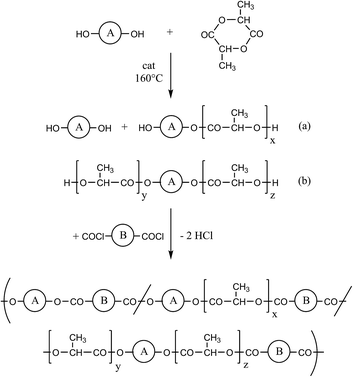
Unsaturated copolyesters of lactide
M. Lahcinia,
S. M. Weidnerb,
J. Oumayamaa,
F. Scheligac and
H. R. Kricheldorf*c
aLaboratory of Organometallic and Macromolecular Chemistry, Faculty, of Sciences, Cadya Ayyad University, Abdelkrim Elkhattati, B.P. 549, Marrakech 4000, Marocco
bBAM, Federal Institute for Material Research and Testing, 1.3 Structure Analysis, Richard Willstätter Strasse 11, D-12489 Berlin, Germany
cInstitut für Technische und Makromolekulare Chemie der Universität, Bundesstr. 45, D-20146 Hamburg, Germany. E-mail: kricheld@chemie.uni-hamburg.de
First published on 23rd September 2016
Abstract
Four classes of unsaturated copolyesters of L-lactide were prepared either from isosorbide or bis(hydroxymethyl)tricyclodecane in combination with fumaric acid or from 1,4-butenediol or 1,4-butynediol with terephthalic acid. All syntheses were performed in such a way that lactide was oligomerized with a diol as the initiator and the resulting oligomers were polycondensed with a dicarboxylic acid dichloride either in a one-pot synthesis or in a two-step procedure. For most copolyesters the SEC measurements gave weight average molecular weights in the range of 30–60 kg mol−1 and dispersities in the range of 4.2–6.2. The MALDI-TOF mass spectra displayed a high content of cycles and indicated an irreversible kinetic course of all polycondensations. Glass-transition temperatures (Tg) above 90 °C were only found for two copolyesters of isosorbide. Addition of bromine to copolyesters of 1,4-butenediol yielded flame retarding biodegradable polymers.
Introduction
With the exception of poly(D,L-lactide) all commercial biodegradable polyesters are crystalline and almost all of them possess low glass-transition temperatures Tg (<0 °C). Their mechanical properties and applications depend on the melting temperature (Tm) and on the extent of crystallinity, or in other words, on the thermal history. This short-coming does not exist in the case of amorphous materials having higher Tg (>90 °C). Hence, numerous widely used engineering plastics are amorphous, for example atactic polystyrene, poly(methyl methacrylate), poly(vinyl chloride), aromatic polycarbonates or poly(ether sulfone)s. Their mechanical properties, notably the heat-distortion temperature, depend on their Tg, which fall into the range of 90–210 °C.In a handful of recent publications, we have reported on syntheses and physical properties of biodegradable copolyesters having Tg values in the range of 80–180 °C.1–5 In three of them syntheses and properties of copolyesters derived from L-lactide and isosorbide were described.1–3 These monomers were selected because they are based on natural resources. Furthermore, isosorbide is known for its tendency to raise the Tg of (co)polyesters compared to α,ώ-alkanediols. Polyesters derived from aliphatic dicarboxylic acids are biodegradable, but possess relatively low Tg (<66 °C),6–9 whereas polyesters of isosorbide and aromatic dicarboxylic acids show high Tg (up to 210 °C), but no biodegradability.10–14
Recently described biodegradable high Tg copolyesters of isosorbide and L-lactide were prepared by a two-step process conducted in an one-pot approach.1–3 The first step consisted in a ring-opening oligomerization (ROP) of lactide initiated by isosorbide. The resulting oligoester diols were condensed with a dicarboxylic acid dichloride in refluxing chlorobenzene (see Scheme 1). This new approach is, of course, too expensive for technical production, but convenient for research purposes, because it requires neither stirring nor vacuum. Moreover, products with weight average molecular weights (Mw) up to 200 kg mol−1 may be obtained.
In this context, the present work served three purposes. Firstly, it should be elucidated if the afore-mentioned synthetic approach can be used for the preparation of unsaturated lactide copolyesters. Unsaturated building blocks are versatile functional groups, which enable a variety of addition reactions and radical grafting or crosslinking. Secondly, it should be found out if unsaturated copolyesters with Tg around or above 50 °C are accessible in this way, so that easy to handle solid materials are obtained. Thirdly, the dispersities of formed copolyesters should be compared with those values predicted by Flory and Odian,15,16 and with much higher values recently found for polyesters prepared by irreversible polycondensations.2–4,14,17
Experimental
Materials
L-Lactide (S-grade) was kindly supplied by Boehringer-Ingelheim AG. It was recrystallized from toluene. Isosorbide 1,4-butynediol and 1,4-butenediol were purchased from Alfa Aesar (Karlsruhe, Germany). Isosorbide was recrystallized from acetone; the other diols were dried over molsieves. 4,8-Bis hydroxymethyl[5.2.10]tricyclodecane (BHMTD, mixtures of isomers) was purchased from Aldrich Co. (Germany) and used as received. Fumaroyl chloride and terephthaloyl chloride were also purchased from Aldrich Co. and used as received. Chlorobenzene, dichloromethane and tetrachloroethylene were distilled over P4O10. Pyridine was distilled over calcium hydride.Polycondensation in refluxing chlorobenzene
Isosorbide (40 mmol) and lactide (80 mmol) were weight into a 100 mL Erlenmeyer flask and 0.1 mL of a 0.5 M solution of SnOct2 in toluene were injected. The reaction vessel was closed with glass stopper and steel spring and immersed into an oil bath preheated to 160 °C. After 2.5 h the reaction mixture was removed from the oil bath and cooled below 50 °C. A solution of fumaroyl chloride (40.4 mmol) in chlorobenzene (23 mL, containing 25% (v/v) tetrachloroethylene) was added. The Erlenmeyer flask was closed with a reflux condenser equipped with a calcium chloride drying tube and immersed again into the oil bath. After 8 h the viscous syrupy reaction mixture was cooled and diluted with dichloromethane (50 mL). After 2 d a homogeneous solution was obtained which was precipitated into a mixture of ligroin and ethanol (3/1, v/v). The resulting gum-like product was washed with fresh ligroin–ethanol mixture for 1 h and dried in vacuo at 65 °C over a period of 2–3 d. All other experiments listed in Table 1 were performed analogously.| Polymer | Molar ratio diol/lact | Yield/% | Mnc/g mol−1 | Mwc/g mol−1 | Ð | Tgd/°C |
|---|---|---|---|---|---|---|
| a Fumaroyl chloride with exact stoichiometry.b 1 mol% excess of fumaroyl chloride.c SEC in chloroform including all oligomers and calibrated with polystyrene.d DSC 10 K min−1, 2nd heating.e Data taken from ref. 1. | ||||||
| 1ae | 1/0 | — | — | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
113.0 | |
| 1b | 1/0.5 | 99.0 | 8500 | 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
4.6 | 100.5 |
| 1c | 1/1 | 99.5 | 9000 | 61![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
6.8 | 91.0 |
| 1d | 1/2 | 99.0 | 8100 | 43![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
5.3 | 76.5 |
| 1e Aa | 1/3 | 98.5 | 6000 | 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
4.9 | 73.0 |
| 1e Bb | 1/3 | 98.0 | 9000 | 39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
4.3 | 73.2 |
| 1f Aa | 1/4 | 97.5 | 8500 | 41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
4.8 | 62.5 |
| 1f Bb | 1/4 | 95.0 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
62![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
5.8 | 65.1 |
The BHMTD diol (25 mmol) was liquefied at 150 °C, weighed into a 100 mL Erlenmeyer flask, and lactide (100 mmol) was added. After injection of 0.1 mL of a 0.5 M SnOct2 solution the polymerization was performed at 160 °C for 2.5 h. After cooling a solution of fumaroyl chloride (25.2 mmol) in chlorobenzene (24 mL, containing 25% (v/v) tetrachloroethylene) was added. The polycondensation was conducted at 160 °C for 8 h. After cooling, the reaction mixture was diluted with dichloromethane (50 mL) and precipitated into a mixture of ligroin and ethanol (3/1, v/v). An overview is shown in Table 2.
| Polymer | Molar ratio diol/lact | Yield/% | Mnc/g mol−1 | Mwc/g mol−1 | Ð | Tgd/°C |
|---|---|---|---|---|---|---|
| a Mixture of chlorobenzene and chloroform (4/1, v/v) at 150 °C.b Mixture of chlorobenzene and tetrachloroethylene (3/1, v/v) at 160 °C.c SEC measurements in chloroform including all oligomers and calibrated with PS.d DSC 10 K min−1, 2nd heating. | ||||||
| 2aa | 1/0.0 | 91.5 | 2400 | 65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
27.0 | 70.0 |
| 2ba | 1/0.5 | 89.0 | 5200 | 29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
5.6 | 60.0 |
| 2cb | 1/1.0 | 87.0 | 6600 | 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
4.3 | 56.6 |
| 2db | 1/2.0 | 88.5 | 7000 | 36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
5.1 | 56.4 |
| 2eb | 1/4.0 | 91.5 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
4.1 | 55.8 |
BHMTD (20 mmol) and lactide (40 mmol) were polymerized with SnOct2 (0.05 mmol) at 160 °C as described above. Molten terephthaloylchloride (10 mmol) was weighed with a hot pipette into a 50 mL Erlenmeyer flask and after crystallization fumaroyl chloride (10 mmol) was added. The acid chlorides were dissolved in chlorobenzene (35 mL) and added to the oligolactides. After warming and shaking for a few minutes a homogeneous solution was obtained which was heated to 160 °C for 8 h. Afterwards the reaction mixture was diluted with dichloromethane (30 mL) and one part of this solution was precipitated into methanol (500 mL). The residual part was concentrated in vacuo and dried at 60 °C in vacuo for 2 d. These experiments are listed in Table 3.
| Polymer | Molar ratio | Mna/g mol−1 | Mwa/g mol−1 | Ð | Tgb/°C | |
|---|---|---|---|---|---|---|
| Diol/lact | F/T | |||||
| a SEC measurements in chloroform including all oligomers.b DSC 10 K min−1, 2nd heating. | ||||||
| 1A | 1/2 | 1/3 | 5000 | 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
4.1 | — |
| B | 1/2 | 1/3 | 6200 | 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
3.5 | 68.5 |
| 2A | 1/2 | 1/1 | 5800 | 31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
5.3 | — |
| B | 1/2 | 1/1 | 7600 | 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
4.1 | 63.4 |
| 3A | 1/3 | 3/1 | 5500 | 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
4.8 | — |
| B | 1/3 | 3/1 | 6300 | 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
4.4 | 57.0 |
| 4A | 1/4 | 0/4 | 5100 | 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
4.3 | — |
| B | 1/4 | 0/4 | 6400 | 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
3.9 | 73.0 |
Polycondensations in refluxing xylene
Isosorbide (40 mmol) and L-lactide (80 mmol) were weighed into a 100 mL round bottom flask and 0.1 mL of a 0.5 M solution of SnOct2 in toluene was injected. After polymerization for 2.5 h at 160 °C the reaction mixture was cooled to approx. 100 °C and a solution of fumaroyl chloride (40.5 mmol) in xylene (mixture of isomers, 25 mL) was added. The reaction vessel was equipped with a reflux condenser and placed into an oil bath heated to 160 °C. After 20 h, the viscous solution was diluted with dichloromethane (50 mL) and after homogenization precipitated into ligroin containing 25% (v/v) of ethanol. All other experiments of Table 4 were performed analogously.| Exp. No. | Diol | Molar ratio diol/lact | Yield/% | Mna/g mol−1 | Mwa/g mol−1 | Ð |
|---|---|---|---|---|---|---|
| a SEC measurements in chloroform including all oligomers. | ||||||
| 1 | Isosorbide | 1/1 | 98.5 | 7200 | 37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
5.1 |
| 2 | Isosorbide | 1/2 | 98.0 | 6500 | 31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
4.8 |
| 3 | Isosorbide | 1/3 | 97.0 | 6700 | 3000 | 4.5 |
| 4 | Isosorbide | 1/4 | 96.5 | 7700 | 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
5.2 |
| 5 | BHMTD | 1/2 | 89.5 | 5200 | 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
5.3 |
| 6 | BHMTD | 1/4 | 91.0 | 7200 | 3100 | 4.3 |
Polycondensations in dichloromethane with addition of pyridine
cis-1,4-Butenediol (40 mmol) and lactide (80 mmol) were weighed into a 50 mL Erlenmeyer flask and 1 mL of a 0.5 M solution of SnOct2 in toluene was injected. After polymerization for 2.5 h at 160 °C the reaction mixture was cooled to room temperature and dissolved in dry dichloromethane (40 mL). This solution was poured into a 100 mL Erlenmeyer flask containing terephthaloyl chloride (40.2 mmol). After dilution with dichloromethane (30 mL), pyridine (100 mmol) was added dropwise with stirring. The reaction mixture was stored at 22–23 °C for 2 d and precipitated into cold methanol (5 °C, 1 L). The precipitated copolyester was dried at 30 °C for 2 d in vacuo (the temperature was initially kept low to avoid methanolytic cleavage of lactide bonds), and afterwards for 1 d at 65 °C. All other copolymerizations listed in Table 6 were performed analogously.| Polymer | Molar ratio diol/lact | Yield/% | Mna/g mol−1 | Mwa/g mol−1 | Ð | Tgb/°C |
|---|---|---|---|---|---|---|
| a SEC measurements in chloroform including all oligomers.b DSC 10 K min−1, 2nd heating. | ||||||
| 4b | 1/0.5 | 98.5 | 4600 | 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
6.1 | 69.5 |
| 4c | 1/1.0 | 98.0 | 6000 | 37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
6.5 | 67.8 |
| 4d | 1/2.0 | 97.0 | 7800 | 53![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
6.8 | 65.2 |
| 4e | 1/3.0 | 96.0 | 7800 | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
6.3 | 62.7 |
| 4f | 1/4.0 | 97.5 | 8600 | 61![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
7.1 | 61.9 |
| Polymer | Molar ratio diol/lact | Yield/% | Mna/g mol−1 | Mwa/g mol−1 | Ð | Tgb/°C |
|---|---|---|---|---|---|---|
| a SEC measurements in chloroform including all oligomers.b DSC 10 K min−1, 2nd heating. | ||||||
| 5b | 1/0.5 | 91.5 | 3000 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
4.1 | 40.5 |
| 5c | 1/1.0 | 86.0 | 2900 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
4.3 | 40.3 |
| 5d | 1/2.0 | 81.5 | 2900 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
4.2 | 40.5 |
| 5e | 1/3.0 | 90.0 | 5000 | 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
5.6 | 48.0 |
| 5f | 1/4.0 | 91.0 | 6100 | 37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
6.1 | 50.0 |
Copolyester 5f of Table 6 (10 mmol) was dissolved in dichloromethane (45 mL) and added at once to bromine (10 mmol) pre-weighed in a 50 mL Erlenmeyer flask. The closed reaction vessel was stored at 22–23 °C for 3 d. Afterwards the yellowish solution was precipitated into 600 mL of a ligroin/ethanol mixture (3/1 v/v). The precipitated polyester was dried at 30 °C in vacuum for 1 d and at 60 °C for another day. All other experiments (listed in Table 7) were performed analogously, whereas in the B-experiments a 10 molar excess of bromine was used.
| Polymera | Mol. ratio diol/lact | Yield/% | Bromine calc./exp. | Mnb/g mol−1 | Mwb/g mol−1 | Tg/°C |
|---|---|---|---|---|---|---|
| a Annotation d, e and f corresponds to polymers listed in Table 6. (A) – Experiments were performed with stoichiometric amounts of bromine and (B) – experiments with 10 mol% excess of bromine.b SEC measurements in chloroform including all oligomers. | ||||||
| 6d (B) | 1/2 | 83 | 24.8/24.2 | 3000 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
40.5 |
| 6e (A) | 1/3 | 84 | 20.5/20.1 | 2900 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
40.3 |
| 6e (B) | 1/3 | 91 | 20.5/20.6 | 2900 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
40.5 |
| 6f (A) | 1/4 | 86 | 17.4/17.0 | 5000 | 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
48.0 |
| 6f (B) | 1/4 | 93 | 17.4/17.6 | 6100 | 37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
50.0 |
Measurements
The 400 MHz 1H NMR spectra were recorded on a Bruker Avance 400 FT spectrometer in 5 mm o.d. sample tubes with CDCl3 as solvent and TMS as internal standard. The DSC measurements were conducted with a Mettler-Toledo DSC 821. A heating rate of 10 K min−1 was used. For Tg determination the 2nd heating trace was evaluated using the Stare-Software 9.00. The MALDI-TOF mass spectra were recorded using a Bruker Autoflex III mass spectrometer in the linear mode using an acceleration voltage of 20 kV. The sample spots were prepared by dropping premixed sample/salt/matrix solutions (10/2/50 v/v/v) containing analyte (2 mg mL−1), potassium trifluoroacetate as salt (2 mg mL−1) and trans-2-[3-(4-tert-butylphenyl)-2-methyl-2-propenylidene]malononitrile (DCTB) 10 mg mL−1 as matrix. The SEC measurements were performed in chloroform at 30 °C on a home-made apparatus equipped with a Merck-Hitachi L-7100 pump, an ERC 7510 RI-detector and a Merck L-5025 column thermostat. Two PSS mixed bed columns (B 10 l) were used, and the WinGPC Unity Software (PSS Mainz, Germany) was applied for evaluation of the elution curves. The sample concentration was 5 mg mL−1.Results
Synthetic methods
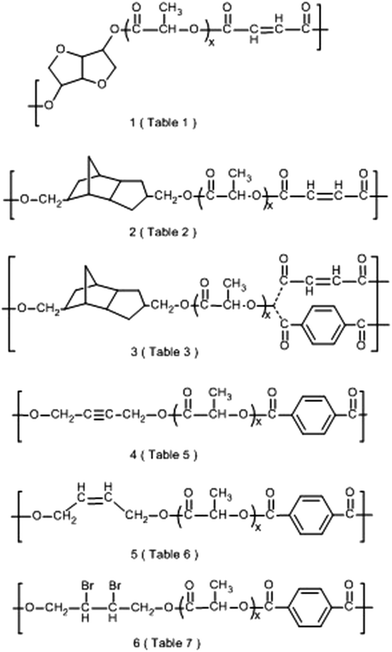
Two types of the polymerization process outlined in Scheme 1 were studied. Both synthetic routes have in common that oligolactides were prepared by ROP of a lactide bulk initiated with a diol and catalyzed with SnOct2. In type (A) these oligolactides were polycondensed with fumaroyl chloride in refluxing chlorobenzene without addition of a HCl acceptor or a catalyst. In version (B) the oligolactides were cooled to room temperature, and a dicarboxylic acid dichloride was added. These reactants were dissolved in dichloromethane and the polycondensation was promoted by dropwise addition of pyridine. It turned out that version (A) was suited for polycondensation of fumaroyl chloride (structures (1) and (2) in Scheme 2), whereas in version (B) fumaroyl chloride decomposes upon addition of pyridine. In contrast version (A) proved useless for preparation of copolyesters (5) derived from cis-1,4-butenediol, because polycondensation of the lactide oligomers with terephthaloyl chloride in refluxing chlorobenzene yielded black tars. However, copolyesters (5) were accessible by version (B), since terephthaloyl chloride did not undergo side reactions with pyridine. 1,4-Butynediol is compatible with both versions, but higher molecular weights were expected from polycondensations according to version (A). Therefore, copolyesters (4) were prepared according to version (A). At this point it should be mentioned that the signals of unreacted diols were detectable in the 1H NMR spectra of the polymerization products (prior to their polycondensation) when the diol/lactide ratios were relatively high (1/05, 1/1.0 and 1/1/2.0). Therefore, the structure of the resulting copolyesters is based on three different diol units (original diol, (a) and (b)) as illustrated in Scheme 1. Hence, the formulas outlined in Scheme 2 are a simplification.Copolyesters derived from fumaric acid
For the first series of copolyesters isosorbide was used as diol in analogy to previous studies, where this diol was combined with aromatic dicarboxylic acid dichlorides. Isosorbide was selected because it is a commercially available monomer based on natural resources, and it favours the formation of products with high glass transition temperatures. The oligomerization of lactide with isosorbide as initiator was performed at those conditions previously described for similar copolyesters.2,3 The subsequent polycondensation with fumaroyl chloride was conducted in a 3/1 (v/v) mixture of chlorobenzene and tetrachloroethylene. The latter solvent was added because its high specific weight prevents that parts of the copolyester separates on the bottom of the reaction vessel. In contrast the upper layer of the reaction mixture maintains a low concentration of reactants, because it is permanently diluted by the refluxing chlorobenzene. An additional benefit of this mixture is the light yellowish colour of the polyesters, instead of the brownish colour typical for most copolyester prepared in neat chlorobenzene. At lactide/diol ratios >0.5/1 soluble copolyesters were obtained, whereas at a ratio of 0.5/1, which is identical with the highest concentration of fumaroyl units in the reaction mixture, gelation was observed. An attempt to synthesize the homopolyester of isosorbide and fumaric acid also resulted in gelation. Furthermore, in polycondensations with a high concentration of fumaroyl chloride (No. 1, Table 2) gelation occurred when isosorbide was substituted by another diol (structure (2), Table 2). Therefore, it may safely be concluded that the fumaroyl structure (and not isosorbide) is responsible for crosslinking.Another problem related to the properties of fumaroyl chloride is its relatively low boiling point of 156 °C, which is not much higher than that of chlorobenzene, and its consequences for the stoichiometry of the reaction mixture caused by a slight loss due to the evolution of HCl. The syntheses of copolyesters 1e and 1f (Table 1) that were studied first were conducted with perfect stoichiometry (A), and with an excess (1 mol%) of fumaroyl chloride (B). The SEC measurements clearly demonstrated that a slight excess of fumaroyl chloride favoured higher molar masses. Therefore all other polycondensations listed in Tables 1 and 2 were conducted with a one molar excess of fumaroyl chloride.
The virgin reaction mixtures were diluted with dichloromethane and precipitated into a mixture of ligroin and ethanol (3/1 v/v) to remove both the catalyst and chlorobenzene. This non-solvent mixture was preferred to methanol, because residual methanol may cleave lactide bonds while drying the products. Despite precipitation, dispersities (Ð) in the range of 4.3–6.8 were found when all oligomers were included in the calculation of number (Mn) and weight average molecular weight (Mw). As demonstrated in previous publications, such high dispersities are quite normal for irreversible polycondensations at high monomer concentrations.2–4,14,17 The concentrations used in this work were only by a factor of 2–3 lower than those of polycondensations in bulk. The mathematical equations developed by Flory or Odian for the calculation of dispersities predict a maximum dispersity of 2 for polycondensations in bulk.15,16 However, those equations were designed for a theory which ignores the formation of cyclic oligomers and polymers. High molar fractions of cycles were indeed found when the copolyesters prepared in this work were subjected to MALDI TOF mass spectrometry (see figures and discussion below). With increasing molar fraction of lactide the cycles contained more and more lactide units. However, cycles exclusively consisting of lactide units were absent what proves that equilibration reactions did not occur. In agreement with previous observations, the polycondensations performed in this work are irreversible polycondensations.2,3
The 1H NMR spectra confirmed a product composition according to the feed ratio. The fumaroyl protons showed a singlet signal for samples rich in lactide (1e and 1f), because most fumaroyl units were flanked by two lactic acid units. When the content of lactide decreased the signal pattern became more complex, because part of the fumaroyl units were attached to isosorbide, whose endo or exo-OH groups caused a slightly different chemical shift of the fumaroyl protons.
For a second series of polycondensations BHMTD was used as diol (structure (2), Table 2). This diol was selected due to its commercial use and because higher Tg polymers were expected compared to polyesters of α,ω-alkanediols. The polycondensations listed in Table 2 revealed the same trends already detected for the first series. The mass spectra again indicated large fractions of cyclics below 6000 Da and the 1H NMR spectra confirmed a composition in agreement with the feed ratio. The fumaroyl protons display a sharp singlet signal at 6.95 ppm when flanked by two lactic acid units and a singlet signal at 6.85 ppm for the homopolyester (2a). The formation of products with relatively low molar masses was against our expectations.
Three more copolyesters based on BHMTD were prepared in such a way that mixtures of fumaroyl chloride and terephthaloyl chloride were used (Table 3). These experiments served three purposes. Firstly, they should illustrate the flexibility of the synthetic approach with regard to the composition of the copolyesters. Secondly, the incorporation of terephthaloyl units should raise the Tg. Thirdly, the influence of precipitation in a non-solvent (methanol) on molecular weights and dispersities should be elucidated. The results listed in Table 3 demonstrate that precipitation enhanced the Mn values by 15–25% and lowered the dispersities by 15–20%. Nonetheless, the molar masses were relatively low compared to the copolyesters of Table 2, most likely because stoichiometric amounts of acid chlorides were used. The Tg values are discussed below.
Since chlorinated solvents are environmentally harmful, several polycondensations were repeated in xylene. The results of these experiments were compiled in Table 4. It was observed that the oligolactides prepared with isosorbide and low lactide contents (1/1 and 1/2) did not dissolve when the solution of fumaroyl chloride in xylene was added. Nonetheless, the polycondensations proceeded, but the resulting copolyester remained insoluble in xylene. All other reaction mixtures listed in Table 4 were homogeneous. The SEC measurements evidenced that the molecular weights of all copolyesters prepared in xylene were lower than those isolated from halogenated solvent mixtures (Tables 1 and 2).
Copolyesters derived from unsaturated diols
An alternative approach to the synthesis of unsaturated copolyesters of lactide consists in the incorporation of unsaturated diols instead of unsaturated dicarboxylic acids. In this work 1,4-butynediol and 1,4-butenediol were used as starting materials because both diols are inexpensive commercial chemicals technically produced from acetylene and formaldehyde. Terephthaloyl chloride was used as reaction partner, because it is more reactive than isophthaloyl chloride. Furthermore, the Tg values of copolyesters derived from terephthalic acid were higher than those derived from isophthalic acid. They should also be higher than those of polyesters of aliphatic dicarboxylic acids. Since numerous polycondensations of oligolactides and dicarboxylic acids in refluxing chlorobenzene had successfully proven this approach, it was applied for the preparation of copolyesters based on butynediol and butenediol too.As demonstrated by the results compiled in Table 5 this method was again successful when 1,4-butynediol was used as comonomer. Because of its low volatility, terephthaloyl chloride was used in perfectly stoichiometric quantities. Yellowish transparent copolyesters with Mw values up to 61![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1 were obtained. In contrast to that, homopolyester 4a precipitated from the reaction mixture as a brownish crystalline powder and proved to be insoluble in all common solvents, so that SEC and NMR measurements were not feasible.
000 g mol−1 were obtained. In contrast to that, homopolyester 4a precipitated from the reaction mixture as a brownish crystalline powder and proved to be insoluble in all common solvents, so that SEC and NMR measurements were not feasible.
The 1H NMR spectra of copolyesters 4b–e and 5b–e proved again that the composition agreed with the feed ratio. The terephthalic acid protons showed a singlet signal at 8.14 ppm regardless of the composition. Characteristic for these copolyesters was a quadruplet signal around 5.38 ppm originating from those lactic acid units directly attached to terephthalic acid, whereas a broad CH signal of all other lactic acid units appears around 5.21 ppm. The MALDI-TOF mass spectra indicated formation of a large number of cyclic oligomers and polymers (s. below).
When cis-1,4-butenediol was used as initiator for the oligomerization of the lactide, the resulting oligoesters were obtained as expected as yellowish transparent viscous liquids. However, their polycondensation with terephthaloyl chloride in refluxing chlorobenzene/tetrachloroethylene yielded black tars. Therefore, the method was modified and the polycondensation step was conducted as a separate step at room temperature in dichloromethane. Pyridine was added dropwise as HCL acceptor and catalyst. Terephthaloyl chloride was added in an excess of 0.5 mol%. However, the molar masses obtained by this method were lower than those obtained before. Due to the formation of pyridine hydrochloride precipitation of the reaction mixtures into ligroin/ethanol mixtures was not feasible and, therefore, these reaction products were precipitated into methanol. As demonstrated by the data compiled in Table 5, satisfactory molecular weights were only obtained for the copolyester with higher lactide contents (5f and 5f). Repetition of the syntheses of 5b and 5e by another member of our research group gave quite similar results. An explanation, why the molecular weight values of 5b–5d were considerably lower than those of 5e and 5f, cannot be given at this time. Homopolyester 5a was synthesized in the same way. Its precipitation resulted in a crystalline material. Thus, the work-up procedure had to be modified. This homopolyester was insoluble in THF and chloroform, so that SEC measurements were not performed.
Modification of unsaturated copolyesters
Since nothing seems to be known about the modification of unsaturated lactide copolyesters, two kinds of modification reactions were performed to illustrate the usefulness of double bonds as functional groups in this polymer class. Radical crosslinking of unsaturated copolyesters of isosorbide was reported by Braun and Bergmann.9 These authors initially prepared copolyesters of mixtures of fumaroyl chloride and sebacoyl chloride. Concentrated solutions of those copolyesters in methyl methacrylate were subjected to benzoyl peroxide initiated radical polymerizations at 80 °C. In this work two solutions of copolyesters 1d and 2c (5 and 10 weight%) in methyl methacrylate were prepared and polymerized with benzoyl peroxide as initiator at a bath temperature of 90 °C. In both cases crosslinked materials were obtained.In contrast to the double bond of fumaric acid, the double bond of cis-butenediol has a relatively high electron density and is, thus, suited for both radical and electrophilic addition reactions. In this work addition of bromine onto the copolyesters 5c, 5d and 5e was studied. These brominations were performed in dichloromethane at 22–23 °C without addition of a catalyst. With samples 5d and 5e two kinds of experiments were conducted, namely addition with a stoichiometric amount of bromine (labeled A in Table 7) and additions with a 10 mol% excess of bromine (labeled B in Table 7). The 1H NMR spectra indicated that with a stoichiometric amount of bromine (A) 2–4% of the double bonds remained unchanged, whereas all butene units were brominated using an excess of bromine (B). The elemental analyses confirmed that the B-samples contained a slightly higher percentage of bromine. Regardless of molecular weight and perfection of the bromine addition, these highly brominated copolyesters of lactide may serve as flame-retardant additives.
MALDI-TOF mass spectrometry
The MALDI TOF mass spectrometry of the homopolyester 2a revealed a high content of cycles in the mass range below m/z 5000 as illustrated in Fig. 1. This result indicated that the stoichiometry was not far from the ideal ratio despite the volatility of fumaroyl chloride and that the conversion was high, certainly above 99%. In contrast the MALDI TOF mass spectra of the homopolyesters 4a and 5a displayed lower fractions of cycles and mainly peaks of linear chains (Fig. 2). Since these homopolyesters (4a and 5a) precipitated from the reaction mixture at moderate conversion, it was not surprising that the fraction of cycles (which increases with the conversion) was relatively low. The mass spectra of the copolyesters 1a–1f and 2a–2f were quite similar and displayed several populations of cyclic copolyesters as exemplarily illustrated for 1f in Fig. 3.However, the mass spectra of the copolyester of butynediol (4b–4e) displayed below m/z 2500 a large fraction of cycles containing one butynediol terephthalate unit and numerous lactide units (C1,y in Fig. 4). At higher masses cycles containing two or three butynediol terephthalate units (C2,y and C3,y) were also detectable (see inset in Fig. 4). In addition to the cycles, peaks of linear chains could also be detected at higher masses. As shown in Fig. 4, linear chains having one CH2OH/CO2H end group (La), two diol end groups (Lb) and two terephthalate end groups (Lc) were found. The mass spectrum of 4f (with the largest fraction of lactide) exclusively displays peaks of cycles containing one diol terephthalate unit up to masses around m/z 6000 (Fig. 5). The mass spectra of the copolyesters 5e and 5f were quite analogous to those of 4e and 4f.
In summary, the high fraction of cycles found in the mass spectra of all copolyesters allow for four important inferences. First, high conversions were achieved. Second, the co-monomer ratios were not far from the ideal stoichiometry. Third, cyclization reactions made a substantial contribution to the limitation of the chain growth and fourth, side reactions were not detectable. This conclusion is not trivial considering the high temperature and the high concentration of HCL in the beginning of the polycondensation.
Size exclusion chromatography (SEC)
The SEC measurements of all copolyesters of this work were conducted in chloroform and calibrated with polystyrene. It is well known that this calibration overestimates the real molar masses of unsubstituted aliphatic polyesters, such as poly(e-caprolactone), or methyl substituted polyesters, such as polylactides, by 50–80% corresponding to correction factors in the range of 0.55–0.67. However, an incorporation of isosorbide and aromatic monomers reduces the extent of overestimation, and for poly(alkylene isophthalate)s correction factors around 0.72 were recently reported.14Hence, for the copolyesters of this work, correction factors in the range of 0.7–0.8 could be expected. The Mn and Mw values listed in Tables 1–6 were calculated for masses above 300 g mol−1, so that all oligomers were included. As discussed previously, operators of SEC apparatus, who are not familiar with polycondensation theory, frequently evaluate the elution curves in such a way that the calculation of Mn and Mw begins at the minimum before the main peak ignoring the majority of oligomers.
Fig. 6 presents the SEC curve of copolyester 4f, which is typical for the elution curves of all copolyesters having Mw values around or above 30 kg mol−1. Characteristic for these curves is a weak maximum of the cyclic oligomers between 700 and 1000 g mol−1 followed by a shallow minimum around 1300–1600 g mol−1. The calculation of molar masses including the oligomer region (starting at 300 g mol−1, left dotted line in Fig. 1) yielded Mn, Mw and Ð values of 8600 g mol−1, 61![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1 and 7.1 (see Table 5). Yet, when the evaluation began at 1600 g mol−1 (right dotted line) values of 21
000 g mol−1 and 7.1 (see Table 5). Yet, when the evaluation began at 1600 g mol−1 (right dotted line) values of 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1, 64
000 g mol−1, 64![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1 and 3.0 are calculated. Mn is now more than 100% higher than the correct value and the dispersity Ð is more than halved. Thus, dispersities of polycondensates below 2.5 either indicate intensive fractionation of the polymer or (more likely) an incorrect evaluation of the SEC measurements. For the vast majority of copolyesters prepared by the “chlorobenzene method” Mw values in the range of 28–62 kg mol−1 were found. These molecular weights are not particularly high but agree with Mw values of most commercial polycondensates.
000 g mol−1 and 3.0 are calculated. Mn is now more than 100% higher than the correct value and the dispersity Ð is more than halved. Thus, dispersities of polycondensates below 2.5 either indicate intensive fractionation of the polymer or (more likely) an incorrect evaluation of the SEC measurements. For the vast majority of copolyesters prepared by the “chlorobenzene method” Mw values in the range of 28–62 kg mol−1 were found. These molecular weights are not particularly high but agree with Mw values of most commercial polycondensates.
 | ||
| Fig. 6 SEC curve of copolyester 4f. The dotted lines indicate the starting points used for the calculation of Mn and Mw. | ||
Differential scanning calorimetry (DSC)
All polyester samples listed in Tables 1–6 were subject of DSC measurements (see Experimental). In previous studies dealing with copolyesters of lactide, isosorbide and various aromatic dicarboxylic acids the Tg values paralleled the molar fraction of isosorbide. However, the Tg values found for the analogous copolyesters of fumaric acid (1b–e, Table 1) do not show such a linear correlation. The Tg values of 1d–f are rather low followed by a steep increase at isosorbide/lactide ratios of 1/1 and 1/0.5. This pattern also disagrees with the Gordon–Taylor equation which correlates Tg with the weight fraction of comonomers. For the homopolyester 1a Braun and Bergmann reported a Tg of 113 °C. However, their polyester showed a low molar mass. For samples of 1a with higher molar masses Tg values between 115–120 °C may be expected. Therefore, copolymers with a composition close to the structure of the homopolyester may have Tg values above 90 °C, as demonstrated by samples 1b and 1c, which were the only examples of unsaturated copolylactides with Tg values above 90 °C in this work.The copolyesters of BHMTD also showed an unusual tendency. The Tg values of all four copolyesters (2b–d) were nearly identical and about 10 °C below the Tg of poly(L-lactide). These results together with the relatively low Tg of the homopolyester 2a demonstrate that BHMTD is inferior to isosorbide, when high Tg values are desired. Somewhat surprising is the finding that Tg of the “polyterephthalate” (4B in Table 3) is only a few degrees higher than that of the “polyfumarate” 2a (Table 2). The logic consequence of this finding is that substitution of fumarate by terephthalate units has only little influence on the Tg. From poly(1,4-butanediol terephthalate) a Tg around 45 °C is known. Hence, it was predictable that the Tg values of copolyesters derived from the rigid 1,4-butynediol will be slightly higher, which was confirmed by the data in Table 4. The Tg values slightly increase with the molar fraction of butynediol. Unfortunately, the DSC trace of the highly crystalline homopolyester 4a did not display a Tg step. Lower Tg values were found for the copolyesters of 1,4-butene diol (Table 5). Thus, it is quite normal that the Tg increases towards the value of neat poly(L-lactide) with increasing amounts of lactide (5e). The addition of bromine raises the Tg values by approximately 20–25 °C, as demonstrated by products 6d–e (Table 6). Since all copolyesters in this work have Tg values around or above 40 °C, they represent easy to handle solids when dry or sticky syrups when containing small amounts of solvent.
Conclusions
The results of this work demonstrate that the previously elaborated synthetic method for the preparation of saturated lactide copolyesters is also useful for the preparation of various types of unsaturated lactide copolyesters. However, when unsaturated diols are used, the relatively low chemical stability of cis-1,4-butenediol requires a modification of the synthetic approach. Instead of a one-pot procedure at high temperature, polycondensation of the oligolactides in a separate step at 20–30 °C is advantageous. For most copolyesters satisfactory molecular weights with Mw values in the range of 28–62 kg mol−1 were achieved. The MALDI-TOF mass spectra revealed that large number fractions of cyclic copolyesters were obtained. This finding allows for the inference that cyclization made a significant contribution to the limitation of chain growth. The C–C double bonds may act as functional groups allowing for radical and electrophilic addition reactions without significant cleavage of lactide bonds. Therefore, these unsaturated copolyesters may be subject to a variety of modifications. However, the incorporation of relatively flexible unsaturated building blocks makes it difficult to achieve glass-transition temperatures above 90 °C.Acknowledgements
M. Lahcini thanks A. v. Humboldt Foundation for a fellowship. All authors thank Mrs R. Laging (BAM, Berlin) for the SEC measurements and Prof. G. Luinstra (TMC, Hamburg) for financial support.Notes and references
- Z. C. Zhang, H. R. Kricheldorf and C. Friedrich, Macromol. Rapid Commun., 2015, 36, 262 CrossRef CAS PubMed.
- H. R. Kricheldorf and S. M. Weidner, Macromol. Chem. Phys., 2013, 214, 726 CrossRef CAS.
- H. R. Kricheldorf and S. M. Weidner, Eur. Polym. J., 2013, 49, 2293 CrossRef CAS.
- S. Chatti, S. M. Weidner, A. Fildier and H. R. Kricheldorf, J. Polym. Sci., Part A: Polym. Chem., 2013, 51, 2464 CrossRef CAS.
- M. Garaleh, T. Yashiro, H. R. Kricheldorf, P. Simon and S. Chatti, Macromol. Chem. Phys., 2010, 211, 1206 CrossRef CAS.
- M. Okada, K. Tsunoda, K. Tachikawa and K. Aoi, J. Appl. Polym. Sci., 2000, 77, 338 CrossRef CAS.
- M. Okada, Y. Okada, A. Tao and K. Aoi, J. Appl. Polym. Sci., 1996, 62, 2257 CrossRef CAS.
- M. Okada, Y. Okada and K. Aoi, J. Polym. Sci., Part A: Polym. Chem., 1995, 33, 2813 CrossRef CAS.
- D. Braun and M. Bergmann, J. Prakt. Chem./Chem.-Ztg., 1992, 334, 298 CrossRef CAS.
- R. Storbeck and M. Ballauff, J. Appl. Polym. Sci., 1996, 59, 1199 CrossRef CAS.
- R. Storbeck, M. Rehahn and M. Ballauff, Makromol. Chem., 1993, 194, 53 CrossRef CAS.
- R. Storbeck and M. Ballauff, Polymer, 1993, 34, 5003 CrossRef CAS.
- J. Thiem and H. Luders, Polym. Bull., 1984, 11, 365 CrossRef CAS.
- H. R. Kricheldorf, S. M. Weidner and F. Scheliga, J. Polym. Sci., Part A: Polym. Chem., 2016, 54, 197 CrossRef.
- P. J. Flory, Principles of Polymer Chemistry, Cornell University Press, 1953 Search PubMed.
- G. Odian, Principles of Polymerization, Wiley Interscience, New York, 4th edn, 2004 Search PubMed.
- H. R. Kricheldorf, S. M. Weidner and M. Lahcini, Macromol. Chem. Phys., 2015, 216, 2095 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |