Influence of morphological and chemical features of biochar on hydrogen peroxide activation: implications on sulfamethazine degradation
Danlian Huang*ab,
Yang Wangab,
Chen Zhangab,
Guangming Zengab,
Cui Laiab,
Jia Wanab,
Lei Qinab and
Yalan Zengab
aCollege of Environmental Science and Engineering, Hunan University, Changsha, Hunan 410082, P. R. China. E-mail: huangdanlian@hnu.edu.cn; Fax: +86 731 88823987; Tel: +86 731 88823987
bKey Laboratory of Environmental Biology and Pollution Control, Hunan University, Ministry of Education, Changsha, Hunan 410082, P. R. China
First published on 28th July 2016
Abstract
This paper investigated how morphological and chemical features of biochars influenced hydroxyl radical (˙OH) generation and sulfamethazine (SMT) degradation in the presence of hydrogen peroxide (H2O2). Effects of SMT adsorption on H2O2 activation by biochar were also studied. In this study, a series of wheat chars were pyrolyzed anaerobically at six heat treatment temperatures (HTT) (300, 400, 500, 600, 700, and 800 °C) and characterized for morphological and chemical features. Higher-temperature biochar led to the higher yield of ˙OH formed during H2O2 activation, which might be due to the better morphological features and higher content of basic character biochar obtained at high temperature. The degradation efficiency of SMT (13.7 μM) went up from 93.4% to 100% and the pseudo-first-order rate constant increased from 0.0211 min−1 to 0.427 min−1 with the increase of charring temperature in H2O2/biochar system. Furthermore, biochars' reactivity toward H2O2 was inhibited by the adsorption of SMT on their surface. The present findings highlighted the influence of morphological and chemical features of biochar on H2O2 activation, and provided a new approach for biochar application to organic contaminant removal.
1. Introduction
Biochar, a fascinating carbon-rich material obtained under low oxygen conditions, has been recognized as a highly efficient adsorbent due to its large surface area, complex porosity and variable surface composition.1–6 Compared with the studies focused on biochar for contaminant absorption, few publications have explored the performance of biochar on organic contaminants degradation, which would provide a broad application prospect for contaminant removal.Porous carbon materials (activated carbons, carbon nanofibers, and graphite, etc.) were used in catalysis either as catalysts or supports, and activation of hydrogen peroxide (H2O2) by activated carbon has been successfully applied to the degradation of dissolved organic pollutants.7–10 Considering the relatively low-cost and similar characteristics of biochar as that of activated carbon, the potentiality of the catalytic activity of biochar toward oxidants (H2O2, persulfate, etc.) has been extensively investigated in several literatures.11–13 Biochar supported nanoscale zerovalent iron was successfully used in catalysis for persulfate activation, promoting the generation of sulfate radicals (˙SO4−) and the degradation of trichloroethylene.13 Previous reports indicated that biochars, with the redox activity and electron shuttling capacity, may be characterized as an electron shuttle for environmental remediation by directly mediating electron transfer processes and then influenced pollutant transformation reactions.14,15 It has been found that the chars (produced from pine needles, wheat, and maize straw) showed a great performance on H2O2 activation, resulting in the generation of hydroxyl radicals (˙OH) and the degradation for 2-chlorobiphenyl completely.11
H2O2 and its final products are eco-friendly during the process of activation.16,17 Additionally, the generation of ˙OH radicals was recognized as the key in chemical oxidizing of organic pollutants in H2O2 activation.18,19 Therefore, it was pressed to define the influencing factors in H2O2 activation by biochar to improve the formation of ˙OH. The H2O2 activation was expected to be initiated on the surface of the char similar to the process of adsorption. While the significant effects of morphological and chemical features on the adsorption behavior of chars have been reported systematically, few studies have been done for understanding how these characteristics of biochar (surface area, porosity, and surface chemistry, etc.) influence H2O2 activation.1,4,20–23
This paper mainly focus on analyzing the relationships between morphological and chemical features of biochars (produced from different charring temperatures and modifications) and their performance in the activation of H2O2, in order to design improved chars. Considering the negative effects on ecosystem and human health from sulfamethazine (SMT) and the established mechanism of SMT degradation by ˙OH, SMT was selected as the target contaminant to evaluate the degradation efficiency of organic contaminants by H2O2/biochar system.24–27
2. Materials and methods
2.1. Chemicals
Sulfamethazine was obtained from Sigma Chemical Company (99%, w/w). Hydrogen peroxide (H2O2, 30%), titanium(IV) oxysulphate (15 wt% in dilute sulphuric acid, 99.99%), salicylic acid (99.5%), 2,3-dihydroxybenzoic acid (2,3-DHBA, 99.8%), and 2,5-dihydroxybenzoic acid (2,5-DHBA, 99.8%) were purchased from J&K Scientific Ltd, China. The rest of the chemicals was purchased from China National Medicines Corporation Ltd (Beijing, China), ultra-pure water (resistivity of 18.2 MΩ cm) was used throughout the experiments. Wheat straws were used in this study, which were collected from a village of Changsha (N27.9179, E113.199), Hunan province, China. It contained approximately 36.9% cellulose, 23.6% hemicellulose and 9.2% lignin according to previous methods.28–302.2. Biochar preparation
Wheat straws were rinsed with ultra-pure water four times and dried overnight, then oven-dried biomass was crushed and screened into 60 mesh. For pyrolysis, the resulting biomass was pyrolyzed with N2 purge (0.5 L min−1) at 10 °C min−1 in a tube furnace. The treated samples were kept at the target temperature between 300 and 800 °C for 2 h. After allowing the char to cool to room temperature, the char was placed in an air-tight container and stored in the dark before use. The chars were designated as W300, W400, W500, W600, W700, and W800, consisted with the pyrolysis temperature, respectively.2.3. Oxygenation of biochar surface
100 mg of biochar sample W800 was added into 100 mL of 16% HNO3 and agitated at 160 rpm at 80 °C for 3 h. The resulting sample was rinsed with ultra-pure water until constant pH, and dried in a vacuum drying oven. Then the oxygenated biochar, which retained the original textural properties and obtained the higher amount of surface functionalities, was available for further study.2.4. Removal of inorganics
100 mg of biochar samples (W300, W500, and W700) were immersed in 100 mL of 16% HCl at 160 rpm at 25 °C for 24 h, the resulting samples were rinsed with ultra-pure water until constant pH, and dried in a vacuum drying oven, resulting in the deashed biochar, which had been removed all of inorganics.2.5. Generation of ˙OH in H2O2/biochar system
A series of experiments were performed in 100 mL conical flasks consisting of 50 mL of reaction solution. First, 50 mg of biochars was hydrated in 48 mL phosphate buffer solution (PBS, 10 mM, pH 7.4), at 25 °C for 10 min. Subsequently, 1.0 mL salicylic acid (SA, the final concentration was 2.0 mM), 1.0 mL H2O2 (the final concentration was 10 mM) were quickly added in sequence to initiate reaction. Then the flasks were in an incubator shaker with a shaking rate of 160 rpm at 25 °C for 2 h in the dark. Periodically, the suspension was filtered with 0.22 μm membranes glass microfiber filters for further analysis, and 1.0 mL ethanol was immediately added into 4.0 mL solution to quench the reaction. All of the experiments were performed in triplicate. Blank experiments without biochar or H2O2 were carried out under identical reaction conditions.2.6. Degradation of SMT in H2O2/biochar system
To evaluate the broad application prospect of H2O2/biochar system on contaminants removal, the degradation efficiency of SMT in this system was studied. Batch experiments were carried out following the procedure described in Section 2.5, except SMT (the final concentration was 13.7 mM) was added instead of SA. Control experiments were performed under identical reaction conditions in the absence of H2O2 or biochar. In this study, the degradation efficiency of SMT was calculated based upon the values of SMT concentrations in “aqueous” and “solid” phases. To quantify the concentration of SMT in “solid” phases, the reacted chars were filtered and extracted by hexyl hydride, and the recovery ratio of SMT was 93–105%.2.7. Characterization methods
Elemental (C, H, N and O) compositions of each char sample were determined using an elemental analyzer (model EA1110, CE Instruments, Milan, Italy). The extractable metals (Ca, Mg, Fe, Zn, and Mn) were determined by Atomic Absorption Spectrometry (Z-2000, Hitachi, Japan) following the acid digestion, the content of K was determined using Flame Photometry (FP640, Inesa, China).11,31Surface area (SBET) and pore volume were determined by N2 adsorption at 77 K and CO2 adsorption at 273 K with a surface area analyzer after outgassing at 473 K (ASAP-2020, Micromeritics Instrument Corporation, USA).32,33 The morphologies of biochars were characterized by environmental scanning electron microscopy (ESEM) (JSM-5600, Japan) at an accelerating voltage of 20 kV. X-ray photoelectron spectroscopy (XPS) was obtained with a Thermo ESCALAB 250XI system with Al Kα radiation at 1486.6 eV.
The pH of biochar samples was measured by combining biochar with ultra-pure water at the ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 (w/v) after being stirred at 160 rpm at 25 °C for 2 h (FE20K, Mettler Toledo, USA). The surface acidity and basicity of chars were determined by “base adsorption” and “acid adsorption”, respectively.34,35 The base or acid uptake of biochar was converted to the content of surface acidity or surface basicity (cmol kg−1), respectively. Moisture, volatile matter (VM), fixed carbon, and ash contents of these chars were measured according to the American Society for Testing and Materials (ASTM) D5124.36
20 (w/v) after being stirred at 160 rpm at 25 °C for 2 h (FE20K, Mettler Toledo, USA). The surface acidity and basicity of chars were determined by “base adsorption” and “acid adsorption”, respectively.34,35 The base or acid uptake of biochar was converted to the content of surface acidity or surface basicity (cmol kg−1), respectively. Moisture, volatile matter (VM), fixed carbon, and ash contents of these chars were measured according to the American Society for Testing and Materials (ASTM) D5124.36
2.8. Analysis methods
The concentrations of hydroxyl radicals were indirectly measured following a salicylic acid (SA) method.11 SA can react with ˙OH, which would cause for the formation of 2,3-dihydroxybenzoic (2,3-DHBA), 2,5-dihydroxybenzoic (2,5-DHBA) and catechol.37 High performance liquid chromatography (HPLC) (Series 1100, Agilent, Germany) equipped with a Zorbax SB-C18 column (4.6 × 250 mm, 5 μm, Waters) was applied to the analysis of SA, 2,3-DHBA and 2,5-DHBA. Details about the analysis and calculation method were reported by Fang et al.11Details about the measurement of SMT were described in our previous study.25 The concentrations of H2O2 were analyzed by UV-vis spectrophotometer (2700, Shimadzu Corporation, Japan) following a colorimetric method.38
3. Results and discussion
3.1. Characteristics of the biochars
With the increasing of heat treatment temperatures (HTT), O and H content decreased whereas C content increased, leading to the reductions in H/C, O/C molar ratios of these samples (Table 1, Fig. 1). H/C and O/C molar ratios have been analyzed to evaluate the degree of aromaticity and polarity of chars, respectively.39 The decrease of H/C value was closely correlated to the stronger carbonization of biochar, which indicated that high carbonizing temperature may result in the formation of higher aromatic ring structures in biochars.22,40 The reduction of O/C value with increasing pyrolysis temperature may cause biochars to be less hydrophilic. In addition, the molar ratio of (O + N)/C of the samples, as a polarity index indicator, decreased with elevation of pyrolysis temperature, which indicated a reduction of the surface polar functional groups. The results of the XPS C1s peaks were presented in Fig. 2, which clearly showed the formation and variation of oxygen-containing functionalities of biochars. The photoelectron peak was resolved into four curves with peaks of approximately 284.8, 286.2, 289.8, 290.5 eV, which represented aliphatic/aromatic carbon (C–C, C–H, and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), C–O, O–C
C), C–O, O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, and carbonate groups,25 respectively. In Table 2, aliphatic/aromatic carbon and carbonate groups might developed whereas C–O decreased as the increase of HTT, and carbonate groups would be strong after 600 °C.
O, and carbonate groups,25 respectively. In Table 2, aliphatic/aromatic carbon and carbonate groups might developed whereas C–O decreased as the increase of HTT, and carbonate groups would be strong after 600 °C.
| Characteristics | W300 | W400 | W500 | W600 | W700 | W800 |
|---|---|---|---|---|---|---|
| a Mean ± standard deviation (SD) in triplicate measurements.b Elemental compositions and atomic ratios are on an dry ash-free basis. | ||||||
| Proximate analysisa | ||||||
| pH | 6.92 ± 0.02 | 7.34 ± 0.04 | 7.69 ± 0.01 | 8.13 ± 0.05 | 8.42 ± 0.03 | 8.53 ± 0.04 |
| Yield (%) | 52.31 ± 0.66 | 38.88 ± 0.22 | 35.52 ± 0.45 | 31.12 ± 0.28 | 29.72 ± 0.21 | 28.42 ± 0.32 |
| Ash (%) | 7.10 ± 0.23 | 11.29 ± 0.17 | 12.42 ± 0.11 | 14.24 ± 0.22 | 14.92 ± 0.12 | 15.29 ± 0.28 |
| VM (%) | 58.49 ± 0.42 | 32.72 ± 0.31 | 20.81 ± 0.16 | 14.59 ± 0.18 | 11.37 ± 0.31 | 9.78 ± 0.14 |
| Fixed C (%) | 31.18 ± 0.44 | 52.11 ± 0.14 | 61.68 ± 0.22 | 65.15 ± 0.34 | 67.90 ± 0.14 | 68.72 ± 0.42 |
| Moisture (%) | 3.23 ± 0.03 | 3.88 ± 0.01 | 5.09 ± 0.05 | 6.02 ± 0.08 | 5.81 ± 0.02 | 6.21 ± 0.04 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||
| Ultimate analysis | ||||||
| Cb (%) | 65.2 | 75.5 | 82.9 | 85.5 | 88.8 | 89.9 |
| Hb (%) | 5.12 | 4.48 | 3.55 | 2.51 | 1.93 | 1.62 |
| Ob (%) | 27.92 | 18.29 | 11.75 | 10.28 | 7.59 | 6.83 |
| Nb (%) | 1.76 | 1.73 | 1.80 | 1.71 | 1.68 | 1.65 |
| Molar H/C | 0.942 | 0.712 | 0.514 | 0.352 | 0.261 | 0.216 |
| Molar O/C | 0.321 | 0.182 | 0.106 | 0.09 | 0.064 | 0.057 |
| Molar (O + N)/C | 0.344 | 0.201 | 0.125 | 0.107 | 0.08 | 0.073 |
| K (g kg−1) | 12.43 | 16.74 | 15.5 | 15.78 | 15.64 | 15.66 |
| Ca (g kg−1) | 15.45 | 19.15 | 20.7 | 20.37 | 20.64 | 19.85 |
| Mg (g kg−1) | 2.12 | 3.15 | 2.84 | 2.83 | 2.54 | 2.57 |
| Fe (g kg−1) | 1.52 | 1.43 | 1.36 | 1.31 | 1.25 | 1.23 |
| Mn (g kg−1) | 0.11 | 0.14 | 0.13 | 0.12 | 0.11 | 0.12 |
| Total (wt%) | 3.16 | 4.06 | 4.05 | 4.04 | 4.01 | 3.94 |
| Peak (eV) | Assessment | Peak concentration (%) | |||||
|---|---|---|---|---|---|---|---|
| W300 | W400 | W500 | W600 | W700 | W800 | ||
| a XPS analysis showed no presence due to the extremely low content. | |||||||
| 284.8 | C–C, C–H, or C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C C |
61.97 | 63.31 | 65.90 | 73.14 | 73.43 | 74.20 |
| 286.2 | C–OH, C–O–C | 38.03 | 36.69 | 26.68 | 20.51 | 17.11 | 16.32 |
| 289.8 | COOH, COOC | N/Da | N/D | 7.42 | N/D | N/D | N/D |
| 290.5 | Carbonate groups | 6.35 | 9.46 | 9.48 | |||
The variations of extractable constitutes (K, Ca, Mg, Fe and Mn) with the increasing charring temperature were presented in Table 1. It was clear that extractable cations obviously changed from 300 °C to 500 °C and trended towards stability for subsequent temperature. As shown in Fig. 3, the acidity of biochars was thought to be the amount and strength of being acid, such as carboxyl, lactone, phenol, etc. The surface alkalinity of biochars was determined by the quality or state of basic sites: (1) some of oxygen functional groups, (2) heteroatom (except oxygen) functional groups, (3) inorganic matters, (4) π-electrons and edge-electron pairs (Fig. 3).41 The surface acidity decreased whereas the surface alkalinity increased with increasing pyrolysis temperature (Table 3), which was consistent with the result of XPS. The trend of pH with increasing HTT was consistent with that of ash content, resulting from the development and migration of the alkalinity and carbonate content.
| Characteristics | W300 | W400 | W500 | W600 | W700 | W800 |
|---|---|---|---|---|---|---|
| a Mean ± standard deviation (SD) in triplicate measurements. | ||||||
| SBET (m2 g−1) | 8.9 | 22.9 | 108.1 | 185.4 | 216.3 | 223.4 |
| Pore volume (cm3 g−1) | 0.006 | 0.018 | 0.058 | 0.089 | 0.095 | 0.105 |
| Surface aciditya (cmol kg−1) | 183.4 ± 3.6 | 130.2 ± 2.8 | 86.4 ± 3.1 | 60.4 ± 2.3 | 54.3 ± 1.3 | 48.9 ± 3.4 |
| Surface basicitya (cmol kg−1) | 84.9 ± 2.9 | 108.4 ± 4.3 | 128.7 ± 5.1 | 139.7 ± 3.1 | 143.6 ± 3.9 | 146.2 ± 4.8 |
The changes in VM and fixed carbon contents occurred mostly at 300–500 °C due to the decomposition of biopolymer including hemicellulose, cellulose, and lignin (Table 1). Given that VM was relative to contents of the labile fraction of biochar, the rapid decline of VM may indicate that the higher temperature led to the higher stabilization.42 The textural characteristics of the biochars were closely related to the pyrolysis temperature, and the results were given in (Table 3, Fig. 4). The extremely low value in SBET (8.9 m2 g−1) and porosity (0.006 cm3 g−1) of W300 suggested that the decomposition of biopolymers was not marked at relatively low HTT. With increasing charring temperature, the formation and release of volatile organic components significantly intensified the development of pore nature and surface area. The results showed that the surface area and porosity of the samples both improved rapidly from 8.9 (W300) to 223.4 (W800) m2 g−1, and 0.006 (W300) to 0.105 (W800) cm3 g−1, respectively.
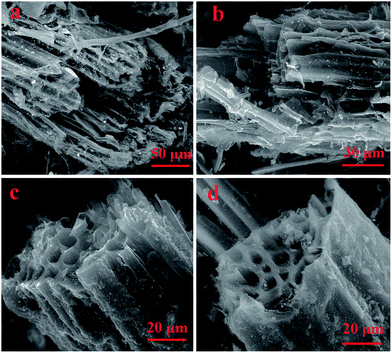 | ||
| Fig. 4 ESEM images of biochars produced through pyrolysis at 300 °C (a), 500 °C (b), 700 °C (c) and 800 °C (d). | ||
The effects of these characteristics on H2O2 activation in H2O2/biochar system would be later discussed in Section 3.3.
3.2. Generation of ˙OH in H2O2/biochar system
To verify the hypothesis that the morphological and chemical features of biochar affect the H2O2 activation, biochars (pyrolyzed at a series of HTT) were utilized as catalyst, the corresponding values in H2O2 decomposition and ˙OH yields were measured in this study. SA, a chemical probe of ˙OH, was used to measure the concentrations of ˙OH radicals generated in H2O2/biochar system. In this part, the rate of H2O2 decomposition was described as eqn (1),| [H2O2] = [H2O2]0e−kdt | (1) |
It has been reported that the morphological and chemical features of biochar were significantly impacted by the pyrolysis temperature,21–23 and the H2O2 activation was initiated on the surface of the biochar, the differences in above-mentioned results might result from the differences in surface area, porous structure, and surface chemistry. Therefore, the influences of those properties in H2O2 activation were further investigated and discussed in the following section.
3.3. Influence of morphological and chemical features
Table 3 revealed that surface acidity decreased while surface alkalinity increased with the increasing pyrolysis temperature. The results showed that the relatively higher temperature enhanced the removal of acidic active sites and promoted the formation of basic active sites, which made access to a great basic character for biochar (development of pH value). As presented in Fig. 6(c) and (d), the changes of surface acidity and basicity of chars were closely connected to the formation of ˙OH. Obviously, there was a negative correlation between the concentrations of trapped [OH] and surface acidity (Fig. 6(c), R2 = 0.843), while a positive correlation for surface basicity (Fig. 6(d), R2 = 0.863). Similarly, several papers has reported that carbon materials with great surface basicity has more activity for H2O2 decomposition.44,45 According to Ribeiro et al.,38 the increasing basicity of carbon materials promoted ˙OH formation from H2O2 decomposition.
As shown in Fig. 7(a) and (b), it was seen that the modified biochars showed lower capacity, which had more acid oxygen-containing functionalities and higher values of surface acidity. The preparation for the modified sample was described in Section 2.3. The sample char W800 was selected, because of the minimum of the acid oxygen-functionalities and stabilized textural properties. Compared with the original biochar, the oxygenated biochar had 8% and 5% decrease in surface area and total pore volume, respectively. Acidity increased from 48.9 to 97.6 cmol kg−1, and basicity decreased from 146.2 to 105.7 cmol kg−1. This suggested that acid oxygen-functionalities had been successfully inserted onto the surface. The reactivity of the original biochar was compared to that of the oxygenated biochar, which had similar textural properties but more acid oxygen-functionalities on the surface. The results in Fig. 7(a) and (b), showed that remarkably difference between the original biochar and the oxygenated biochar in the H2O2 activation and ˙OH formation. It can be concluded that the oxygenated biochar had lower catalytic activity for H2O2 activation and the formation of ˙OH than original biochar.
Summarizing, the H2O2 decomposition rate may increase in the presence of strong basic functionalities and weak acid oxygen-functionalities on the biochar surface. Considering the acid oxygen-functionalities had an electron withdrawing capacity,9,45 the process of the electron transfer would be inhibited and led to the decrease of ˙OH formation in H2O2/biochar system. On the contrary, basic functionalities acting as proton sinks would exhibit a strong positive charge on the surface of biochar, improving the electron transfer effects.38,41,46 Thus, these functional groups could be recognized as active sites for H2O2 decomposition and contributed to enhance the generation of ˙OH radicals.
A new research demonstrated that inorganics (Ca, K, Na, Si, P, and Mg) were observed in the poplar wood biochar, and biochar with higher contents of inorganics had higher catalytic performance in methane-cracking reactions.48 Fang et al.12 noted that changing in the types and concentrations of metals may significantly alter the ability to activate persulfate of biochar. Therefore, we investigated that whether inorganics played an important role in activating H2O2. The deashed chars, which produced from the procedure described in Section 2.4 and removed more than 95% of inorganic fractions, were compared to the original char samples by measuring the yields of the trapped ˙OH. As presented in Fig. 7(c), the trapped ˙OH concentrations decrease 18%, 23%, 24% compared to that of the original char samples, W300, W500, and W700, respectively. These results suggested that the inorganic matters, which only made up approximately 4% (w/w) of biochars, surely did a considerable contribution to the generation of ˙OH radicals from the H2O2/biochar system. Additionally, this also indicated that the deashed biochars had catalytic activity. As a result, the presence of the surface area/porous texture, which had no loss in the acid treatment, played an important role in the process that the biochar activates H2O2.
Given that the importance of inorganics in H2O2 activation of char was remarkable, we explored whether ash, made up of only inorganics, would exhibit a better performance in activating H2O2 than that of char. W800 was heated in an open crucible to 800 °C for 45 min to produce the ash. Compared with the original char sample, this production lost more than 99% of the organic fractions (carbon) and was made up of the metals and minerals. The experiments were carried out according to the procedure described in Section 2.5. The ash exhibited relatively poor performance in activating H2O2 than the char, as demonstrated by the low yields of trapped [OH] in Fig. 7(a), indicating that the presence of organic carbon was essential to the generation of ˙OH radicals in the H2O2/biochar system. Some possible explanations could be proposed for these: (1) interactions between organic and inorganic fractions may contribute to the process that the char activates H2O2; (2) carbon can be considered as supports, on which inorganics are dispersed randomly, associated with their excellent surface nature; (3) some active sites adhered closely to the surface on organic carbon were linked with the H2O2 decomposition.
3.4. Degradation kinetics of SMT
To evaluate the removal efficiency of organic pollutants in H2O2/biochar system, the experiments for SMT degradation were carried out following the procedure described in Section 2.6. Fig. 8 showed that the degradation efficiency of 13.7 μM SMT was 93.2% for W300/H2O2, 100% for W500/H2O2, and 100% for W700/H2O2, respectively, during reaction time of 2 h. Oppositely, only 6.3%, 8.6%, 9.3% of SMT was removed in the system without H2O2, which was mostly depended on the adsorption on char under the identical reaction conditions. Additionally, less than 5% of SMT disappeared in control experiments without biochar. The degradation of SMT can be described by following equation (eqn (2)),| [SMT] = [SMT]0e−kobst | (2) |
| Biochar | W300 | W400 | W500 | W600 | W700 | W800 |
|---|---|---|---|---|---|---|
| SMT degradation (%) | 93.2 | 94.7 | 100 | 100 | 100 | 100 |
| kobs (min−1) | 0.0211 | 0.0237 | 0.0324 | 0.0379 | 0.0411 | 0.0427 |
| R2 | 0.914 | 0.926 | 0.957 | 0.931 | 0.963 | 0.925 |
According to several reports,24,26,49–53 the ˙OH radicals could attack the pyrimidine ring, aromatic ring or sulfonamide bond [N–S] of SMT molecule. The pyrimidine ring was broken by the cleavage of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond and C–N bond, resulting in the formation of 4-amino-N-carbamimido-yl-benzenesulfonamide, formic acid (HCOOH), acetic acid (CH3COOH).26,49–51 The cleavage of sulfonamide bond [N–S], as the main pathway for SMT degradation, would lead to the formation of 4,6-dimethylpyrimidin-2-amine and sulfanilic acid.24,26,49–52 Meanwhile, sulfate (SO42−) could be released during this process. Sulfanilamide group could be oxidized to 4-aminophenol and then to 4-nitrophenol, which could be explained by the strong negative charge between aromatic ring and the nitrogen atom.49–53 As the reaction progress, some of intermediates could be completely degraded to CO2 and H2O.49–53 The possible pathways responsible for SMT degradation in this system could be proposed and described in Fig. 9.
N bond and C–N bond, resulting in the formation of 4-amino-N-carbamimido-yl-benzenesulfonamide, formic acid (HCOOH), acetic acid (CH3COOH).26,49–51 The cleavage of sulfonamide bond [N–S], as the main pathway for SMT degradation, would lead to the formation of 4,6-dimethylpyrimidin-2-amine and sulfanilic acid.24,26,49–52 Meanwhile, sulfate (SO42−) could be released during this process. Sulfanilamide group could be oxidized to 4-aminophenol and then to 4-nitrophenol, which could be explained by the strong negative charge between aromatic ring and the nitrogen atom.49–53 As the reaction progress, some of intermediates could be completely degraded to CO2 and H2O.49–53 The possible pathways responsible for SMT degradation in this system could be proposed and described in Fig. 9.
Such the adsorption and degradation of SMT may be forced to coexist in this system. Thus how the surface adsorption of SMT would inhibit/accelerate the biochars' reactivity toward H2O2 and SMT degradation should be studied. For this purpose, the effects of adsorption time on biochar were investigated before using as a catalyst for H2O2 activation. As expected in Fig. 10(a) and (b), both the degradation of SMT and the decomposition of H2O2 were suffered markedly following the adsorption time extended, as demonstrated the visible decline in the pseudo-first-order rate constants of SMT degradation (kobs) and H2O2 decomposition (kd). The results were in agreement with Fang et al.,11 which aimed at that of 2-chlorobiphenyl. The inhibition of H2O2 activation reactions might result from the variations in morphological and chemical features of biochar during the process of adsorption. More SMT molecules adhered to surface and entered into the pore of biochar, which may cause the reduction of surface availability and the blocking of the pore. In our previous study,25 π–π electron-donor–acceptor interaction and other physical-chemistry processes occurred on surface of chars during SMT adsorption, leading to the reduction in both electrons availability and active sites on the surface of biochar. Therefore, the accessibility of H2O2 was hindered as the adsorption time extended, the degradation of SMT was inhibited at the same time.
4. Conclusions
In this study, biochar exhibited favorable catalytic performance in H2O2 activation to generate ˙OH, promoting SMT degradation in aqueous solutions. There were linear relationships between the catalytic performance on H2O2 activation and morphological/chemical features of biochars, such as surface area (R2 = 0.975), porosity (R2 = 0.967), surface acidity (R2 = 0.843), and surface basicity (R2 = 0.863). Furthermore, the process that the biochar activates H2O2 was significantly hindered by the surface adsorption of SMT. Our findings provides a new approach for removal of organic contaminants, efficiently utilizing the performance of biochar as the catalyst in H2O2 activation.Acknowledgements
This work was supported by the Program for the National Natural Science Foundation of China (51378190, 51278176, 51408206, 51579098, 51521006), the National Program for Support of Top-Notch Young Professionals of China (2014), the Fundamental Research Funds for the Central Universities, the Program for New Century Excellent Talents in University (NCET-13-0186), the Program for Changjiang Scholars and Innovative Research Team in University (IRT-13R17), Scientific Research Fund of Hunan Provincial Education Department (No. 521293050).References
- M. Ahmad, A. U. Rajapaksha, J. E. Lim, M. Zhang, N. Bolan, D. Mohan, M. Vithanage, S. S. Lee and Y. S. Ok, Chemosphere, 2014, 99, 19–33 CrossRef CAS PubMed.
- J. Lehmann, J. Gaunt and M. Rondon, Mitigation and Adaptation Strategies for Global Change, 2006, 11, 395–419 CrossRef.
- E. Marris, Nature, 2006, 442, 624–626 CrossRef CAS PubMed.
- X. Tan, Y. Liu, G. Zeng, X. Wang, X. Hu, Y. Gu and Z. Yang, Chemosphere, 2015, 125, 70–85 CrossRef CAS PubMed.
- Y. Sun, B. Gao, Y. Yao, J. Fang, M. Zhang, Y. Zhou, H. Chen and L. Yang, Chem. Eng. J., 2014, 240, 574–578 CrossRef CAS.
- D. Kołodyńska, R. Wnętrzak, J. Leahy, M. Hayes, W. Kwapiński and Z. Hubicki, Chem. Eng. J., 2012, 197, 295–305 CrossRef.
- S. G. Huling, E. Kan, C. Caldwell and S. Park, J. Hazard. Mater., 2012, 205, 55–62 CrossRef PubMed.
- N. H. Ince and I. G. Apikyan, Water Res., 2000, 34, 4169–4176 CrossRef CAS.
- P. Serp and J. L. Figueiredo, Carbon materials for catalysis, Wiley Online Library, 2009 Search PubMed.
- S. G. Huling, P. K. Jones and T. R. Lee, Environ. Sci. Technol., 2007, 41, 4090–4096 CrossRef CAS PubMed.
- G. Fang, J. Gao, C. Liu, D. D. Dionysiou, Y. Wang and D. Zhou, Environ. Sci. Technol., 2014, 48, 1902–1910 CrossRef CAS PubMed.
- G. Fang, C. Liu, J. Gao, D. D. Dionysiou and D. Zhou, Environ. Sci. Technol., 2015, 49, 5645–5653 CrossRef CAS PubMed.
- J. Yan, L. Han, W. Gao, S. Xue and M. Chen, Bioresour. Technol., 2015, 175, 269–274 CrossRef CAS PubMed.
- A. Kappler, M. L. Wuestner, A. Ruecker, J. Harter, M. Halama and S. Behrens, Environ. Sci. Technol. Lett., 2014, 1, 339–344 CrossRef CAS.
- L. Klüpfel, M. Keiluweit, M. Kleber and M. Sander, Environ. Sci. Technol., 2014, 48, 5601–5611 CrossRef PubMed.
- D. D. Dionysiou, M. T. Suidan, I. Baudin and J.-M. Laîné, Appl. Catal., B, 2004, 50, 259–269 CrossRef CAS.
- J. A. Khan, X. He, N. S. Shah, H. M. Khan, E. Hapeshi, D. Fatta-Kassinos and D. D. Dionysiou, Chem. Eng. J., 2014, 252, 393–403 CrossRef CAS.
- T. M. El-Morsi, M. M. Emara, H. M. A. El Bary, A. S. Abd-El-Aziz and K. J. Friesen, Chemosphere, 2002, 47, 343–348 CrossRef CAS PubMed.
- B. A. Smith, A. L. Teel and R. J. Watts, Environ. Sci. Technol., 2004, 38, 5465–5469 CrossRef CAS PubMed.
- K. Sun, K. Ro, M. Guo, J. Novak, H. Mashayekhi and B. Xing, Bioresour. Technol., 2011, 102, 5757–5763 CrossRef CAS PubMed.
- C. Lattao, X. Cao, J. Mao, K. Schmidt-Rohr and J. J. Pignatello, Environ. Sci. Technol., 2014, 48, 4790–4798 CrossRef CAS PubMed.
- M. Uchimiya, L. H. Wartelle, K. T. Klasson, C. A. Fortier and I. M. Lima, J. Agric. Food Chem., 2011, 59, 2501–2510 CrossRef CAS PubMed.
- M. Xie, W. Chen, Z. Xu, S. Zheng and D. Zhu, Environ. Pollut., 2014, 186, 187–194 CrossRef CAS PubMed.
- M. Pérez-Moya, M. Graells, G. Castells, J. Amigó, E. Ortega, G. Buhigas, L. M. Pérez and H. D. Mansilla, Water Res., 2010, 44, 2533–2540 CrossRef PubMed.
- C. Zhang, C. Lai, G. Zeng, D. Huang, C. Yang, Y. Wang, Y. Zhou and M. Cheng, Water Res., 2016, 95, 103–112 CrossRef CAS PubMed.
- Y. Feng, J. Liu, D. Wu, Z. Zhou, Y. Deng, T. Zhang and K. Shih, Chem. Eng. J., 2015, 280, 514–524 CrossRef CAS.
- Y.-q. Gao, N.-y. Gao, Y. Deng, Y.-q. Yang and Y. Ma, Chem. Eng. J., 2012, 195, 248–253 CrossRef.
- D.-L. Huang, G.-M. Zeng, C.-L. Feng, S. Hu, X.-Y. Jiang, L. Tang, F.-F. Su, Y. Zhang, W. Zeng and H.-L. Liu, Environ. Sci. Technol., 2008, 42, 4946–4951 CrossRef CAS PubMed.
- D.-L. Huang, G.-M. Zeng, C.-L. Feng, S. Hu, C. Lai, M.-H. Zhao, F.-F. Su, L. Tang and H.-L. Liu, Bioresour. Technol., 2010, 101, 4062–4067 CrossRef CAS PubMed.
- C. Zhang, L. Liu, G.-M. Zeng, D.-L. Huang, C. Lai, C. Huang, Z. Wei, N.-J. Li, P. Xu and M. Cheng, Biochem. Eng. J., 2014, 91, 149–156 CrossRef CAS.
- D. Wang, W. Zhang and D. Zhou, Environ. Sci. Technol., 2013, 47, 5154–5161 CrossRef CAS PubMed.
- M. Teixidó, J. J. Pignatello, J. L. Beltrán, M. Granados and J. Peccia, Environ. Sci. Technol., 2011, 45, 10020–10027 CrossRef PubMed.
- D. Zhu, S. Kwon and J. J. Pignatello, Environ. Sci. Technol., 2005, 39, 3990–3998 CrossRef CAS PubMed.
- H. Boehm, Carbon, 1994, 32, 759–769 CrossRef CAS.
- C.-H. Cheng and J. Lehmann, Chemosphere, 2009, 75, 1021–1027 CrossRef CAS PubMed.
- A. Standard, ASTM International, West Conshohocken, PA, 2004 Search PubMed.
- Y. Li, T. Zhu, J. Zhao and B. Xu, Environ. Sci. Technol., 2012, 46, 10302–10309 CAS.
- R. S. Ribeiro, A. M. Silva, J. L. Figueiredo, J. L. Faria and H. T. Gomes, Carbon, 2013, 62, 97–108 CrossRef CAS.
- X. Zhao, W. Ouyang, F. Hao, C. Lin, F. Wang, S. Han and X. Geng, Bioresour. Technol., 2013, 147, 338–344 CrossRef CAS PubMed.
- W. Wu, M. Yang, Q. Feng, K. McGrouther, H. Wang, H. Lu and Y. Chen, Biomass Bioenergy, 2012, 47, 268–276 CrossRef CAS.
- J. M. Tascón, Novel carbon adsorbents, Elsevier, 2012 Search PubMed.
- J. Lehmann and S. Joseph, Biochar for environmental management: science, technology and implementation, Routledge, 2015 Search PubMed.
- A. Rey, J. Zazo, J. Casas, A. Bahamonde and J. Rodriguez, Appl. Catal., A, 2011, 402, 146–155 CrossRef CAS.
- R. S. Ribeiro, N. A. Fathy, A. A. Attia, A. M. Silva, J. L. Faria and H. T. Gomes, Chem. Eng. J., 2012, 195, 112–121 CrossRef.
- V. P. Santos, M. F. Pereira, P. Faria and J. J. Órfão, J. Hazard. Mater., 2009, 162, 736–742 CrossRef CAS PubMed.
- R. S. Ribeiro, A. M. Silva, J. L. Figueiredo, J. L. Faria and H. T. Gomes, Appl. Catal., B, 2016, 402(1), 146–155 Search PubMed.
- L. Helsen and A. Hacala, J. Hazard. Mater., 2006, 137, 1438–1452 CrossRef CAS PubMed.
- N. B. Klinghoffer, M. J. Castaldi and A. Nzihou, Fuel, 2015, 157, 37–47 CrossRef CAS.
- Y. Liu, J. Hu and J. Wang, Radiat. Phys. Chem., 2014, 96, 81–87 CrossRef CAS.
- Y. Fan, Y. Ji, D. Kong, J. Lu and Q. Zhou, J. Hazard. Mater., 2015, 300, 39–47 CrossRef CAS PubMed.
- Y. Liu and J. Wang, J. Hazard. Mater., 2013, 250, 99–105 CrossRef PubMed.
- A. G. Trovó, R. F. Nogueira, A. Agüera, A. R. Fernandez-Alba, C. Sirtori and S. Malato, Water Res., 2009, 43, 3922–3931 CrossRef PubMed.
- Z. Guo, F. Zhou, Y. Zhao, C. Zhang, F. Liu, C. Bao and M. Lin, Chem. Eng. J., 2012, 191, 256–262 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |









