC showed complete abrogation of gold nanoparticle-induced apoptosis in cancer cells. In the presence of H2O2, the elevation of intracellular ROS was exponentially potentiated. The results also showed that gold nanoparticles were able to generate ROS and that nanoparticle-induced apoptotic cell death in A431 cells can be mediated by ROS signalling.
3.11 Effect of Vitis vinifera seed AuNPs on the identification of apoptosis by Annexin V-FITC staining
Treatment of A431 skin cancer cells with the IC50 concentration of Vitis vinifera seed gold nanoparticles induced apoptosis; the cells were stained with Annexin V-propidium iodide, visualized, and quantified (Fig. 13 and 14). After 24 h treatment, the cells entered the apoptotic stage, and some cells were necrotic. More necrotic cells were found in staurosporine and fluorouracil treated cells, while Vitis vinifera seed nanoparticles showed significant (p < 0.01) necrotic cells compared to the control. The percentage of apoptotic cells treated with seed gold nanoparticles was 11.69%, while the percentage of necrotic cells was 31.71%. Control untreated cells showed intact cells; the A431 cells were stained with Annexin V-FITC conjugate, which is a fluorescent probe that binds to cells in early apoptosis, where phosphatidylserine is translocated to the external portion of the membrane.3.12 Effect of Vitis vinifera seed AuNPs on nuclear morphology
A431 cells were treated with Vitis vinifera seed gold nanoparticles for 24 h, stained for nuclear morphological changes using acridine orange/ethidium bromide, and visualized under a fluorescence microscope. The results obtained from the AO/EtBr staining are presented in Fig. 15 and 16. Staining of the control cells showed cells with round, green nuclei; however, early apoptotic cells had fragmented DNA, appearing as a green color in the nuclei. Late apoptotic cells are stained orange, necrotic cells are stained red, and the DNA is fragmented. The results exhibited increase in early and late apoptotic cells after treatment with seed gold nanoparticles, which decreased the number of viable cells immensely. Treatment with seed nanoparticles produced significantly more necrotic cells (30.60%, p < 0.01) and the percentage of apoptotic cells was 43.48 (p < 0.01), showing higher toxicity to the cancer cells. Vitis vinifera seed nanoparticle-treated A431 cells showed more nuclear morphological changes compared to the control cells.Staurosporine was used to induce apoptosis; the results showed 80.21% of apoptotic cells, whereas fluorouracil, which was used as a standard drug, showed 78.21% apoptotic and 15.4% necrotic cells. Most of the gold nanoparticle-treated A431 cells showed typical characteristics, such as the formation of apoptotic bodies and plasma membrane blebbing. The seed gold nanoparticle-treated cells were largely stained red. These results indicated that cell death in most of the cells occurred due to apoptosis and secondary necrosis.
3.13 Effect of Vitis vinifera seed gold nanoparticles on the mitochondrial membrane potential
A431 cells treated with Vitis vinifera seed gold nanoparticles showed green fluorescence, indicating apoptotic cells with low mitochondrial Δψm, whereas the untreated control cells showed healthy cells with high mitochondrial Δψm with intense red fluorescence due to electrophoretic accumulation in the mitochondria (Fig. 17). Rhodamine-123, a lipophilic dye, was used to assess the membrane potential which accumulates within mitochondria in a potential dependent manner. When Vitis vinifera seed nanoparticles at a concentration of 24.2 μM, according to the IC50, were added to the cells, disruption of the membrane potential was evident as the fluorescence emission of rhodamine-123 dye changes from red to green.The orange-red fluorescence intensity was greater in the control cells than in the Vitis vinifera seed gold nanoparticle-treated A431 cells; the fluorescence intensity decreased to nearly 75% in the AuNP-treated group. Staurosporine was used as a positive control; it showed green fluorescence, while fluorouracil, which was used as a standard drug, also showed significant green fluorescence (Fig. 18). These results proved that Vitis vinifera seed coated gold nanoparticles when treated against A431 cells showed disruption and loss of mitochondrial membrane potential. Grape seed nanoparticles were able to induce significant (p < 0.05) loss of membrane potential in A431 skin cancer cells.
4. Discussion
The synthesis of metal nanoparticles with definite sizes and shapes for biomedical applications is in increasing demand, as it involves cost-effective, dependable and environment-friendly procedures. The present study revealed that the seeds of a naturally occurring fruit, i.e., Vitis vinifera (grapes), can play a significant role in the reduction and stabilization of gold nanoparticles with varied shapes and sizes with high monodispersion. The present method is a distinctive green route for the synthesis of gold nanoparticles with suitable structural moieties. Extracts of V. vinifera seed were found to be rich in phytochemicals such as flavonoids and polyphenols, which are powerful antioxidants.45 In the present study, these compounds exhibited a vital role as complex reducing, capping and stabilizing agents in the green synthesis process. Furthermore, the distinctive feature of the present biogenic green route is that changes in parameters, such as temperature, and in vitro stability studies can be effectual in the synthesis of gold nanoparticles with finely modified structures.In this study, the synthesis of gold nanoparticles using fruits was found to be beneficial, by which the phytochemicals acted as both reducing and capping agents during the process, producing gold nanoparticles that are more bio-friendly and leading to the development of biocompatible gold nanoparticles for therapeutic purposes.46
The appearance of a deep pinkish red color after addition of V. vinifera seed extracts in gold solution is a clear indication of the formation of gold nanoparticles in the reaction mixture. The color exhibited by the gold nanoparticles was due to surface plasma resonance (SPR), which is the excitation of all the free electrons within the conduction band, leading to oscillation of the electrons.47
According to the TEM images, most particles were spherical in shape and were found to be in a nearly monodispersed form, as reported in other studies using plant extracts.48 The crystal sizes calculated using X-ray diffraction data were comparable with the results reported by Philip49 in the synthesis of Au and Ag nanoparticles with palm oil mill effluent and mushroom. The particle size histogram of the gold nanoparticles showed sizes slightly larger than those found by TEM analysis. This is because the particle size analyzer yields estimates of the hydrodynamic diameters of the nanoparticles, which are different from the geometrical diameters obtained from TEM analysis. This could be due to the contrast between the gold nanoparticles and the organic layers surrounding the nanoparticles, which is much greater and results in the appearance of larger particles when particle size distribution is obtained using light scattering techniques.50 The increased particle size of the AuNPs may be attributed to the phytochemical coatings on the gold nanosurfaces, which causes substantial changes in the hydrodynamic radius. These results are supported by the studies of Ghosh et al.,38 who synthesized gold nanoparticles using Gnidia glauca; their nanoparticles were spherical and ∼10 nm in size, while the particle distribution showed particles in the range between 50 and 150 nm.
The presence of small and large nanoparticles was evidently due to agglomeration and nucleation to form newer, larger particles, which occurred sequentially throughout the reaction process. From the FTIR spectra, it is apparent that most of the biocompatible components involved in the synthesis of Vitis vinifera seed AuNPs are flavonoids, phenols, proteins and water-soluble alkaloids. The protein molecules act as surface coating molecules which prevent internal agglomeration of the particles. The FTIR absorption spectra of the Vitis vinifera seed gold nanoparticles before and after reduction of Au3+ show the presence of different peaks before and after the synthesis of the nanoparticles, indicating that Vitis vinifera seed gold nanoparticles may play a role in stabilizing AuNPs by adsorbing on the surface of bioreduced AuNPs; several other studies are in accordance with the results of this study.51–54
The results indicated that the conductivity of the gold colloids was preserved, reflecting the fact that gold nanoparticles are possibly encapsulated by the seed extract, which protects the activity of the particles over a long period of time.50 The constant negative zeta potential after 30 days suggested that the gold nanoparticles were stable and that most of the nanoparticles did not aggregate. The results were in accordance with the average diameters of the gold nanoparticles as analyzed using TEM.
In the present study, the cytotoxic effects of Vitis vinifera seed AuNPs 50 ± 5 nm in size were investigated and their possible effects on cell death in A431 cells were also determined. The cytotoxicity of the seed nanoparticles were confirmed by MTT assay, and the concentration that produced 50% cell death of A431 cells was non-toxic to normal cells (HaCaT); this substantiates the chemotherapeutic activity of the gold nanoparticles. This cytotoxicity of the gold nanoparticles can be attributed to the synergistic effects of the phenolic moieties, which are assumed to have anti-proliferative activities.55 The IC50 concentration strongly indicated that Vitis vinifera seed AuNPs had a potent cytotoxic effect on skin cancer cell lines. Many studies report that the cytotoxicity and quantitative uptake of gold nanoparticles is dependent on their sizes and shapes.56
Morphology changes that occurred in the A431 cells are due to the toxicity of the gold nanoparticles that were suspended in the culture medium at their IC50 concentration. Some cells lost their morphology as they entered the apoptosis pathway, and some cells displayed roundedness. The results are in agreement with previous studies where A549 cells were visualized by phase contrast microscope after incubation with gold nanoparticles and were found to be round, characteristic of stressed cells, whereas BHK21 and HepG2 cells remained unaffected by gold nanoparticle treatment.40
To date, literature has suggested that cancer cells are under increased oxidative stress compared to normal cells, which is associated with increased generation of reactive oxygen species (ROS) and alterations in metabolic activity.57,58 ROS are chemically active molecules that can impose severe cellular damage; because cancer cells are already under increased ROS stress, they are vulnerable to further ROS insults, which can also provide a unique opportunity to kill malignant cells and may also have significant therapeutic implications.57,59
Thus, our study showed that ROS generation is due to the exhaustion of antioxidant defense systems and impairment of mitochondrial electron transport chains.60 This generates increased ROS, causing loss of mitochondrial membrane potential and mediating mitochondrial damage and apoptosis in a p53-independent manner.61,62 Thus, Vitis vinifera seed gold nanoparticles exerted their mechanism of action for anticancer effects through the generation of ROS intermediates, probably triggering the apoptotic cascade for cell death.
Many researchers have identified mitochondria as target organelles with regard to the cellular effects of nanoparticles, and various studies have showed that nanoparticles can elicit damage to nuclear DNA.63 The highly organized process of programmed cell death is called apoptosis,64,65 and it is characterized by a set of morphological changes and biochemical steps, including translocation of phosphatidylserine from the inner to the outer layer of the plasma membrane and the formation of apoptotic bodies as a result of chromatin condensation and fragmentation of the cell.66 The characteristic morphological changes of apoptosis include membrane blebbing, cell shrinkage, chromatin condensation and degradation of DNA.67
Vitis vinifera seed gold nanoparticle-treated A431 cells underwent apoptosis and secondary necrosis, which may be attributed to the increased production of ROS in A431 cells and the loss of membrane potential. Investigation of gold nanoparticle-induced apoptosis has also been reported, e.g., HepG2 cells treated with 82.91 nM gold nanoparticles and A549 cells treated with 144.16 nM gold nanoparticles showed an increase in apoptotic cells and propidium iodide-positive dead cells.68 Thus, the Vitis vinifera seed gold nanoparticles acted as apoptotic inducing agents and were able to trigger apoptosis in A431 skin cancer cells without causing any damage to normal cells. A431 cells treated with Vitis vinifera seed gold nanoparticles revealed morphological features such as membrane blebbing, cell shrinkage and chromatin condensation after 24 h treatment. These findings are in agreement with other studies, where isodeoxyelephantopin isolated from the chloroform extract of Elephantopus scaber showed anticancer effects against KB cells, which revealed chromatin condensation and nuclear fragmentation.67
In this study, a decrease in the mitochondrial membrane potential in A431 cells after treatment with Vitis vinifera seed gold nanoparticles was observed by fluorescence microscopy. These results were in agreement with other studies, which showed that gold nanoparticles (8.17 μg mL−1) synthesized using Abelmoschus esculentus (L.) pulp extract showed significant elevation of intracellular ROS and decreasing mitochondrial membrane potential, which indicates the involvement of apoptosis in cell death.60 This result confirmed that Vitis vinifera seed AuNPs were able to decrease membrane potential so that the process of apoptosis followed the mitochondrial pathway, which is considered to be an important mediator of cell apoptosis.69
Ismail et al.70 biosynthesized gold nanoparticles using grape seed extract with a size below 20 nm and a metal ion concentration of 0.13 mM, while our study showed the particle size of the gold nanoparticles to be below 55 nm with 1 mM metal ion concentration. Although studies have demonstrated the anticancer activity of grape seed extract, it is highly useful to synthesize Vitis vinifera seed gold nanoparticles, as the nanoparticles can selectively target cancer cells; also, the phenolic moieties that form the capping on the nanoparticles may serve as anticancer agents, providing better efficacy in killing cancer cells. Biofunctionalized gold nanoparticles act as carriers in drug delivery systems wherein the gold nanoparticle core is generally nontoxic and inert.17 Vitis vinifera seed phytochemicals, having additional antioxidant properties, could be toxic to skin cancer cells when they are adsorbed onto gold nanoparticles. Nanoparticle-mediated delivery can be useful to enhance the bioavailability and to limit the toxicity of chemopreventive agents.71,72 The apoptotic and cytotoxic activities could be attributed to the synergistic effect of the gold nanoparticles functionalized with Vitis vinifera seed water-soluble phenolic moieties. At such a low concentration of gold nanoparticles, the anticancer activity cannot be attributed only to the phenolic groups of the Vitis vinifera seed extract. The shape, size and various inherent properties of the gold nanoparticles may also contribute to the synergistic effect.73 Research studies have reported the cytotoxicity, quantitative uptake into cells and anticancer effects of gold nanoparticles, attributing these effects to the size,16,74 shape16 and concentration of the nanoparticles.75 The cytotoxicity of AuNPs may also be due to the variability of the cell lines used for the study and the dosing parameters.40 However, it is still unknown whether the surface coating or the size of the gold nanoparticles is responsible for the cytotoxicity.
The present study also demonstrated that in vitro treatment with Vitis vinifera seed gold nanoparticles can inhibit the growth of A431 skin cancer cells by inducing cytotoxicity and induce apoptosis by exhibiting morphological changes. Apoptosis-inducing agents have the potential to be developed as new anti-tumor drugs that specifically target tumor cells, as apoptotic cell death does not induce an inflammatory response.76 Hence, based on the results obtained from the in vitro studies, it is quite evident that bioconjugated gold nanoparticles synthesized using Vitis vinifera seed have better therapeutic potential compared to chemically synthesized nanoparticles.77,78 It may be valuable to explore biosynthesized gold nanoparticles as a possible source of unique and novel anticancer drugs.
Conflict of interest
Authors have declared that no competing interests exist.Acknowledgements
The authors express their gratitude to Dr Paul Dhinakaran, Chancellor, Dr Sundar Manoharan S, Vice chancellor, Dr Joseph Kennedy, Registrar, Dr Patrick Gomes, Former Director, School of Biotechnology and Health Sciences, and Dr J. Jannet Vennila, Director, School of Biotechnology and Health Sciences, Karunya University for providing the necessary facilities for carrying out the experiments.References
- R. T. Greenlee, M. B. Hill-Harmon, T. Murray and M. Thun, Ca-Cancer J. Clin., 2001, 51, 15–36 CrossRef CAS PubMed.
- H. S. Black, J. Invest. Dermatol., 2004, 122, xiii–xiv CrossRef CAS PubMed.
- D. van Berlo, A. Wessels, A. W. Boots, V. Wilhelmi, A. M. Scherbart, K. Gerloff, F. J. van Schooten, C. Albrecht and R. P. Schins, Free Radical Biol. Med., 2010, 49, 1685–1693 CrossRef CAS PubMed.
- R. Arvizo, R. Bhattacharya and P. Mukherjee, Expert Opin. Drug Delivery, 2010, 7, 753–763 CrossRef CAS PubMed.
- H. Ohigashi and A. Murakami, BioFactors, 2004, 22, 49–55 CrossRef CAS PubMed.
- K. K. Wong, Recent Pat. Anti-Cancer Drug Discovery, 2009, 4, 28–35 CrossRef CAS PubMed.
- L. Qiao and B. C. Wong, Drug Resist. Updates, 2009, 12, 55–64 CrossRef CAS PubMed.
- R. Seigneuric, L. Markey, D. S. A. Nuyten, C. Dubernet, C. T. A. Evelo, E. Finot and C. Garrido, Curr. Mol. Med., 2010, 10, 640–652 CrossRef CAS PubMed.
- P. C. Chen, S. C. Mwakwari and A. K. Oyelere, Nanotechnol. Sci. Appl., 2008, 1, 45–66 CAS.
- P. Xu, E. A. Van Kirk, W. J. Murdoch, Y. Zhan, D. D. Isaak, M. Radosz and Y. Shen, Biomacromolecules, 2006, 7, 829–835 CrossRef CAS PubMed.
- K. S. Soppimath, L. H. Liu, W. Y. Seow, S. Q. Liu, R. Powell, P. Chan and Y. Y. Yang, Adv. Funct. Mater., 2007, 17, 355–362 CrossRef CAS.
- X. Wang, Y. Wang, Z. G. Chen and D. M. Shin, Cancer Res. Treat., 2009, 41, 1–11 CrossRef CAS PubMed.
- E. E. Connor, J. Mwamuka, A. Gole, C. J. Murphy and M. D. Wyatt, Small, 2005, 1, 325–327 CrossRef CAS PubMed.
- P. K. Jain, W. Huang and M. A. El-Sayed, Nano Lett., 2007, 7, 2080–2088 CrossRef CAS.
- J. Xiao and L. Qi, Nanoscale, 2011, 3, 1383–1396 RSC.
- B. D. Chithrani, A. A. Ghazani and W. C. W. Chan, Nano Lett., 2006, 6, 662–668 CrossRef CAS PubMed.
- G. Han, P. Ghosh and V. M. Rotello, Nanomedicine, 2007, 2, 113–123 CrossRef CAS PubMed.
- C. K. Kim, P. Ghosh and V. M. Rotello, Nanoscale, 2009, 1, 61–67 RSC.
- S. Muthuirulappan and S. P. Francis, Asian Pac. J. Cancer Prev., 2013, 14, 2213–2216 CrossRef PubMed.
- G. M. Cragg and D. J. Newman, Ann. Appl. Biol., 2003, 143, 127–133 CrossRef CAS.
- L. M. Alias, S. Manoharan, L. Vellaichamy, S. Balakrishnan and C. R. Ramachandran, Exp. Toxicol. Pathol., 2009, 61, 205–214 CrossRef CAS PubMed.
- V. L. Singleton, Tannins and the qualities of wines, in Plant Polyphenols, ed. P. E. Laks and R. W. Hemingway, Plenum Press, New York, 1992, pp. 859–80 Search PubMed.
- J. M. Souquet, V. Cheynier and M. Moutounet, Bulletin of the International Organisation of Vine and Wine, 2000, 73, 835–836 Search PubMed.
- I. Novak, P. Janeiro, M. Seruga and A. M. Oliveira-Brett, Anal. Chim. Acta, 2008, 630, 107–115 CrossRef CAS PubMed.
- G. Sharma, A. K. Tyagi, R. P. Singh, D. C. Chan and R. Agarwal, Breast Cancer Res. Treat., 2004, 85, 1–12 CrossRef CAS PubMed.
- X. Ye, R. L. Krohn, W. Liu, S. S. Joshi, C. A. Kuszynski, T. R. McGinn, M. Bagchi, H. G. Preuss, S. J. Stohs and D. Bagchi, Mol. Cell. Biochem., 1999, 196, 99–108 CrossRef CAS PubMed.
- S. M. Meeran and S. K. Katiyar, Exp. Dermatol., 2007, 16, 405–415 CrossRef CAS PubMed.
- A. M. Engelbrecht, M. Mattheyse, B. Ellis, B. Loos, M. Thomas, R. Smith, S. Peters, C. Smith and K. Myburgh, Cancer Lett., 2007, 258, 144–153 CrossRef CAS PubMed.
- C. Agarwal, R. P. Singh and R. Agarwal, Carcinogenesis, 2002, 23, 1869–1876 CrossRef CAS PubMed.
- D. Bagchi, M. Bagchi, S. J. Stohs, D. K. Das, S. D. Ray, C. A. Kuszynski, S. S. Joshi and H. G. Pruess, Toxicology, 2000, 148, 187–197 CrossRef CAS PubMed.
- V. Mohansrinivasan, D. C. Subathra, M. Deori, A. Biswas and N. S. Jemimah, Braz. Arch. Biol. Technol., 2015, 58, 540–546 CrossRef CAS.
- E. Q. Xia, G. F. Deng, Y. J. Guo and H. B. Li, Int. J. Mol. Sci., 2010, 11, 622–646 CrossRef CAS PubMed.
- J. D. Newman and W. A. MacCrehan, Langmuir, 2009, 25, 8993–8998 CrossRef CAS PubMed.
- A. Taleb, C. Petit and M. P. Pileni, J. Phys. Chem. B, 1998, 102, 2214–2220 CrossRef CAS.
- C. Krishnaraj, R. Ramachandran, K. Mohan and P. T. Kalaichelvan, Spectrochim. Acta, Part A, 2012, 93, 95–99 CrossRef CAS PubMed.
- J. Kasthuri, K. Kathiravan and N. Rajendiran, J. Nanopart. Res., 2009, 11, 1075–1085 CrossRef CAS.
- K. B. Narayanan and N. Sakthivel, Mater. Lett., 2008, 62, 4588–4590 CrossRef CAS.
- S. Ghosh, S. Patil, M. Ahire, R. Kitture, D. D. Gurav, A. M. Jabgunde, S. Kale, K. Pardesi, V. Shinde, J. Bellare, D. D. Dhavale and B. A. Chopade, J. Nanobiotechnol., 2012, 10, 17 CrossRef CAS PubMed.
- T. Mosmann, J. Immunol. Methods, 1983, 65, 55–63 CrossRef CAS PubMed.
- H. K. Patra, S. Banerjee, U. Chaudhuri, P. Lahiri and A. K. Dasgupta, Nanomedicine, 2007, 3, 111–119 CAS.
- R. P. Rastogi, S. P. Singh, D. P. Hader and R. P. Sinha, Biochem. Biophys. Res. Commun., 2010, 397, 603–607 CrossRef CAS PubMed.
- Y. L. Cheng, W. L. Chang, S. C. Lee, Y. G. Liu, C. J. Chen, S. Z. Lin, N. M. Tsai, D. S. Yu, C. Y. Yen and H. J. Harn, Life Sci., 2004, 75, 1579–1594 CrossRef CAS PubMed.
- S. K. Dash, S. Chattopadhyay, T. Ghosh, S. Tripathy, S. Das, D. Das and S. Roy, ISRN Oncol., 2013, 2013, 709269 Search PubMed.
- D. Ribble, N. B. Goldstein, D. A. Norris and Y. G. Shellman, BMC Biotechnol., 2005, 5, 12 CrossRef PubMed.
- J. G. Nirmala and R. T. Narendhirakannan, Int. J. Pharm. Pharm. Sci., 2011, 3, 242–249 Search PubMed.
- S. Mukherjee, V. Sushma, S. Patra, A. K. Barui, M. P. Bhadra, B. Sreedhar and C. R. Patra, Nanotechnology, 2012, 23, 455103 CrossRef PubMed.
- S. Zeng, X. Yu, W.-C. Law, Y. Zhang, R. Hu, X.-Q. Dinh, H.-P. Ho and K.-T. Yong, Sens. Actuators, B, 2013, 176, 1128–1133 CrossRef CAS.
- S. L. Smitha, K. M. Nissamudeen, D. Philip and K. G. Gopchandran, Spectrochim. Acta, Part A, 2008, 71, 186–190 CrossRef CAS PubMed.
- D. Philip, Spectrochim. Acta, Part A, 2009, 73, 374–381 CrossRef PubMed.
- P. Elia, R. Zach, S. Hazan, S. Kolusheva, Z. E. Porat and Y. Zeiri, Int. J. Nanomed., 2014, 9, 4007–4021 Search PubMed.
- M. Sastry, A. Ahmad, M. I. Khan and R. Kumar, Curr. Sci., 2003, 85, 162–170 CAS.
- S. S. Shankar, A. Rai, A. Ahmad and M. Sastry, J. Colloid Interface Sci., 2004, 275, 496–502 CrossRef CAS PubMed.
- K. B. Narayanan and N. Sakthivel, Adv. Colloid Interface Sci., 2010, 156, 1–13 CrossRef CAS PubMed.
- http://www.wellesley.edu/Chemistry/chem211lab/OrgoLabManual/Appendix/Instruments/InfraredSpec/Chem211%20IR%20Lit%20Value%20Table.pdf.
- S. Kawaii, Y. Tomono, E. Katase, K. Ogawa and M. Yano, Biosci., Biotechnol., Biochem., 1999, 63, 896–899 CrossRef CAS PubMed.
- B. D. Chithrani and W. C. Chan, Nano Lett., 2007, 7, 1542–1550 CrossRef CAS PubMed.
- E. O. Hileman, J. Liu, M. Albitar, M. J. Keating and P. Huang, Cancer Chemother. Pharmacol., 2004, 53, 209–219 CrossRef CAS PubMed.
- T. L. Riss and R. A. Moravec, Assay Drug Dev. Technol., 2004, 2, 51–62 CrossRef CAS PubMed.
- Y. Zhou, E. O. Hileman, W. Plunkett, M. J. Keating and P. Huang, Blood, 2003, 101, 4098–4104 CrossRef CAS PubMed.
- M. M. Rahaman Mollick, B. Bhowmick, D. Mondal, D. Maity, D. Rana, S. K. Dash, S. Chattopadhyay, S. Roy, J. Sarkar, K. Acharya, M. Chakraborty and D. Chattopadhyay, RSC Adv., 2014, 4, 37838–37848 RSC.
- H. Hug, S. Strand, A. Grambihler, J. Galle, V. Hack, W. Stremmel, P. H. Krammer and P. R. Galle, J. Biol. Chem., 1997, 272, 28191–28193 CrossRef CAS PubMed.
- W. P. Tsang, S. P. Chau, S. K. Kong, K. P. Fung and T. T. Kwok, Life Sci., 2003, 73, 2047–2058 CrossRef CAS PubMed.
- K. Unfried, C. Albrecht, L.-O. Klotz, A. Von Mikecz, S. Grether-Beck and R. P. F. Schins, Nanotoxicology, 2007, 1, 52–71 CrossRef CAS.
- L. Qiao and B. C. Wong, Drug Resist. Updates, 2009, 12, 55–64 CrossRef CAS PubMed.
- S. W. Fesik, Nat. Rev. Cancer, 2005, 5, 876–885 CrossRef CAS PubMed.
- G. Kroemer, L. Galluzzi, P. Vandenabeele, J. Abrams, E. S. Alnemri, E. H. Baehrecke, M. V. Blagosklonny, W. S. El-Deiry, P. Golstein, D. R. Green, M. Hengartner, R. A. Knight, S. Kumar, S. A. Lipton, W. Malorni, G. Nunez, M. E. Peter, J. Tschopp, J. Yuan, M. Piacentini, B. Zhivotovsky and G. Melino, Cell Death Differ., 2009, 16, 3–11 CrossRef CAS PubMed.
- F. A. Kabeer, G. B. Sreedevi, M. S. Nair, D. S. Rajalekshmi, L. P. Gopalakrishnan and R. Prathapan, Asian J. Pharm. Clin. Res., 2012, 6, 51–56 CrossRef.
- M. Singh, M. Kumar, S. Manikandan, N. Chandrasekaran, A. Mukherjee and A. K. Kumaraguru, J. Nanomed. Nanotechnol., 2014, S5, 009 Search PubMed.
- N. Zhang, X. Kong, S. Yan, C. Yuan and Q. Yang, Cancer Sci., 2010, 101, 2375–2383 CrossRef CAS PubMed.
- E. H. Ismail, M. M. H. Khalil, F. A. A. Seif and F. El-Magdoub, Progress in Nanotechnology and Nanomaterials, 2014, 3, 1–12 Search PubMed.
- N. Nishiyama, Nat. Nanotechnol., 2007, 2, 203–204 CrossRef CAS PubMed.
- I. A. Siddiqui, V. M. Adhami, D. J. Bharali, B. B. Hafeez, M. Asim, S. I. Khwaja, N. Ahmad, H. Cui, S. A. Mousa and H. Mukhtar, Cancer Res., 2009, 69, 1712–1716 CrossRef CAS PubMed.
- D. Raghunandan, B. Ravishankar, G. Sharanbasava, D. B. Mahesh, V. Harsoor, M. S. Yalagatti, M. Bhagawanraju and A. Venkataraman, Cancer Nanotechnol., 2011, 2, 57–65 CrossRef CAS PubMed.
- Y. Pan, S. Neuss, A. Leifert, M. Fischler, F. Wen, U. Simon, G. Schmid, W. Brandau and W. Jahnen-Dechent, Small, 2007, 3, 1941–1949 CrossRef CAS PubMed.
- T. Mironava, M. Hadjiargyrou, M. Simon, V. Jurukovski and M. H. Rafailovich, Nanotoxicology, 2010, 4, 120–137 CrossRef CAS PubMed.
- M. A. Rahman, S. C. Bachar and M. Rahmatullah, Pak. J. Pharm. Sci., 2010, 23, 256–258 Search PubMed.
- G. Han, P. Ghosh and V. M. Rotello, Adv. Exp. Med. Biol., 2007, 620, 48–56 CrossRef PubMed.
- H. Y. El-Kassas and M. M. El-Sheekh, Asian Pac. J. Cancer Prev., 2014, 15, 4311–4317 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2016 |