Compatibility studies between an amphiphilic pH-sensitive polymer and hydrophobic drug using multiscale simulations
Yan Wangab,
Jia Wei Renc,
Can Yang Zhangd,
Meng Chan Hea,
Zhi Min Wu*b and
Xin Dong Guo*a
aBeijing Laboratory of Biomedical Materials, College of Materials Science and Engineering, Beijing University of Chemical Technology, Beijing, 100029, P. R. China. E-mail: xdguo@buct.edu.cn
bSchool of Chemical Engineering, Xiangtan University, Xiangtan 411105, P. R. China. E-mail: xdwuzm@xtu.edu.cn
cNorth China Electric Power University Hospital, Beijing 102206, China
dDepartment of Pharmaceutical Sciences, College of Pharmacy, Washington State University, Spokane, Washington 99210, USA
First published on 12th October 2016
Abstract
The compatibility of an amphiphilic pH-sensitive polymer (docosahexaenoic acid–histidine–lysine, DHA–HisXLys10) and hydrophobic drug (doxorubicin, DOX) was investigated using multiscale simulations at different pH conditions, including Blends and dissipative particle dynamics simulations. Some important elements obtained from the computer simulations were analyzed, such as Flory–Huggins interaction parameters, binding energy distributions, phase diagrams and radius distribution function. In conclusion, the pH values and the number of pH-sensitive segments (histidine) significantly influence the compatibility of DHA–HisXLys10 and DOX, resulting in different drug loading capacity and system structural stability. According to the simulation results, the compatibility of the systems at pH > 6.0 is better than that at pH < 6.0. Moreover, when the number of histidine residues is 10 or 15, the compatibility is best at pH > 6.0. Using DPD simulation, the compatibility of DHA–HisXLys10 (X = 10 or 15) and DOX is optimal when the pH is higher than 6.0, which falls in line with the results obtained from Blends simulation. Overall, when the number of histidine residues is 10 or 15 and the pH is larger than 6.0, DOX and DHA–HisXLys10 have better compatibility. So it is obvious that polymeric micelles self-assembled from DHA–His10Lys10/DHA–His15Lys10 as an ideal drug carrier have higher drug-loading capacity and more excellent stability. This work has demonstrated that multiscale simulations could be a powerful method to investigate the compatibility between polymers and drugs.
1. Introduction
In recent years, increasing attention has been paid to cancer which threatens human’s health and lives severely.1 Many anticancer drugs have high activity in vitro, but their applications in the clinic are widely hindered because of inferior water-solubility, low bioavailability and serious side effects, etc.2,3 Therefore, in order to overcome these shortcomings, many different kinds of drug delivery systems, such as liposomes,4 polymeric micelles5 and nanoparticles,6 have been developed in recent years.7,8 Notably, a host of polymeric micelles self-assembled from amphiphilic copolymers with typical core–shell structure are commonly used as potential drug vectors.9,10 Hydrophobic drugs could be entrapped in the micellar core by a hydrophobic interaction. A polymeric micelle is a thermodynamically and dynamically stable system, which shows many good performances and unique properties, such as high drug loading capacity, low toxicity and side effects, good biocompatibility and biodegradation, etc.11–13 For these reasons, developing effective polymeric micelles to be used as hydrophobic anticancer drug delivery vectors is a research hotspot now.12,14 The pH value is generally 7.4 in the normal tissues, but there is an acidic environment in the endosomes/lysosomes in tumor tissue.15,16 Therefore, pH-sensitive polymeric micelles are one of most effective and ideal drug delivery systems, resulting from smart and effective targeted drug delivery and sustained controlled drug release.17–20A potential and successful pH-sensitive polymeric micelle applied in practice as a drug delivery vector should satisfy the following conditions at least: (i) exhibit high drug loading capacity and ideal encapsulation efficiency, in order to enhance the drug therapeutic efficacy; (ii) release the drug in the targeted sites efficiently rather than in normal ones, in order to reduce the side effects; (iii) show high stability, good biocompatibility and be barely cytotoxic. There are many factors, such as structure of the amphiphilic polymer, physicochemical properties of the drugs, and so on, affecting the performance of a drug-loading system. Among them, compatibility between vector and drug, which directly affects the system stability, drug-loading capacity and drug release kinetics, is the key element.21–23 Therefore, in order to obtain potential and successful polymeric micelles, the study of the compatibility between polymers and drugs is imperative.
Quite a few works have been performed to research compatibility using different methods. Vaghani et al.24 studied the compatibility of clarithromycin in hydrogels composed of cross-linking chitosan and PVP by Fourier transform infrared (FTIR) spectroscopic analysis, differential scanning calorimetry (DSC) and powder X-ray diffraction (p-XRD) study. They confirmed the stability of clarithromycin in the hydrogels. Allen’s group21 predicted polymer–drug compatibility by physicochemical analyses of polymer–drug pairs and comparing the difference in total and partial solubility parameters of the system. Maximiano et al.25 applied a variety of techniques, including DSC, FTIR and p-XRD, to evaluate the compatibility of a drug (benznidazole, BNZ) with excipients hydroxyethylcellulose, polyethylene glycol, and hydroxypropyl-beta-cyclodextrin. Finally, they confirmed the incompatibility of BNZ with only the polyethylene glycol which should not be used in the development of solid dosage forms containing BNZ. However, it is usually time-consuming and costly to study the compatibility of a polymer and hydrophobic drug using these approaches. So it is very important to seek a fast and effective way for evaluating polymer–drug compatibility. Computer simulation seems to be the desired method of choice, which can allow us to avoid cumbersome and inefficient trial and error formulation studies. For instance, Phillip and his coworkers26 applied a molecular dynamics (MD) simulation method to successfully predict the compatibility of water-insoluble drugs and block copolymers. Eslami et al.27 investigated the compatibility of tacrine with poly(n-butylcyanoacrylate) or chitosan using MD simulation. According to the simulation results, the tacrine molecule exhibited higher compatibility with PBCA than chitosan. Lan and coworkers28 investigated the compatibilization of cyclotriphosphazene N3P3[NH(CH2)(3)Si(OCH2CH3)(3)](6) (APESP) in flame retarded polypropylene (PP)/ammonium polyphosphate (APP) composites by molecular dynamics (MD) and the dissipative particle dynamics (DPD) simulation method. It was found that the compatibility of APP in the PP matrix was significantly improved due to the loading of the cyclotriphosphazene derivative APESP, compared with the loading of gamma-aminopropyltriethoxysilane (APES) or hexachlorocyclotriphosphazene.
With regard to the micelles self-assembled from an amphiphilic pH-sensitive polymer, the hydrophobic core is the binding site with hydrophobic drugs by hydrophobic interaction, and the hydrophilic shell has a protective effect outside of the system. The solubility of the pH-sensitive segment could be influenced by the pH values of the environment and the hydrophobic proportion of the pH-sensitive polymer could be affected by the number of pH-sensitive segments. So the compatibility between the micelles and drugs can be influenced by the number of pH-sensitive segments and the pH values. In this work, the compatibility of amphiphilic pH-sensitive polymers and hydrophobic drugs is investigated by a multiscale simulation method. Amphiphilic pH-sensitive copolymers with different numbers of histidine (H) residues conjugated with docosahexaenoic acid (DHA) and lysine (K) have been designed and synthesized before in our lab.29 DHA was selected as the hydrophobic core, K was selected as the hydrophilic shell, and H was selected as the pH-sensitive segment. A series of DHA–HisXLys10 were used as model polymers. Doxorubicin (DOX), a widely used hydrophobic anticancer drug, was selected as the model drug. Fig. 1 illustrates the chemical structures of DOX (Fig. 1A) and polymer DHA–HisXLys10 (Fig. 1B). The compatibility of vectors and drugs is investigated by changing the number of the histidine residues and the pH values using multiscale simulation, including Blends simulation and dissipative particle dynamics (DPD) simulation. The study of the compatibility of pH-sensitive polymers and hydrophobic drugs can help in the search for a drug carrier that is more stable and effective, and guide the development of amphiphilic pH-sensitive biomaterials for anticancer drug delivery.
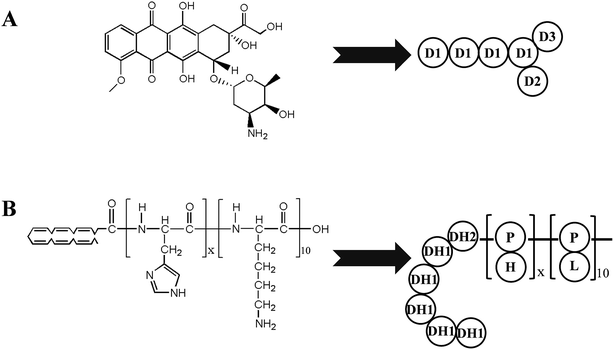 | ||
| Fig. 1 Chemical structures of doxorubicin (A) and docosahexaenoic acid conjugated peptides (DHA–HisXLys10) (B). | ||
2. Simulation methodology
2.1 Blends simulation
The Blends module in the Material Studio 6.0 software (Accelrys) was utilized to study the compatibility of DOX and DHA–HisXLys10, greatly reducing the need for laboratory experimentation. We can analyze the run results to predict the compatibility, such as the Flory–Huggins chi parameter, binding energies and the phase diagrams for the two components. The reason why we choose this module is that this module has high efficiency and unique superiority. Its high efficiency lies in that it provides a way to shorten the discovery process by estimating the miscibility behavior of polymer–polymer mixtures. This method can predict the thermodynamics of mixing directly from the chemical structures of the two components and, therefore, requires only their molecular structures and a force field as input in the Blends simulation. Its unique superiority lies in that it combines a modified Flory–Huggins model30,31 and molecular simulation techniques32 to calculate the compatibility of polymer–polymer mixtures. Two important extensions to the Flory–Huggins model are employed:(i) Blends incorporates an explicit temperature dependence on the interaction parameter. This is accomplished by generating a large number of pair configurations and calculating the binding energies followed by temperature averaging the results using the Boltzmann factor and calculating the temperature-dependent interaction parameter.
(ii) Blends is an off-lattice calculation, meaning that molecules are not arranged on a regular lattice as in the original Flory–Huggins theory. The coordination number is explicitly calculated for each of the possible molecular pairs using molecular simulations.
These two extensions to the classical Flory–Huggins theory of mixing are documented in publications by Blanco33 and Fan et al.32 By substituting the temperature-dependent interaction parameter, χ, in the Flory–Huggins expression, the free energy is known for all compositions and temperatures. From this, the phase diagram of the mixture can then be determined by loading the critical point, the coexisting curve (binodal), and the stability curve (spinodal) in the two-phase diagram.
Based upon the theory of Blends simulation, the following expressions are a few critical factors of the Blends module. The Flory–Huggins interaction parameter, χ, is defined as:
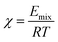 | (1) |
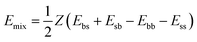 | (2) |
In evaluating binding energies, Blends distinguishes the components by using the role property: one component has a base role, the other has a screen role. A given base–screen combination can give four potentially different pairs, each of which will have an associated binding energy value:33
(i) Base–base pair (Ebb).
(ii) Screen–screen pair (Ess).
(iii) Base–screen pair (Ebs).
(iv) Screen–base pair (Esb).
The last two pairs are equivalent. Blends simulation is only used to calculate the energy of base–screen pairs and then uses this value for the energy of a screen–base pair.
During the whole Blends simulation process, we set the force field to Dreiding and changed the ‘charges to charge’ using ‘QEq’ for the computation of Flory–Huggins interactions. In addition, the radial distribution function can be used as an aiding tool for estimating compatibility. But in general, Blends allows us to efficiently sample the mixing, binding, and coordinating properties between specific molecules. To enable efficient screening of multiple systems in a single calculation, each molecule is assigned the role of a base or screen. All tasks in Blends begin by finding all combinations of molecules assigned a base role with all molecules assigned a screen role. Blends then applies the task to each base–screen mixture in turn.
In addition, to simplify the simulating calculation and advance the accuracy of the simulation, the compatibility forecast of DOX and DHA–HisXLys10 can be performed by evaluating the compatibility of DOX with different number chains of histidine using the Blends module. In the content of the polymer DHA–HisXLys10, DHA (docosahexaenoic acid) is an indispensable highly unsaturated fatty acid for humans and a major component for the development or maintenance of the cells of the nervous system; lysine is an essential amino acid to the human body and can promote human development and enhance the body’s immune function; histidine is an important amino acid that inhibits tumor development. DHA and lysine are excellent biomaterials with good biocompatibility and have high thermodynamic and kinetic stability.34,35 So there is no obvious change of the compatibility of DOX with DHA and lysine when the molecular weight of DHA and lysine in polymer DHA–HisXLys10 are confirmed. But histidine contains imidazole groups, and their isoelectric point (IEP) is 6.0. At pH < 6.0 imidazole groups can easily trap protons and have positive charge, which makes histidine show hydrophilicity; at pH > 6.0 it is harder for the group to trap protons, making histidine show hydrophobicity. So, the compatibility of DOX with histidine would be affected by the pH values due to the transformation of the hydrophobic and hydrophilic performance of histidine. Moreover, the number of histidine residues as pH-sensitive residues in the polymer DHA–HisXLys10 also impacts on the compatibility between DOX and histidine at the same pH value. Thus, in the Blends simulation, the compatibility of DOX with different numbers of histidine residues at different pH values was assessed. Furthermore, it should be noted that imidazole groups in histidine residues can trap protons and have positive charge with on the decrease of the pH from pH > 6.0 to pH < 6.0. So, at pH < 6.0, we attached a positive charge to imidazole groups of histidine residues. Hence achieving a different pH in Blends simulations.
2.2 DPD simulation
In order to further probe into the miscibility details of DOX and DHA–HisXLys10 (X = 5, 10, 15, 20), the DPD program was used to verify the proposed Blends simulation at the mesoscopic level. DPD is a simulation method for particle dynamics. The motion of particles is calculated by solving the so-called equations of motion over a certain time span. The equations of motion describe how particles move under the influence of forces. In DPD the motion of the particle is simulated at constant temperature, and the forces include those of a special thermostat. DPD uses a stochastic and momentum conserving thermostat, which distinguishes the method from Brownian or Molecular Dynamics.In DPD simulations, individual atoms or molecules are lumped together into quasi-particles (beads). That is to say, DOX and DHA–HisXLys10 were represented by beads to model the whole molecular structure. The characteristics of beads result in the value of repulsion parameters. The parameter aij of conservative force between different types of particles is referred to as the DPD repulsion parameter. In order to calculate the parameter aij, a linear relationship between the repulsive parameter (aii) and Flory–Huggins parameter (χij) was proposed by Groot and Warren:36
| aij = aii + 3.27χij | (3) |
| aiiρ = 75kBT | (4) |
| aij | W | DOX1 | DOX2 | DOX3 | DH1 | DH2 | P | L | H1 | H2 |
|---|---|---|---|---|---|---|---|---|---|---|
| W | 25.0 | |||||||||
| DOX1 | 98.9 | 25.0 | ||||||||
| DOX2 | 65.1 | 20.3 | 25.0 | |||||||
| DOX3 | 46.2 | 31.0 | 30.8 | 25.0 | ||||||
| DH1 | 51.2 | 30.1 | 29.1 | 27.8 | 25.0 | |||||
| DH2 | 31.0 | 28.8 | 28.0 | 24.2 | 39.2 | 25.0 | ||||
| P | 27.5 | 26.7 | 26.2 | 24.3 | 40.6 | 21.8 | 25.0 | |||
| L | 21.0 | 22.7 | 23.5 | 21.0 | 23.8 | 21.5 | 20.8 | 25.0 | ||
| H1 | 31.5 | 19.4 | 27.1 | 23.2 | 40.4 | 23.6 | 23.8 | 21.3 | 25.0 | |
| H2 | 4.7 | 7.0 | 5.6 | 5.1 | 8.8 | 7.3 | 5.3 | 102.0 | 4.6 | 25.0 |
In this work, a simulation box with grid dimensions of 30 × 30 × 30 rc3 (rc is the DPD length unit), with periodic boundary conditions was employed. All DPD simulations were performed for 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 time steps with an integration time step of 0.05 to get thermodynamic equilibrium.39,40 The simulations were completed using the DPD program incorporated in the commercial software Materials Studio 6.0 (Accelrys Inc.).
000 time steps with an integration time step of 0.05 to get thermodynamic equilibrium.39,40 The simulations were completed using the DPD program incorporated in the commercial software Materials Studio 6.0 (Accelrys Inc.).
3. Results and discussion
3.1 Flory–Huggins parameters
The Flory–Huggins parameter χ was put forth to estimate the miscibility behavior of binary mixtures, such as solvent–solvent, polymer–solvent, and polymer–polymer mixtures. Thus, the χ value could also be used to describe the compatibility of components in our systems. According to Flory–Huggins theory, lower χ values indicate higher affinity and better compatibility between the components. Herein, the χ values of the systems (DOX and different number chains of histidine) were calculated using the Blends module at different pH values, as shown in Fig. 2.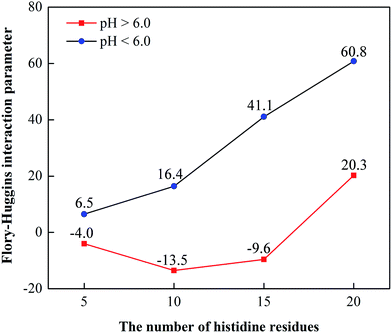 | ||
| Fig. 2 Plots of the computed Flory–Huggins interaction parameters (χ) between DOX and different number of histidine residues (5, 10, 15, 20) using Blends simulation at different pH values. | ||
As seen from Fig. 2, when the number of histidine residues is fixed, the χ values at pH < 6.0 are always greater than those at pH > 6.0, indicating that the compatibility between DOX and histidine is better at pH > 6.0 than at pH < 6.0. The reason could be that histidine segments change from hydrophobic to hydrophilic ones with the decrease in pH value due to the protonation of imidazole groups. Moreover, the χ values decrease from −4.0 to −13.5 as the number of histidine residues increases from 5 to 10 at pH > 6.0, indicating the compatibility increases. An increase in the compatibility can result in increased stability and a larger drug-loading content of the DHA–His10Lys10 micelle. As the number of histidine residues continues to increase to 15 and 20, the χ values increase from negative to positive, showing the compatibility is getting steadily worse. The most likely reason is that the steric hindrance of the longer histidine residues decreases the affinity between DOX and histidine residues. When the pH is lower than 6.0, with the increase of the number of histidine segments from 5 to 20, the χ values increase from 6.5 to 60.8, demonstrating the compatibility becomes worse gradually. This is due to the steric hindrance resulting from the longer histidine segments. Based on the analysis above, when the pH is higher than 6.0 and the number of histidine residues is 10, the compatibility of the system is the best. Furthermore, when the number of histidine residues is equal, the difference of χ values between pH > 6.0 and pH < 6.0 notated as Δχ can reflect the pH sensitivity of the histidine residues. Larger Δχ means stronger pH sensitivity which shows that micelles formed from a block copolymer containing histidine can reach the lesion location accurately and rapidly, as well as improve the bioavailability of drugs. Moreover, it has also been shown that the structural stability of the polymeric micelle varies with its pH sensitivity and stronger pH sensitivity leads to worse structural stability when the pH is lower than 6.0, which can promote drug release from micelles. Apparently, we can see that Δχ is the largest when the number of histidine residues is 15, demonstrating that the pH sensitivity of DHA–His15Lys10 micelles is the best (Fig. 2).
We can draw a conclusion that when the number of histidine residues is 10 or 15 and the pH is larger than 6.0, the compatibility and pH sensitivity of the system are better according to the simulation results. The conclusion indicates that DHA–His10Lys10 or DHA–His15Lys10 micelles as desired drug carriers can display high drug loading efficiency, good stability and high bioavailability.
3.2 Binding energy distributions
Binding energy in the Blends simulation is a measure of the energy of interaction between two components and a great tool to distinguish the stand or fall compatibility. In the simulation, histidine residues and DOX molecules are set to base and screen role, respectively. During the process, the mixture of DOX and histidine can appear as three different pairs, which are base–base, base–screen, and screen–screen combinations. The binding energies corresponding to these three combinations represent Ebb (red line), Ebs (blue line), and Ess (green line), respectively. If Ebb, Ebs and Ess have very similar distributions, this is a good indicator that the structures of the mixture will be compatible. The simulation results of binding energy distributions for DOX molecules and different numbers of histidine residues at different pH values are plotted in Fig. 3. As can be seen from Fig. 3, the Ebb, Ebs, and Ess in each plot all have very similar distributions, so the distribution shape of the binding energies can’t be taken as criteria for evaluating the compatibility of DOX and histidine. In this case, the correlation coefficient of any two curves (Ebb, Ebs, and Ess) in each plot is used to evaluate the curve’s correlation. In this work, the average values of correlation coefficients in each plot is calculated to measure similarity of curves and assess the compatibility between DOX and histidine segments. The calculated results corresponding to binding energy distribution plots are listed in Table 2. For the convenience of discussion, the average values of correlation coefficients are identified as ‘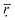 ’.
’.
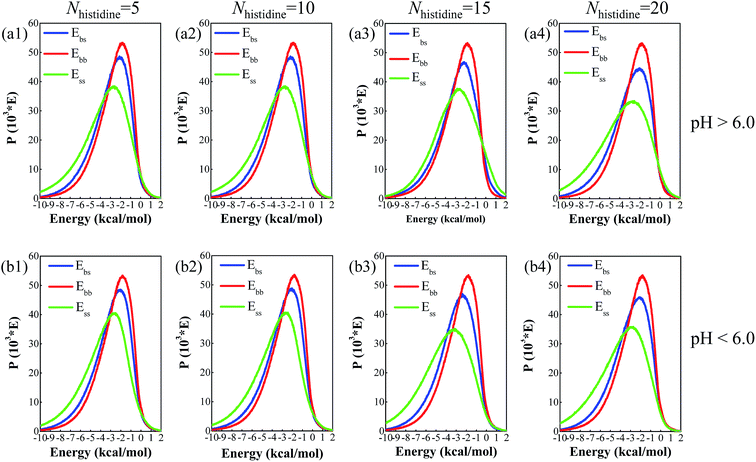 | ||
| Fig. 3 The binding energy distribution curves of DOX molecules and different numbers of histidine residues at pH > 6.0 (a) and pH < 6.0 (b). | ||
| Nhistidine = 5 | Nhistidine = 10 | Nhistidine = 15 | Nhistidine = 20 | |
|---|---|---|---|---|
| pH > 6.0 | 0.9435 | 0.9938 | 0.9604 | 0.9363 |
| pH < 6.0 | 0.9270 | 0.9266 | 0.9073 | 0.8718 |
Based on the correlation coefficient definition, we can know that the closer that 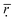 is to 1, the higher the comparability degree among the binding energy distribution curves is. As can be seen from the Table 2 the
is to 1, the higher the comparability degree among the binding energy distribution curves is. As can be seen from the Table 2 the 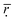 get larger when the number of histidine residues increases from 5 to 10 at pH > 6.0, showing that the compatibility between DOX and histidine residues becomes better. However, the
get larger when the number of histidine residues increases from 5 to 10 at pH > 6.0, showing that the compatibility between DOX and histidine residues becomes better. However, the 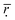 shows a decreasing trend when the number of histidine segments continues to increase from 10 to 15 and from 15 to 20, indicating that the compatibility became worse gradually. The steric hindrance of the longer histidine blocks is the main cause for this phenomenon. At pH < 6.0 an increase in the number of histidine segments is shown to decrease
shows a decreasing trend when the number of histidine segments continues to increase from 10 to 15 and from 15 to 20, indicating that the compatibility became worse gradually. The steric hindrance of the longer histidine blocks is the main cause for this phenomenon. At pH < 6.0 an increase in the number of histidine segments is shown to decrease 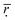 , which is an indication of the lower compatibility of DOX with longer histidine blocks. This is because the number of histidine residues increases, which can produce space steric hindrance. In addition, by comparing all the data in Table 2, the interesting observation is that the minimum value for
, which is an indication of the lower compatibility of DOX with longer histidine blocks. This is because the number of histidine residues increases, which can produce space steric hindrance. In addition, by comparing all the data in Table 2, the interesting observation is that the minimum value for 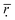 at pH > 6.0 is bigger than the maximum value of
at pH > 6.0 is bigger than the maximum value of 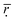 at pH < 6.0. This illustrates the compatibility at pH > 6.0 is better than that at pH < 6.0, which is attributed to the transformation of histidine residues from hydrophobic to hydrophilic ones as the pH decreases. From the analysis above, the compatibility is best when the number of histidine residues is 10 and the pH is larger than 6.0, which means DHA–His10Lys10 micelles at pH > 6.0 have the advantages of both good stability and high drug loading efficiency.
at pH < 6.0. This illustrates the compatibility at pH > 6.0 is better than that at pH < 6.0, which is attributed to the transformation of histidine residues from hydrophobic to hydrophilic ones as the pH decreases. From the analysis above, the compatibility is best when the number of histidine residues is 10 and the pH is larger than 6.0, which means DHA–His10Lys10 micelles at pH > 6.0 have the advantages of both good stability and high drug loading efficiency.
3.3 Phase diagram
Phase diagrams are useful to illustrate the compatibility of binary mixtures, which is derived from the free energy of mixing. They give information on which temperatures the mixture is miscible or unstable. A phase diagram generally contains three pieces of information: critical points (red), binodal (blue), and spinodal (green). Using the Blends simulation, the results of the phase diagram for DOX and different numbers of histidine residues (5, 10, 15 and 20) under different pH are depicted in Fig. 4. In the phase diagrams, a critical point marks the start of a coexistence region, meaning that if the mixture has more critical points, it also has more coexistence regions. The coexistence region is bound by the binodal (blue lines in Fig. 4). Meanwhile the spinodal (green lines in Fig. 4) separates the coexistence region into two regions. In the region between the binodal and the spinodal, the mixture is metastable. But in the region bounded by the spinodal, the mixture is unstable. So we can estimate the compatibility at different pH values by comparing the size of the region (S) between binodal and spinodal when the phase diagram plots of Fig. 4 have the same numbers of histidines and critical points. A larger area of S reflects that the mixture of DOX and histidine exhibits better compatibility. Table 3 lists the values of the area of the region mentioned above, which correspond to the eight phase diagram plots in Fig. 4.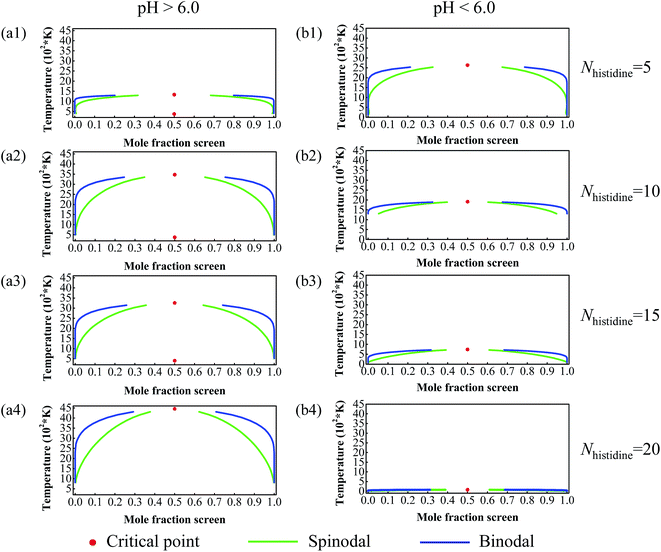 | ||
| Fig. 4 The phase diagrams plots for binary systems composed of DOX and different numbers of histidine residues at pH > 6.0 (a) and pH < 6.0 (b). | ||
| Nhistidine = 5 | Nhistidine = 10 | Nhistidine = 15 | Nhistidine = 20 | |
|---|---|---|---|---|
| pH > 6.0 | 0.9432 | 3.5426 | 3.4746 | 5.5438 |
| pH < 6.0 | 2.6506 | 2.1314 | 1.1038 | 0.1158 |
As shown in Fig. 4a1–a3, there are 2 critical points at pH > 6.0, when the number of histidine residues increases from 5 to 15. So, the compatibility between DOX and histidine segments would be differentiated through a comparison of the S values in Table 3. It is obvious that higher S values indicate better compatibility in terms of phase diagram theory. The S values firstly increase (0.9432–3.5426) and then decrease (3.5426–3.4746) with the increase of the number of histidine residues, demonstrating the compatibility is best when the number of histidine residues is 10 (Table 3). As the number of histidine blocks increases to 20 (Fig. 4a4), the number of critical points is reduced from two to one though the S value is the highest (5.5438), suggesting that the compatibility is poor. We believe that the result is caused because of the steric hindrance of longer histidine blocks. When the pH < 6.0 (Fig. 4b1–b4), the number of critical points within the four phase diagram plots is 1, showing poor compatibility. Meanwhile, as seen from Table 3, S values decrease as the number of histidine segments increases, showing the compatibility of the system is getting poorer and poorer. The results mean that an increase in the chain number of histidines can result in a decrease in the DOX-loading capacity and the structural stability of the DHA–HisXLys10 micelles. Furthermore, it is worth pointing out that the numbers of critical points in Fig. 4a4 and b1 are equal to 1, but the value of S for Fig. 4a4 is larger than that for Fig. 4b1. Consequently we can come to the conclusion that the compatibility between DOX and histidine at pH > 6.0 is better than that at pH < 6.0, which is possibly due to the protonation of imidazole groups, resulting in the transformation of histidine segments from hydrophobic to hydrophilic with pH lowering. Herein, it needs to be noted that only when the number of critical phase diagram points are same can the S values be used for comparison. As can be seen from Fig. 4, the number of critical points at pH > 6.0 for the peptides with 5 histidine residues is two, which is more than that at pH < 6.0 (the number is one). Thus the compatibility of DOX and 5 histidine residues at pH > 6.0 is better than at pH < 6.0.
In summary, the compatibility is best when the number of histidine residues is 10 and the pH is higher than 6.0, resulting in DHA–His10Lys10 micelles that have the highest drug-loading capacity and excellent stability.
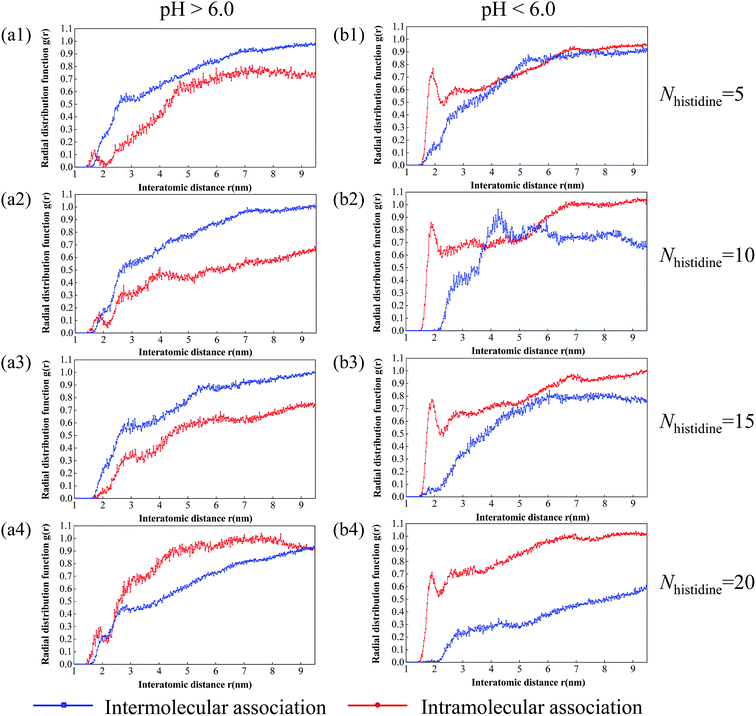
3.4 Radial distribution function
To further understand the compatibility between DOX and histidine segments, we examined the radial distribution function (i.e., RDF or g(r)). RDF is used to describe the spatial correlation between any two molecules or segments of molecules, which is of utmost importance for compatibility assessment. Besides, the RDF for pure substances may afford insights on the relative distribution and strength of interactions. In this work, we calculated the RDFs of those two cases for the pairs of atoms consisting of oxygen atoms of DOX molecules and hydrogen atoms of DOX molecules and histidine blocks. In total, eight RDF plots (Fig. 5) are generated for DOX with different numbers of histidine residues at different pH values. Here, the blue line in the plots corresponds to the intermolecular association between the oxygen atoms of DOX molecules and hydrogen atoms of the histidine blocks, whereas the red line in the plots signifies the intramolecular association among the oxygen atoms and hydrogen atoms of the DOX molecules themselves. In addition, the integral area of the RDF curve can reflect the coordination number (CN) which is able to describe the closeness between atoms accurately. Herein, the difference of CN between inter-RDF curves and intra-RDF curves (ΔCN) is used to estimate the compatibility of DOX and histidine residues, as shown in Table 4. The higher ΔCN values represent the closer combination of oxygen atoms of DOX molecules and hydrogen atoms of histidine blocks, as well as better the compatibility of DOX with histidine residues.| Nhistidine = 5 | Nhistidine = 10 | Nhistidine = 15 | Nhistidine = 20 | |
|---|---|---|---|---|
| pH > 6.0 | 1.5152 | 2.3550 | 1.8596 | −1.2606 |
| pH < 6.0 | −0.6644 | −1.5713 | −1.6266 | −4.0614 |
When the pH is higher than 6.0, the ΔCN values increase from 1.5152 to 2.3550 and then decrease to a negative value (−1.2606) with the number of histidine segments increasing from 5 to 20 (Table 4). The result indicates that DOX molecules have the best compatibility with histidine residues when the number of histidine residues is 10 and the pH is greater than 6.0. As the number of histidine segments increases, ΔCN decreases at pH < 6.0, showing that the compatibility continually gets worse. Steric hindrance brought by adding histidine segments contributes to the poorer compatibility. Furthermore, we also observe from Table 4 that the ΔCN values are negative at pH < 6.0, indicating that the compatibility is poor. The main reason is that histidine residues are converted to hydrophilic ones by the protonation of imidazole groups with the decrease of pH value. By summarizing the above simulated results, the best compatibility of DOX with histidine residues can be obtained when the number of histidine residues is 10 and the pH is larger than 6.0, meanwhile, the micelles formed from polymer DHA–His10Lys10 by self-assembling have the highest stability and drug-loading capacity.
3.5 DPD simulations
DPD simulations were performed to further explore the compatibility of DOX and histidine segments at different pH values. The DPD method can well simulate drug delivery systems on the mesoscopic level and be helpful for us to observe directly the morphologies of drug-loaded micelles and the drug distributions inside the micelles which can be utilized to evaluate the compatibility of DOX and DHA–HisXLys10. In DPD simulations, the system composed of 8% DHA–HisXLys10, 2% DOX, and 90% water was analyzed. The simulated results and the corresponding cross-section views at pH > 6.0 and pH < 6.0 are depicted in Fig. 6.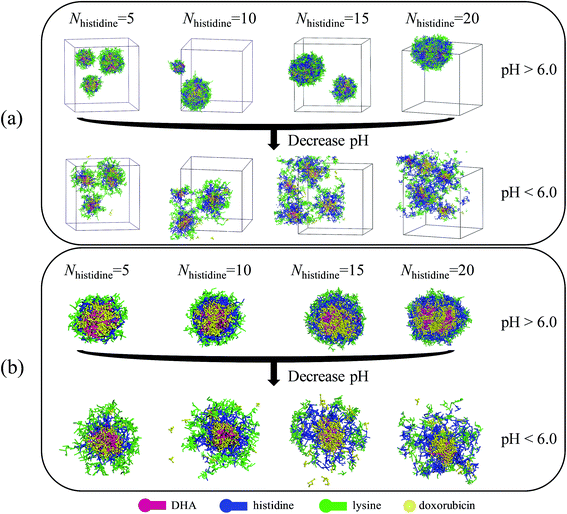 | ||
| Fig. 6 Typical simulated snapshots of DOX-loaded micelles (a) and the corresponding section view (b) at pH > 6.0 and pH < 6.0. The number of histidine residues is 5, 10, 15 and 20. | ||
As shown in Fig. 6a, at pH > 6.0, DOX molecules and polymer molecules can form spherical micelles with a dense structure. This result implies that DOX molecules have good compatibility with DHA–HisXLys10 molecules at pH > 6.0, which ensures certain stability and drug loading of micelles. When the pH value is decreased to lower than 6.0, the drug-loaded micelles experience a structural transformation from dense to swollen. This is reasonable because the protonation of imidazole groups, leading to the situation where histidine residues are translated to hydrophilic ones at lower pH condition. The micelles with swollen structure are very unstable and have poor drug loading, as well as poor compatibility between DOX and DHA–HisXLys10 molecules. However the swollen micellar structure facilitates the release of DOX at pH < 6.0. Above we have discussed the effect of different pH values on compatibility of DOX with DHA–HisXLys10. Then we have discussed the influence of the number of histidine residues on the compatibility of DOX with DHA–HisXLys10.
Fig. 6b shows DOX molecules distributed in both the pH-sensitive layer and the core of the micelles at pH > 6.0. When the number of histidine residues increases from 5 to 10, the DOX molecules display the trend of moving to the core of the micelles, which can improve the stability of micelles and increase the drug loading efficiency of micelles. There is no obvious variation of DOX distribution in micelles when the number of histidine segments is increased from 10 to 15. When the number of histidine residues increases to 20, many DOX molecules distribute in the pH-sensitive layer of the micelles and have a tendency to move to the surface of the micelles, resulting in poor stability of the micelles and low drug-loading efficiency. At pH < 6.0 the structure of drug-loaded micelles becomes more swollen on increasing the number of histidine residues, leading to a lower DOX loading ability and less stability of the micelles. The result shows that the compatibility of DOX with DHA–HisXLys10 becomes worse with increasing histidine segment number. The major reason for the differences of compatibility is steric hindrance resulting from longer histidine residues. However, from the perspective of drug controlled release, the poorer compatibility of DOX with DHA–HisXLys10, and the worse structural stability of DOX-loaded micelle is of benefit to the drug release from the micelles. In conclusion, DOX has better compatibility with DHA–HisXLys10 when the number of histidine segments is 10 or 15 and the pH is higher than 6.0, which is in agreement with the results obtained from the Blends simulations.
4. Conclusions
In this work, multiscale simulations are carried out to study the compatibility of a hydrophobic drug with a pH-sensitive polymer at different pH values. The assessment of compatibility consists of convenient and effective methods to choose drug-loaded micelles with good stability and high drug loading efficiency. In our work, the pH conditions and the number of pH-sensitive residues (histidine) can affect remarkably the compatibility between DOX and DHA–HisXLys10. The compatibility of DOX with different numbers of histidine residues under different pH is investigated through the Flory–Huggins interaction parameters, the binding energy distributions, phase diagrams and radius distribution function. These simulation results indicate that the compatibility at pH > 6.0 is better than that at pH < 6.0. Besides, at pH > 6.0 the compatibility is best when the number of histidine blocks is 10 or 15. In DPD simulations, DOX and DHA–His10Lys10/DHA–His15Lys10 have better compatibility when the pH is higher than 6.0, which is in agreement with the results of Blends simulation. All the simulation results indicate that the compatibility of DOX and DHA–HisXLys10 is best when the number of histidine residues is 10 or 15 and pH is greater than 6.0, suggesting that the DOX-loaded micelles have higher drug-loading capacity and more excellent stability. Accordingly, the research is of great significance in selecting the optimal pH-sensitive polymer as hydrophobic drug carrier, and in addition multiscale simulations may develop as a potentially effective approach for the study on the compatibility of blend systems.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (51473017, 51673019, 21406187), the Fundamental Research Funds for the Central Universities (buctrc201406), the Innovation and Promotion Project of Beijing University of Chemical Technology, and the long-term subsidy mechanism from the Ministry of Finance and the Ministry of Education of PRC.References
- M. M. Yallapu, M. Jaggi and S. C. Chauhan, Curr. Pharm. Des., 2013, 19, 1994–2010 CAS.
- R. D. Dubey, N. Alam, A. Saneja, V. Khare, A. Kumar, S. Vaidh, G. Mahajan, P. R. Sharma, S. K. Singh, D. M. Mondhe and P. N. Gupta, Int. J. Pharm., 2015, 492, 80–91 CrossRef CAS PubMed.
- P. Venkatesan, N. Puvvada, R. Dash, B. N. P. Kumar, D. Sarkar, B. Azab, A. Pathak, S. C. Kundu, P. B. Fisher and M. Mandal, Biomaterials, 2011, 32, 3794–3806 CrossRef CAS PubMed.
- T. T. T. N. Nguyen, J. Ostergaard, S. Sturup and B. Gammelgaard, Anal. Bioanal. Chem., 2012, 402, 2131–2139 CrossRef CAS PubMed.
- M. Hruby, C. Konak and K. Ulbrich, J. Controlled Release, 2005, 103, 137–148 CrossRef CAS PubMed.
- S. Manju and K. Sreenivasan, Langmuir, 2011, 27, 14489–14496 CrossRef CAS PubMed.
- X. Zhou, X. D. Li, T. Mao, J. X. Zhang and X. H. Li, Soft Matter, 2011, 7, 6264–6272 RSC.
- M. Studenovsky, O. Sedlacek, M. Hruby, J. Panek and K. Ulbrich, Anticancer Res., 2015, 35, 753–757 CAS.
- A. Sahu, U. Bora, N. Kasoju and P. Goswami, Acta Biomater., 2008, 4, 1752–1761 CrossRef CAS PubMed.
- B. J. Chun, C. C. Fisher and S. S. Jang, Phys. Chem. Chem. Phys., 2016, 18, 6284–6290 RSC.
- Y. Bae, T. A. Diezi, A. Zhao and G. S. Kwon, J. Controlled Release, 2007, 122, 324–330 CrossRef CAS PubMed.
- Y. N. Cui, J. H. Sui, M. M. He, Z. Y. Xu, Y. Sun, J. Liang, Y. J. Fan and X. D. Zhang, ACS Appl. Mater. Interfaces, 2016, 8, 2193–2203 CAS.
- S.-l. Lin, X.-f. Wen, Z.-q. Cai, P.-h. Pi, D.-f. Zheng, J. Cheng, L.-j. Zhang, Y. Qian and Z.-r. Yang, Phys. Chem. Chem. Phys., 2011, 13, 17323–17332 RSC.
- E. K. Peter, K. Lykov and I. V. Pivkin, Phys. Chem. Chem. Phys., 2015, 17, 24452–24461 RSC.
- D. J. Adams, M. L. Wahl, J. L. Flowers, B. Sen, M. Colvin, M. W. Dewhirst, G. Manikumar and M. C. Wani, Cancer Chemother. Pharmacol., 2006, 57, 145–154 CrossRef CAS PubMed.
- D. J. Adams and L. R. Morgan, Curr. Med. Chem., 2011, 18, 1367–1372 CrossRef CAS PubMed.
- J. Chen, X. Z. Qiu, J. Ouyang, J. M. Kong, W. Zhong and M. M. Q. Xing, Biomacromolecules, 2011, 12, 3601–3611 CrossRef CAS PubMed.
- S. Matsumoto, R. J. Christie, N. Nishiyama, K. Miyata, A. Ishii, M. Oba, H. Koyama, Y. Yamasaki and K. Kataoka, Biomacromolecules, 2009, 10, 119–127 CrossRef CAS PubMed.
- J. Z. Du, L. Fan and Q. M. Liu, Macromolecules, 2012, 45, 8275–8283 CrossRef CAS.
- C. Y. Zhang, D. Xiong, Y. Sun, B. Zhao, W. J. Lin and L. J. Zhang, Int. J. Nanomed., 2014, 9, 4923–4933 CrossRef PubMed.
- J. B. Liu, Y. H. Xiao and C. Allen, J. Pharm. Sci., 2004, 93, 132–143 CrossRef CAS PubMed.
- J. P. L. Dwan’Isa, L. Rouxhet, V. Preat, M. E. Brewster and A. Arien, Pharmazie, 2007, 62, 499–504 Search PubMed.
- A. Choucair and A. Eisenberg, J. Am. Chem. Soc., 2003, 125, 11993–12000 CrossRef CAS PubMed.
- S. S. Vaghani and M. M. Patel, Drug Dev. Ind. Pharm., 2011, 37, 1160–1169 CrossRef CAS PubMed.
- F. P. Maximiano, K. M. Novack, M. T. Bahia, L. L. de Sa-Barreto and M. S. S. da Cunha, J. Therm. Anal. Calorim., 2011, 106, 819–824 CrossRef CAS.
- S. Patel, A. Lavasanifar and P. Choi, Biomacromolecules, 2008, 9, 3014–3023 CrossRef CAS PubMed.
- M. Eslami, S. J. Nikkhah, S. M. Hashemianzadeh and S. A. S. Sajadi, Eur. J. Pharm. Sci., 2016, 82, 79–85 CrossRef CAS PubMed.
- Y. H. Lan, D. H. Li, R. J. Yang, W. S. Liang, L. X. Zhou and Z. W. Chen, Compos. Sci. Technol., 2013, 88, 9–15 CrossRef CAS.
- Y. Wang, Q. Y. Li, X. B. Liu, C. Y. Zhang, Z. M. Wu and X. D. Guo, ACS Appl. Mater. Interfaces, 2015, 7, 25592–25600 CAS.
- K. S. Schweizer and J. G. Curro, J. Chem. Phys., 1989, 91, 5059 CrossRef CAS.
- K. F. Freed, J. Phys. A: Math. Theor., 1985, 18, 871 CrossRef CAS.
- C. F. Fan, B. D. Olafson and M. Blanco, Macromolecules, 1992, 25, 3667–3676 CrossRef CAS.
- M. Blanco, J. Comput. Chem., 1991, 12, 237–247 CrossRef CAS.
- M. Baumgartner, S. Sturlan, E. Roth, B. Wessner and T. Bachleitner-Hofmann, Int. J. Cancer, 2004, 112, 707–712 CrossRef CAS PubMed.
- H. Deng, Z. Yin, T. Jiang, H. Liu, X. Fan, M. Wang, X. Ma, Z. Fan, C. Zheng and K. Deng, Colloid Polym. Sci., 2015, 293, 2341–2348 CAS.
- R. D. Groot and P. B. Warren, J. Chem. Phys., 1997, 107, 4423–4435 CrossRef CAS.
- R. D. Groot and T. J. Madden, J. Chem. Phys., 1998, 108, 8713–8724 CrossRef CAS.
- P. J. Hoogerbrugge and J. Koelman, Europhys. Lett., 1992, 19, 155–160 CrossRef.
- S. Z. Ling, Q. Y. You, D. G. Xin, S. Yao, Q. Yu and J. Z. Li, J. Colloid Interface Sci., 2011, 363, 114–121 CrossRef PubMed.
- X. Guo, L. Zhang, Q. Yu and Z. Jian, Chem. Eng. J., 2007, 131, 195–201 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |