DOI:
10.1039/C6RA16127H
(Paper)
RSC Adv., 2016,
6, 73197-73202
Extract-mediated synthesis of Ag@AgCl nanoparticles using Conium maculatum seeds: characterization, antibacterial activity and cytotoxicity effect against MCF-7 cell line†
Received
21st June 2016
, Accepted 27th July 2016
First published on 28th July 2016
Abstract
In this study, silver/silver chloride nanoparticles (Ag@AgCl-NPs) were successfully synthesized using silver nitrate (AgNO3) via an eco-friendly and simple green approach using the seed extract of Conium maculatum (SECM) at room temperature. The biologically synthesized Ag@AgCl-NPs were characterized by UV-vis spectroscopy, transmission electron microscopy (TEM), scanning electron microscopy (SEM), energy dispersive X-ray spectroscopy (EDS) and X-ray diffraction (XRD). Electron microscopic imaging showed that the size of the Ag@AgCl-NPs was about 20 nm, with the particles spherical in shape. Moreover, the crystalline structure of the synthesized nanoparticles was confirmed using the XRD pattern. The potential antibacterial activity of the NPs against standard strains of human pathogens (Gram-positive and negative bacteria) and in vitro anticancer activity against the MCF-7 cell line were investigated. The results demonstrated the ability of the synthesized nanoparticle to kill the cancer cells and inhibit the growth of the human pathogenic and standard strains.
1. Introduction
Nanotechnology has wide-ranging significant potential applications. While juxtaposed with bulk materials, nanoparticles (NPs) supply a wide extent of exclusive chemical, optical, mechanical, electronic, and magnetic properties and features.1 A variety of chemical and physical practices such as laser ablation,2 lithography,3 photochemical reduction,4 electrochemical,5 radiation6 and biological procedures have been applied for synthesis and integration of metal nanoparticles. Despite all the reached and beneficial facts, the majority of these methods are companioned with many problems, such as addressing various toxic and hazardous chemicals that foul and taint the nanoparticles' surface7 and impede the efficacy of these nanoparticles toward medical applications.8
For the purpose of preventing adverse consequences during the synthesis and application of nanoparticles, there is a rising demand to develop eco-friendly and cost-effective methods for orchestrating nanomaterials that are not considered as toxic or mephitic within the whole procession. One doable approach to put an end to these toxic and hazardous chemicals is to utilize biomaterials and biological systems, including bacteria, fungi, yeasts, algae, or plants.9–12 Recently this very method has received upsurging attention on account of its cost-effectiveness, austerity, and inherent safety.7,13 That plant extracts are quite capable to diminish metal ions has been known since the early 1900s, despite the fact that the essence of the reducing agents involved was not well fathomed. In view of its simplicity, the use of live plants or whole plant extract and plant tissue for reducing metal salts to NPs has attracted noticeable attention during the last 30 years.14–17 Of all the metal nanoparticles, silver nanoparticles play a significant role in the territories of biology and medicine12,18 for the reason of possessing higher levels of antibacterial and anticancer activities,19,20 treatment of diseases, food preservation, and purification of water.21
For instance, extracts of Calotropis procera,22 Phoenix dactylifera,12 Melia azedarach,23 Aloevera,24 Sinapis arvensis,20 Camellia sinensis,25 Sesbania drummondii26 and leaf extract of neem7 are some of the plant materials that are applied to the biosynthesis of different kinds of nanoparticles including silver nanoparticles (Ag NPs), silver chloride nanoparticles (AgCl-NPs) and the combination of both particles (Ag@AgCl-NPs). In this very current study, the extract is simply intermingled with solution of AgNO3 at room temperature. The reduction reaction is fulfilled after a few minutes. In this article, we have reported a green simple one-step approach to biosynthesizing of Ag@AgCl-NPs using seed extract of Conium maculatum. The obtained nanoparticles have been characterized with spectroscopy and imaging techniques. Additionally, the antibacterial and anticancer activities of the synthesized Ag@AgCl-NPs have been investigated.
2. Results and discussion
2.1. UV-vis spectra analysis
One of the most quite captivating features of NPs is their optical properties. UV-vis absorption spectroscopy is a utile technique to characterize synthesized NPs. For metallic NPs, absorption wavelengths in the range of 200–800 nm are commonly and standardly applied for the characterization.27 It has been demonstrated that Ag@AgCl-NPs exhibit brownish colors emanated from the agitation of surface plasmon resonance (SPR) of the NPs.28 This color strongly depends on the concentration and size of the nanoparticles. In the spectrum of this where the plasmonic resonance peak at 441 nm is assigned to Ag29 while and the peak observed at 272 nm is corresponded to AgCl-NPs.30 UV-vis absorption spectra of the control and Ag@AgCl-NPs 2 mM synthesized here using the extract of Conium maculatum is depicted in Fig. 1. This verifies the synthesis of silver@silver chloride as nanoparticles.31
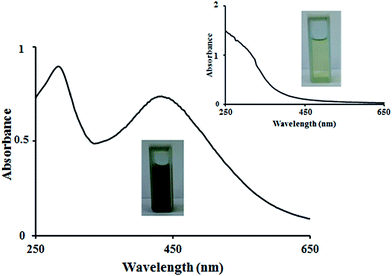 |
| | Fig. 1 UV-vis absorption spectrum of silver@silver chloride nanoparticles and control (inset) produced by the seed extract of Conium maculatum. | |
2.2. X-ray diffraction analysis
Fig. 2 shows the XRD pattern of the biosynthesized Ag@AgCl NPs. The purity and size of the NPs were determined with X-ray diffraction using Kα radiation of Cu (λ = 0.15406 nm) in the angle range of 35–80°. The XRD pattern corroborates the crystalline structure of the NPs. In this diffractogram, peaks observed at 38.17°, 44.21°, 64.48° and 77.48° are corresponded to (111), (200), (220), and (311) planes of silver and characteristic peaks appeared at 46.22°, 54.78° and 57.43° are assigned to (220), (311) and (222) planes of silver chloride profile32,33 confirming the successful biosynthesis of Ag@AgCl-NPs. The crystallite average size of the synthesized nanoparticles has been assessed using Scherrer formula:34,35
D = 0.891λ/β![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ θ |
where D is the crystallite size, λ is the wavelength of the X-ray source (0.15406 nm), β is the FWHM, and θ is the angle of diffraction. With reference to the formula, the average size of the Ag@AgCl-NPs has been calculated to be 23 nm. Some unassigned intense diffraction peaks are also demonstrated in the spectrum, which can be associated with the crystallization of bioorganic phases on the surface of the NPs.
 |
| | Fig. 2 XRD spectra of Ag@AgCl nanoparticles produced by the seed extract of Conium maculatum. | |
2.3. Transmission electron microscopy (TEM) analysis
In addition, transmission electron microscopy (TEM) was used for the determination of shape, average size, and particle size distribution of the NPs. As can be seen from Fig. 3, the synthesized particles are spherical. Furthermore, the analysis of particles distribution determined an average size of the Ag@AgCl-NPs to be 19.77 nm (Fig. 4.) and approximately the synthesized Ag@AgCl-NPs are homogeneous in nature. Hydrothermal,36 in situ anion exchange37 and in situ photoactive38 methods are the mostly used methods which have been developed to synthesize Ag@AgCl NPs. However, these techniques are relatively time consuming and need toxic chemicals, external halide sources and additional stabilizing agents.
 |
| | Fig. 3 TEM images of Ag@AgCl nanoparticles produced by the seed extract of Conium maculatum. | |
 |
| | Fig. 4 Histogram of the Ag@AgCl nanoparticles produced by the seed extract of Conium maculatum. | |
2.4. Scanning electron microscopy and energy-dispersive X-ray spectroscopy (SEM-EDS) analyses
Fig. 5A illustrates the morphology of the synthesized nanoparticles obtained using SEM analysis. As can be seen, a large number of clusters of NPs was synthesized. The composition of these clusters was identified with EDS spectrum (Fig. 5B) in which two marked peaks were appeared at around 3 keV which was a characteristic of silver atoms, and a peak was observed at 2.6 keV which was ascribed to chlorine.39 From the EDS results, it was found the synthesized NPs was composed of about 22% AgCl and 78% Ag.
 |
| | Fig. 5 (A) SEM image and (B) EDS analysis of the synthesized Ag@AgCl NPs. | |
2.5. Antibacterial activity
Antimicrobial activities of the synthesized Ag@AgCl NPs at 300 ppm against Staphylococcus aureus, which was isolated from clinical sample, Listeria monocytogenes-1298 and Pseudomonas-MU2e, were assessed by evaluating the presence of inhibition zone (IZ). To investigate the effect of AgCl impurity on the antibacterial activity of the synthesized NPs, a 66 ppm AgCl solution (according to the EDS results) was prepared and its antibacterial activity was also evaluated. The inhibition zone values are listed in Tables 1–3. As it is clear from the results, the Staphylococcus aureus species isolated from the patients are sensitive to the NPs similar to the other tested species, Listeria monocytogenes-1298 and Pseudomonas-MU2e. Considering the results obtained from AgCl (table), AgCl shows antibacterial activity against the tested species, as well. However, since the activity of Ag@AgCl NPs are greater than that of AgCl alone, the presence of Ag NPs improves its antibacterial effect.
Table 1 Diameters of the inhibition zones corresponded to human pathogenic after treating agent
| Bacteria |
Inhibition zone (mm) |
| Ag/AgCl-NPs 300 ppm |
AgCl 66 ppm |
Control negative |
Control positive |
| Water |
Amoxicillin (10 μg) |
Erythromycin (10 μg) |
| S. aureus |
16 |
7 |
NA |
12 |
28 |
Table 2 Diameters of the inhibition zones corresponded to standard strain (Gram-positive bacteria) after treating with agent
| Bacteria |
Inhibition zone (mm) |
| Ag/AgCl-NPs 300 ppm |
AgCl 66 ppm |
Control negative |
Control positive |
| Water |
Amoxicillin (10 μg) |
Penicillin (10 μg) |
| L. monocytogenes 1298 |
13 |
6 |
NA |
19 |
0 |
Table 3 Diameters of the inhibition zones corresponded to standard strain (Gram-negative bacteria) after treating with agent
| Bacteria |
Inhibition zone (mm) |
| Ag/AgCl-NPs 300 ppm |
AgCl 66 ppm |
Control negative |
Control positive |
| Water |
Ciprofloxacin (10 μg) |
Gentamicin (10 μg) |
| P. MU2e |
15 |
8 |
NA |
18 |
27 |
2.6. Anticancer activity
In vitro, cytotoxicity of the Ag@AgCl-NPs was evaluated against MCF-7 breast cancer cell lines at various concentrations. MCF-7 cells were exposed to SECM, AgCl, SECM + Ag@AgCl-NPs, and doxorubicin (as positive control), separately (0.1, 0.5, and 1 μg ml−1 of each one respectively) for 24 H. When the results of cell viability were determined using MTT essay, we found that SECM + Ag@AgCl-NPs and SECM indicated the highest and lowest anticancer activities, respectively. The result demonstrated that cell viability decreased when SECM along with Ag@AgCl-NPs was used. As seen in Fig. 6, cell viability declined when the concentration of the synthesized Ag@AgCl-NPs increased (0.1, 0.5, and 1 μg ml−1). This decline in the cell viability with rise in Ag@AgCl-NPs concentration suggests that more number of Ag@AgCl-NPs could accumulate inside the cells, resulting in an enhanced stress and ultimately leading to the cell death. These results clearly show the enhanced effectiveness of the Ag@AgCl-NPs which was synthesized biologically mediated by SECM against cancer cells. Moreover, treating the cells with SECM containing AgCl resulted in decreased cell viability compared with SECM alone. However, this decrease was less than that observed when treatment was performed with SECM + Ag@AgCl NPs.
 |
| | Fig. 6 Effect of different doses of seed extract of Conium maculatum, seed extract of Conium maculatum + AgCl, seed extract of Conium maculatum + Ag@AgCl nanoparticles and doxorubicin on MCF-7 cancer cells viability determined by MTT assay. The components have a dose-dependent toxic effect on the cancer cells. Data are expressed as mean ± SEM; n = 4 well for each group; *P < 0.05, **P < 0.01 and ***P < 0.001 vs. control (non-treated) cells; +P < 0.05 and +++P < 0.001 vs. SECM-treated cells; $P < 0.05 and $$$P < 0.001 vs. Doxorubicin-treated cells; ###P < 0.001 vs. SECM + AgCl. | |
3. Experimental
3.1. Materials and instruments
AgNO3 was purchased from Merck. Seeds of Conium maculatum were collected from Kerman, Iran. Deionized water was used in all experiments. Cell culture reagents, Dulbecco's modified Eagle's medium (DMEM), penicillin streptomycin solution, trypsin EDTA, and fetal bovine serum (FBS) were obtained from Bios era, East Sussex, UK. The flasks and dishes were acquired from SPL Life Sciences Inc. (Gyeonggi-Do, South Korea). 2-2,5-Diphenyl-2-tetrazolium bromide (MTT) was purchased from Sigma (St. Louis, MI, USA). All of the reagents and solvents were procured both from Sigma. UV-Vis spectrophotometer (scan drop 250-211Fo75) was supplied from Analyticgena (Germany). Transmission electron microscope (TEM) images were obtained using LEO912-AB, LEO Company. The morphology of the nanoparticles was also analysed using field emission scanning electron microscopy (FESEM) (SUPRA 55-CARL ZEISS, Germany). The elemental composition of the particles was analyzed by energy dispersive X-ray spectroscopy (EDS). The XRD patterns of Ag@AgCl-NPs were obtained using X-ray diffractometer (X'PERTPRO, PANalytical, Germany) equipped with Cu Kα (l = 1.54056 Å) radiation source in the range of 35–80°, operating at 40 kV and 40 mA.
3.2. Synthesis of nanoparticles
Seeds of Conium maculatum were washed with deionized water, and then were dried at a given temperature for three days. To prepare an extract suitable and befitting for the synthesis of the Ag@AgCl-NPs, 5 g of the seeds was added to 100 ml deionized water. This mixture was kept under vigorous stirring at 80 °C for 25 min and then centrifuged twice at 4500 rpm for 20 min. The obtained extract was filtered through a Whatman-40 filter paper. A clear light-brown extract was resulted, which was stored in refrigerator at 4 °C. For the synthesis of Ag@AgCl-NPs, 50 ml of the extract was added to 100 ml aqueous solution containing 2 mM of AgNO3. Within the next a few minutes, the Ag@AgCl-NPs were synthesized, which were stable for a long time. The obtained Ag@AgCl-NPs solution was then stored at 28 °C for next experiments.
3.3. Antibacterial activity
Antimicrobial activity of the synthesized nanoparticles and AgCl was investigated against one type of Staphylococcus aureus, which was isolated from some patients stricken with urinary tract infection (UTI), and two standard strains namely Listeria monocytogenes-1298 and Pseudomonas-MU2e, which were obtained from Department of Microbiology, Shahid Bahonar University of Kerman, Kerman, Iran. Amoxicillin, erythromycin, penicillin, ciprofloxacin, and gentamicin as positive controls were obtained from MAST Company.
So as to assay the antibiotic susceptibility of the compounds, agar disk diffusion method was employed.40 With respect to CLSI protocol, samples were placed in the Mueller Hinton Agar (MHA) surface and then were incubated at 35 °C. In the final stage, inhibition zone was measured for all tested compounds after 24 h. Referring to well-diffusion method, we made wells on the MHA surface using sterile Pasteur pipette and then 100 μl of the Ag@AgCl-NP and AgCl solutions, and water was poured into the wells. After incubation at 37 °C for 24 h, the zone of inhibition was demarcated.
3.4. Cell viability assay
MCF-7, the breast carcinoma cell lines were grown in DMEM with 10% fetal bovine serum and 1% penicillin–streptomycin at standard culture condition. Cells were plated at 5000 cells per cm in 96 cell culture dishes under the culture conditions. After 24 h, cells were fed with fresh medium and were treated with unprocessed extract of SECM containing Ag@AgCl-NPs, AgCl and doxorubicin as positive control. After 48 h, the cells were treated with Methyl Thiazol Tetrazolium (MTT) and incubated in culture condition for 1.5 h. Formazan crystals, formed by mitochondrial reduction of MTT, were solubilized in DMSO, and absorbance was read at 490 nm, using an Elisa Reader (DRG, USA).
4. Conclusion
Due to being green, low-cost, fast, and simple, the biosynthesis of metal nanoparticles are always preferred. Therefore, in this study and for the first time, we have reported the green synthesis of Ag@AgCl-NPs using seed extract of Conium maculatum. SECM contains compounds, which can act as reducing and capping agents for the synthesis of the nanoparticles. The synthesized NPs have been characterized by UV-visible spectroscopy, transmission electron microscopy (TEM), scanning electron microscopy (SEM), energy dispersive X-ray spectroscopy (EDS) and X-ray diffraction (XRD). The average size of the synthesized NPs has been calculated to be 23 nm. Moreover, these NPs exhibited potent antimicrobial properties against human pathogenic bacteria and standard strains, and anticancer activity against MCF-7 cell line. In addition, it is suggested that the synthesized NPs can induce DNA damage through the generation of reactive oxygen species (ROS). Thus, we have proposed the potential therapeutic uses of the synthesized Ag@AgCl NPs.
Acknowledgements
Authors gratefully acknowledge the financial support provided for this work by the Shahid Bahonar University of Kerman.
References
- B. Bhushan, in Springer handbook of nanotechnology, Springer, 2004, pp. 497–541 Search PubMed.
- T. Tsuji, T. Kakita and M. Tsuji, Appl. Surf. Sci., 2003, 206, 314–320 CrossRef CAS.
- T. R. Jensen, M. D. Malinsky, C. L. Haynes and R. P. Van Duyne, J. Phys. Chem. B, 2000, 104, 10549–10556 CrossRef CAS.
- H. Lu, S. Liu, X. Wang, X. Qian, J. Yin and Z. Zhu, Mater. Chem. Phys., 2003, 81, 104–107 CrossRef CAS.
- B. Yin, H. Ma, S. Wang and S. Chen, J. Phys. Chem. B, 2003, 107, 8898–8904 CrossRef CAS.
- N. M. Dimitrijevic, D. M. Bartels, C. D. Jonah, K. Takahashi and T. Rajh, J. Phys. Chem. B, 2001, 105, 954–959 CrossRef CAS.
- S. S. Shankar, A. Rai, A. Ahmad and M. Sastry, J. Colloid Interface Sci., 2004, 275, 496–502 CrossRef CAS PubMed.
- M. Ramar, B. Manikandan, P. N. Marimuthu, T. Raman, A. Mahalingam, P. Subramanian, S. Karthick and A. Munusamy, Spectrochim. Acta, Part A, 2015, 140, 223–228 CrossRef CAS PubMed.
- E. Castro-Longoria, A. R. Vilchis-Nestor and M. Avalos-Borja, Colloids Surf., B, 2011, 83, 42–48 CrossRef CAS PubMed.
- R. Rajan, K. Chandran, S. L. Harper, S.-I. Yun and P. T. Kalaichelvan, Ind. Crops Prod., 2015, 70, 356–373 CrossRef CAS.
- M. S. Nejad, M. Khatami and G.-H. Shahidi-Bonjar, IET Nanobiotechnol., 2016, 10, 33–38 CrossRef PubMed.
- M. Khatami and S. Pourseyedi, IET Nanobiotechnol., 2015, 9, 184–190 CrossRef PubMed.
- J. Huang, Q. Li, D. Sun, Y. Lu, Y. Su, X. Yang, H. Wang, Y. Wang, W. Shao and N. He, Nanotechnology, 2007, 18, 105104 CrossRef.
- B. Ankamwar, J. Chem., 2010, 7, 1334–1339 CAS.
- K. Kandasamy, N. M. Alikunhi, G. Manickaswami, A. Nabikhan and G. Ayyavu, Appl. Nanosci., 2013, 3, 65–73 CrossRef CAS.
- S. Iravani, Green Chem., 2011, 13, 2638–2650 RSC.
- A. T. Marshall, R. G. Haverkamp, C. E. Davies, J. G. Parsons, J. L. Gardea-Torresdey and D. van Agterveld, Int. J. Phytorem., 2007, 9, 197–206 CrossRef CAS PubMed.
- M. Rai, A. Yadav and A. Gade, Biotechnol. Adv., 2009, 27, 76–83 CrossRef CAS PubMed.
- A. K. Mittal, D. Tripathy, A. Choudhary, P. K. Aili, A. Chatterjee, I. P. Singh and U. C. Banerjee, Mater. Sci. Eng., C, 2015, 53, 120–127 CrossRef CAS PubMed.
- M. Khatami, S. Pourseyedi, M. Khatami, H. Hamidi, M. Zaeifi and L. Soltani, Bioresources and Bioprocessing, 2015, 2, 1–7 CrossRef.
- S. Silver, FEMS Microbiol. Rev., 2003, 27, 341–353 CrossRef CAS PubMed.
- S. A. Babu and H. G. Prabu, Mater. Lett., 2011, 65, 1675–1677 CrossRef CAS.
- R. Sukirtha, K. M. Priyanka, J. J. Antony, S. Kamalakkannan, R. Thangam, P. Gunasekaran, M. Krishnan and S. Achiraman, Process Biochem., 2012, 47, 273–279 CrossRef CAS.
- S. P. Chandran, M. Chaudhary, R. Pasricha, A. Ahmad and M. Sastry, Biotechnol. Prog., 2006, 22, 577–583 CrossRef CAS PubMed.
- A. R. Vilchis-Nestor, V. Sánchez-Mendieta, M. A. Camacho-López, R. M. Gómez-Espinosa, M. A. Camacho-López and J. A. Arenas-Alatorre, Mater. Lett., 2008, 62, 3103–3105 CrossRef CAS.
- N. C. Sharma, S. V. Sahi, S. Nath, J. G. Parsons, J. L. Gardea-Torresde and T. Pal, Environ. Sci. Technol., 2007, 41, 5137–5142 CrossRef CAS PubMed.
- C. S. Kumar, UV-VIS and Photoluminescence Spectroscopy for Nanomaterials Characterization, Springer, 2013 Search PubMed.
- P. Mulvaney, Langmuir, 1996, 12, 788–800 CrossRef CAS.
- F. Cheng, J. W. Betts, S. M. Kelly and A. L. Hector, Mater. Sci. Eng., C, 2015, 46, 530–537 CrossRef CAS PubMed.
- V. A. Kumar, Y. Nakajima, T. Uchida, T. Hanajiri and T. Maekawa, Mater. Lett., 2016, 176, 169–172 CrossRef CAS.
- H. Alishah, S. P. Seyedi, S. Y. Ebrahimipour and S. Esmaeili-Mahani, J. Cluster Sci., 2016, 27, 421–429 CrossRef CAS.
- S. Ashokkumar, S. Ravi, V. Kathiravan and S. Velmurugan, Spectrochim. Acta, Part A, 2015, 134, 34–39 CrossRef CAS PubMed.
- A. Miri, M. Sarani, M. R. Bazaz and M. Darroudi, Spectrochim. Acta, Part A, 2015, 141, 287–291 CrossRef CAS PubMed.
- A. Patterson, Phys. Rev., 1939, 56, 978 CrossRef CAS.
- S. Y. Ebrahimipour, I. Sheikhshoaie, J. Castro, W. Haase, M. Mohamadi, S. Foro, M. Sheikhshoaie and S. Esmaeili-Mahani, Inorg. Chim. Acta, 2015, 430, 245–252 CrossRef CAS.
- L. Xinhui, H. Jinlin, L. Jiajia, H. Yong, S. Yong, Y. Haijian, T. Guoxiu and Q. Haisheng, Mater. Lett., 2013, 91, 129–132 CrossRef.
- J. Cao, B. D. Luo, H. L. Lin, B. Y. Xu and S. F. Chen, J. Hazard. Mater., 2012, 217, 107–115 CrossRef PubMed.
- D. Lihong, T. Shanshan, Z. Junyi, Z. Peiying, Z. Lifan and T. Fengwei, Mater. Lett., 2013, 91, 245–248 CrossRef.
- V. Gopinath, S. Priyadarshini, N. M. Priyadharsshini, K. Pandian and P. Velusamy, Mater. Lett., 2013, 91, 224–227 CrossRef CAS.
- R. Schwalbe, L. Steele-Moore and A. C. Goodwin, Antimicrobial susceptibility testing protocols, CRC Press, 2007 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra16127h |
|
| This journal is © The Royal Society of Chemistry 2016 |
Click here to see how this site uses Cookies. View our privacy policy here.  *c
*c

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos
cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ
θ




