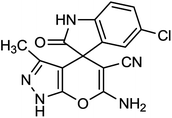
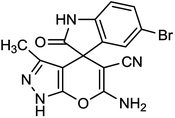
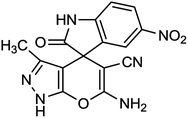
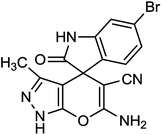
Green synthesis of tetrahydrobenzo[b]pyrans, pyrano[2,3-c]pyrazoles and spiro[indoline-3,4′-pyrano[2,3-c]pyrazoles catalyzed by nano-structured diphosphate in water†
Behrooz Maleki*a,
Negar Nasiria,
Reza Tayebeea,
Amir Khojastehnezhad*b and
Hossien Ali Akhlaghia
aDepartment of Chemistry, Hakim Sabzevari University, Sabzevar, 96179-76487, Iran. E-mail: malekibehrooz@gmail.com; Fax: +98 44010300; Tel: +98 5144013324
bYoung Researchers and Elite Club, Mashhad Branch, Islamic Azad University, Mashhad, Iran. E-mail: akhojastehnezhad@yahoo.com
First published on 16th August 2016
Abstract
A green and recoverable nano-structured diphosphate (Na2CaP2O7) was synthesized and fully characterized using FT-IR spectra, X-ray diffraction patterns (XRD), scanning electron microscopy (SEM), and energy-dispersive X-ray spectroscopy (EDX) analysis. The nanostructured catalyst has been successfully used as reusable nanostructured catalyst for green, simple and efficient synthesis of tetrahydrobenzo[b]pyrans, pyrano[2,3-c]pyrazoles and spiro[indoline-3,4′-pyrano[2,3-c]pyrazoles in aqueous media. The catalyst shows environmentally benign character, which can be easily prepared, stored, and recovered several times without obvious significant loss of catalytic activity.
Introduction
One important symbol of green chemistry is reducing the use of organic solvents because of the economical and environmental concerns associated with them. Water plays an essential role in life processes and also a medium for the organic synthesis.1,2 Water is clean, non-toxic, and hazard-free in handling, non-inflammable, cheap and a readily available solvent. Furthermore, because of its highly polarity, high surface tension, high specific heat capacity and network of hydrogen bonds, water plays a significant role in many reactions.3 In 1980, Breslow discovered that Diels–Alder reactions could be performed in water with a huge acceleration.4,5 This discovery led to a considerable interest of synthetic organic chemists in the study of using water as a reaction solvent.6,7 To date, a great number of organic reactions have been carried out in water successfully.8The pyran ring system is present in numerous biologically active natural products as well as many synthetic compounds.9 Tetrahydrobenzo[b]pyrans and pyrano[2,3-c]pyrazoles have a broad spectrum of biological and pharmacological activity, such as anticancer, anti-coagulant, antimicrobial, anti-inflammatory, anti-anaphylactic, and molluscicidal activity.10–13
Conventionally, the synthesis of tetrahydrobenzo[b]pyrans has been performed through a one-pot reaction of three components in the presence of various catalysts such as the Fe3O4@SiO2–imid–PMA nanoparticle,14 choline hydroxide based ionic liquid [Ch][OH],15 red sea sand under microwave or ultrasonic irradiation,16 hexadecyltrimethyl ammonium bromide (HTMAB),17 sodium bromide (NaBr),18 ionic liquids,19–22 tetra-methyl ammonium hydroxide,23 molecular iodine (I2),24 N-methylimidazole,25 sodium selenite,26 tetrabutylammonium bromide (TBAB),27 amine or amino acid,28 potassium phosphate (K3PO4),29 magnetic core–shell titanium dioxide nanoparticles (Fe3O4@SiO2@TiO2),30 nano α-Al2O3 supported ammonium dihydrogen phosphate (NH4H2PO4/Al2O3),31 phenylboronic acid [PhB(OH)2],32 cerium(iii) chloride (CeCl3·7H2O),33,34 nanosized TiO2,35 silica coated magnetite–polyoxometalate nanoparticles (Fe3O4@SiO2@NH-NH2–H3PW12O40),36 meglumine,37 magnetic La0.7Sr0.3MnO3 nanoparticles,38 RuBr2(PPh3)4,39 lactose,40 and silica coated magnetic NiFe2O4 nanoparticles supported H3PW12O40 (NFS-PWA).41
However, a number of these methods suffer from certain drawbacks such as poor yields, difficult workups, long reaction times, high temperatures, the utilization of alternative energy source (microwave or ultrasonic), and the use of volatile or hazardous organic solvents. Therefore, it seemed desirable to develop a more efficient and a general method for the synthesis of tetrahydrobenzo[b]pyrans and pyrano[2,3-c]pyrazoles.
On the basis of the information obtained from the nano-structured diphosphate Na2CaP2O7 (DIPH), we predicted that the DIPH can be used as an efficient catalyst for the promotion of the reactions which requires the use of a catalyst to speed-up. So, we were interested to investigate the applicability of this reagent in the promotion of the synthesis tetrahydrobenzo[b]pyrans and pyrano[2,3-c]pyrazoles.
For the present study, the synthesis of the DIPH has been carried out from Na2CO3, CaCO3 and NH4H2PO4 in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 proportion, respectively, by following the literature procedure.42 In recent years, the DIPH has been used as catalyst in organic synthesis43,44 as it has been shown to be non-toxic, green and stable, inexpensive, safe, non-volatile, non-corrosive and re-usable.
2 proportion, respectively, by following the literature procedure.42 In recent years, the DIPH has been used as catalyst in organic synthesis43,44 as it has been shown to be non-toxic, green and stable, inexpensive, safe, non-volatile, non-corrosive and re-usable.
In our progressive program to develop efficient and environmentally benign protocols for the synthesis of various products,45–59 we tried to report a highly efficient method for the synthesis of tetrahydrobenzo[b]pyrans and pyrano[2,3-c]pyrazoles catalyzed by a DIPH in water (Scheme 1).
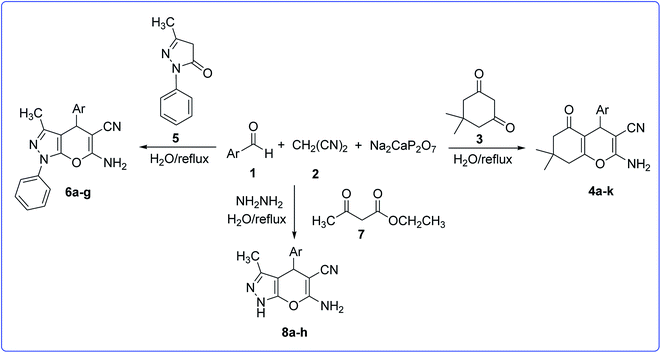 | ||
| Scheme 1 Synthesis of tetrahydrobenzo[b]pyrans and pyrano[2,3-c]pyrazoles catalyzed by a DIPH in water. | ||
Nanostructured diphosphate (Na2CaP2O7) was characterized by several methods including FT-IR, SEM, XRD and EDX. The existence of P2O7 units was confirmed by the symmetrical vibration bands of P–O–P at 720 cm−1 as well as the anti-symmetric vibration bands at 890 cm−1. The related vibrations of the PO4 groups were shared between two fields: a field of symmetrical vibrations at 990 cm−1 and 1030 cm−1 and another that ranged from 1130 to 1280 cm−1 (Fig. S1†).42–44 Scanning Electronic Microscopy (SEM) was used to study the morphology of the surface of Na2CaP2O7 (Fig. S2†). Also, to confirm the formation of Na2CaP2O7 nanostructure, the XRD patterns of sample was studied (Fig. S3†). The XRD pattern of Na2CaP2O7 nanostructure indicates that these nanostructures have synthesized well and all of the major peaks matching with the previous literatures.42–44 Moreover, the elemental analysis obtained from the EDX spectra of catalyst (Fig. S4†), shows the existence of all of the elements in the Na2CaP2O7 nanostructures and these results are in agreement with other results.
At first, we selected the benzaldehyde (1 mmol), dimedone (1 mmol), and malononitrile (2 mmol) as model substrates to establish optimum reaction conditions in the presence of DIPH (Table 1). When the reaction was performed under solvent-free conditions, a low yield of target product was obtained (Entry 1). To find the best solvent for this transformation, the present three-component reaction was screened in methanol, ethanol, and water (Table 2, Entries 2–4). Among these solvents, water was found to be the best one and afforded the highest yield (Entry 5). Then, the amount of the catalyst was evaluated in the model reaction in refluxing water (Entries 6–7). The results showed that 20 mol% of DIPH was the best choice for completing the reaction (Entry 5) and the use of excessive catalyst had no impact either on the rate of the reaction or on the product yield (Entry 7). When the reaction was attempted without a catalyst, it was found that no product was obtained even after 1 h (Entry 8).
| Entry | Catalyst (mol%) | Conditions | Time (min) | Yieldb (%) |
|---|---|---|---|---|
| a Benzaldehyde (1 mmol), dimedone (1 mmol), malononitrile (1 mmol) in refluxing water.b Isolated yields. | ||||
| 1 | 20 | Solvent-free/100 °C | 15 | 72 |
| 2 | 20 | MOH/reflux | 10 | 80 |
| 3 | 20 | EtOH/reflux | 10 | 82 |
| 4 | 20 | EtOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water/reflux water/reflux |
10 | 88 |
| 5 | 20 | Water/reflux | 10 | 94 |
| 6 | 10 | Water/reflux | 10 | 84 |
| 7 | 30 | Water/reflux | 10 | 94 |
| 8 | None | Water/reflux | 60 | None |
| Entry | Ar | Product | Time (min) | Yielda (%) | Mp (°C) | Lit. Mp (°C) | [Ref.] |
|---|---|---|---|---|---|---|---|
| a Isolated yields. | |||||||
| 1 | Ph | 4a | 10 | 94 | 231–233 | 231–233 | 18 |
| 2 | 4-ClC6H4 | 4b | 10 | 90 | 207–209 | 203–206 | 21 |
| 3 | 4-CH3C6H4 | 4c | 10 | 89 | 212–214 | 210–213 | 23 |
| 4 | 3-ClC6H4 | 4d | 10 | 92 | 230–232 | 230–232 | 23 |
| 5 | 3-MeOC6H4 | 4e | 10 | 90 | 192–194 | 188–190 | 20 |
| 6 | 2-CH3C6H4 | 4f | 15 | 86 | 212–214 | 211–212 | 41 |
| 7 | 4-CNC6H4 | 4g | 10 | 95 | 226–228 | 225–228 | 23 |
| 8 | 4-NO2C6H4 | 4h | 10 | 95 | 177–179 | 177–179 | 23 |
| 9 | 3-NO2C6H4 | 4i | 10 | 94 | 210–212 | 211–214 | 18 |
| 10 | 4-BrC6H4 | 4j | 10 | 91 | 202–204 | 203–205 | 26 |
| 11 | 2,4-ClC6H4 | 4k | 10 | 90 | 183–185 | 180–182 | 27 |
| 12 | Ph | 6a | 10 | 93 | 164–166 | 169–171 | 17 |
| 13 | 4-ClC6H4 | 6b | 15 | 86 | 180–182 | 175–177 | 17 |
| 14 | 4-BrC6H4 | 6c | 15 | 90 | 187–188 | 185–186 | 31 |
| 15 | 4-CH3C6H4 | 6d | 20 | 80 | 174–177 | 176–178 | 31 |
| 16 | 4-CH3OC6H4 | 6e | 20 | 82 | 171–173 | 170–172 | 31 |
| 17 | 3-NO2C6H4 | 6f | 15 | 90 | 189–191 | 190–192 | 31 |
| 18 | 4-NO2C6H4 | 6g | 20 | 87 | 195–197 | 197–199 | 31 |
| 19 | Ph | 8a | 10 | 92 | 244–245 | 244–245 | 19 |
| 20 | 4-CH3OC6H4 | 8b | 15 | 86 | 213–215 | 211–213 | 19 |
| 21 | 4-BrC6H4 | 8c | 15 | 85 | 246–248 | 248–250 | 19 |
| 22 | 2-Thienyl-C6H4 | 8d | 10 | 92 | 225–226 | 224–226 | 37 |
| 23 | 4-Pyridin-C6H4 | 8e | 20 | 90 | 215–217 | 216–217 | 37 |
| 24 | 3-BrC6H4 | 8f | 15 | 90 | 224–226 | 222–223 | 19 |
| 25 | 4-NO2C6H4 | 8g | 10 | 90 | 250–252 | 248–249 | 37 |
| 26 | 3-NO2C6H4 | 8h | 15 | 89 | 228–230 | 232–233 | 22 |
Under these optimal conditions (Table 1, Entry 4), the scope and specificity of this protocol was further investigated. At first, a broad range of structurally diverse aldehydes were treated with malononitrile and dimedone (see Table 2) in order to synthesis of tetrahydrobenzo[b]pyrans derivatives 4a–k. As shown in Table 2, all reactions proceeded efficiently and the desired products were obtained from high to excellent yields in relatively short times without any formation of by-products. The reaction of aromatic aldehyde carrying electron donating or electron-withdrawing groups was also successfully carried out with this method (Table 2, Entries 1–11). The reactions showed corresponding compounds in excellent yields.
Encouraged by these results, we extended this study for three component synthesis of pyrano[2,3-c]pyrazoles (6a–g) in the presence of a catalytic amount of DIPH. Considering the reaction time and the yield, the DIPH (20 mol%) was selected as the optimum catalyst used in the refluxing water. Using the optimized reaction conditions, a range of substituted pyrano[2,3-c]pyrazoles were synthesized (Table 2, Entries 12–18). Moreover, the one-pot, four-component synthesis of pyrano[2,3-c]pyrazoles could be successfully used for the synthesis of corresponding compounds (8a–h). This method was found to be effective for aromatic aldehydes bearing either electron donating or electron-withdrawing substituents (Entries 19–21, 24–26) as well as for the heterocyclic aldehyde (Entries 22, 23).
Spiro heterocycles, especially spirocyclic oxindole nucleus, are a substantial category of natural alkaloids that possess various biological and pharmacological activities, such as spirotryptostatin A, B, which are isolated from the fermentation broth of Aspergillus fumigatus, inhibits of cell cycle at G2/M phase and koumine,60 which is one of the alkaloids isolated from the Gelsemium sempervirens plant that has antitumor and analgesic activities.61 Chitosenine as a Gardneria multiflora oxindole alkaloid has ganglioblocking action.62 These interesting properties promoted us to widen the applicability of this procedure with isatins.63
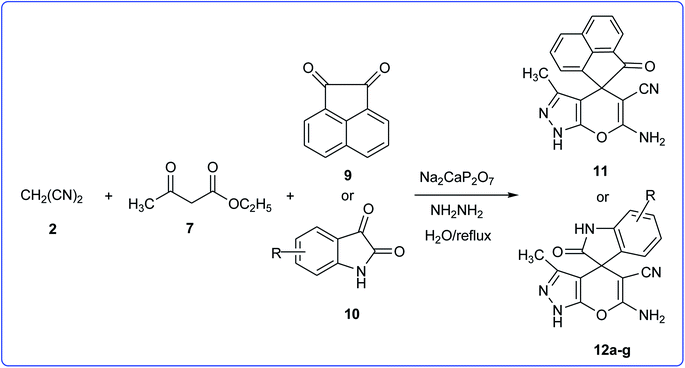
To explore the scope of this reaction to form a spiroheterocyclic compound, we investigated the use of acenaphthylene-1,2-dione (9) or isatin (10) as a substrate to react with the hydrazine hydrate, malononitrile, ethyl acetoacetate under the optimized conditions. As expected the reaction proceeded well to afford spiro[acenaphthylene-1,4′-pyrano[2,3-c]pyrazole (11) in 60 min with 64% yield and spiro[indoline-3,4′-pyrano[2,3-c]pyrazole] derivatives (12a–g) (Scheme 2 and Table 3). These successful results clearly indicate that the present catalytic approach is extendable to a wide variety of substrates.
The reusability of the catalyst was also investigated. For this purpose, the same model reaction was again studied under optimized conditions. After the completion of the reaction, the acetone was added to the reaction mixture to dissolve the product. Then, the catalyst was removed by filtration. It was re-used directly in the model reaction to give 4a in yields of 94%, 92%, 91%, 91%, 90% and 89% for six consecutive runs at 10 min.
In order to illustrate the efficiency of our procedure, results for the preparation of tetrahydrobenzo[b]pyrans and pyrano[2,3-c]pyrazoles previously reported are compared with our data (Table 4). The present method using DIPH as catalyst offers several advantages such as excellent yields, a simple procedure, short reaction times, facile work-up and greener conditions. The green aspect of the DIPH catalyst has been discussed in terms of being non-corrosive, presenting fewer disposals problem, facile isolation of the products, and recyclability.
| Product | Catalyst | Reaction conditions | Time (min) | Yielda (%) | [Ref.] |
|---|---|---|---|---|---|
| a Isolated yields. | |||||
| 4a | Fe3O4@SiO2–imid–PMA | H2O/reflux | 20 | 94 | 14 |
| Red sea sand | EtOH/reflux | 360 | 84 | 16 | |
| [Ch][OH] | H2O/80 °C | 60 | 92 | 15 | |
| NaBr/MWI | Solvent-free, 70 °C | 10 | 91 | 18 | |
| DIPH | H2O/reflux | 10 | 94 | This study | |
| 8a | [bmim]OH | Solvent-free/60 °C | 10 | 88 | 19 |
| L-Proline/[Bmim]BF4 | Solvent-free/50 °C | 10 | 90 | 22 | |
| NH4H2PO4/Al2O3 | EtOH/reflux | 15 | 80 | 31 | |
| NFS-PWA | EtOH/reflux | 45 | 90 | 41 | |
| DIPH | H2O/reflux | 10 | 92 | This study | |
Conclusion
In conclusion, we have developed a green and simple protocol for the synthesis of tetrahydrobenzo[b]pyran and pyrano[2,3-c]pyrazole derivatives via a three or four component condensation reaction in the presence of DIPH in water. This method was found not only to be applied to aromatic and heteroaromatic aldehydes, but also to be useful for the synthesis of ketone-derived dihydropyrano[2,3-c]pyrazole, spiro[indoline-3,4′-pyrano[2,3-c]pyrazole] and spiro[acenaphthylene-1,4′-pyrano[2,3-c]pyrazole]. This procedure provides several advantages such as high yields, wide scope of substrates, short reaction times, non-volatile, non-corrosive, absence of side-reactions, simple work-up procedure, non-toxic, evasion of hazardous catalysts or solvents, and the minimization of cost and waste generation owing to the recycling of the catalyst.Experimental section
All reagents were obtained from commercial sources and were used without purification. IR spectra were recorded as KBr pellets on a Shimadzu 435-U-04 spectrophotometer. 1H and 13C NMR spectra were determined on a Bruker DRX-300 Avance spectrometer in DMSO-d6 or CDCl3, and shifts are given in δ downfield from tetramethylsilane (TMS) as an internal standard. Melting points were determined using an Electrothermal 9200 apparatus and are uncorrected.General procedure for the synthesis of tetrahydrobenzo[b]pyrans (4a–k)
The catalyst, DIPH (20 mol%), was added to a mixture of the aldehydes (1 mmol), malononitrile (1.2 mmol), and dimedone (1 mmol), and 1 ml of water in a 5 ml flask fitted with a reflux condenser. The resulting mixture was heated to reflux (an oil bath) for the appropriate time (see Table 2) with stirring (spin bar). After the completion of the reaction as determined by TLC (hexane–ethyl acetate, 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), the acetone (2 ml) was added and the mixture stirred for 2 min. The catalyst was removed by filtration and washed with acetone and calcined at 500 °C for 1 h before re-use. Then the resulting crude reaction mixture was poured onto crushed ice and the precipitated solid was collected and recrystallized from ethanol (96%, 5 ml) to afford the pure tetrahydrobenzo[b]pyrans derivatives 4a–k. The products were identified by their melting points, 1H-NMR, and IR spectroscopies.
1), the acetone (2 ml) was added and the mixture stirred for 2 min. The catalyst was removed by filtration and washed with acetone and calcined at 500 °C for 1 h before re-use. Then the resulting crude reaction mixture was poured onto crushed ice and the precipitated solid was collected and recrystallized from ethanol (96%, 5 ml) to afford the pure tetrahydrobenzo[b]pyrans derivatives 4a–k. The products were identified by their melting points, 1H-NMR, and IR spectroscopies.
General procedure for the synthesis of pyrano[2,3-c]pyrazoles (6a–g)
The catalyst, DIPH (20 mol%), was added to a mixture of the aldehydes (1 mmol), malononitrile (1.2 mmol), and 3-methyl-1-phenyl-2-pyrazoline-5-one (1 mmol), and 1 ml of water in a 5 ml flask fitted with a reflux condenser. The resulting mixture was heated to reflux (an oil bath) for the appropriate time (see Table 2) with stirring (spin bar). After the completion of the reaction as determined by TLC (hexane–ethyl acetate, 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), the acetone (2 ml) was added and the mixture stirred for 2 min. The products and catalyst were isolated as described above and recrystallized from 96% ethanol (20 ml) to afford pyrano[2,3-c]pyrazoles 6a–g.
1), the acetone (2 ml) was added and the mixture stirred for 2 min. The products and catalyst were isolated as described above and recrystallized from 96% ethanol (20 ml) to afford pyrano[2,3-c]pyrazoles 6a–g.
General procedure for the synthesis of pyrano[2,3-c]pyrazoles (8a–h)
The catalyst, DIPH (20 mol%), was added to a mixture of the aldehydes (1 mmol), malononitrile (1.2 mmol), hydrazine hydrate (2 mmol), ethylacetoacetate (1 mmol), and 1 ml of water in a 5 ml flask fitted with a reflux condenser. The resulting mixture was heated to reflux (an oil bath) for the appropriate time (see Table 2) with stirring (spin bar). After the completion of the reaction as determined by TLC (hexane–ethyl acetate, 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), the acetone (2 ml) was added and the mixture stirred for 2 min. The products and catalyst were isolated as described above and recrystallized from 96% ethanol (20 ml) to afford pyrano[2,3-c]pyrazoles 8a–h.
1), the acetone (2 ml) was added and the mixture stirred for 2 min. The products and catalyst were isolated as described above and recrystallized from 96% ethanol (20 ml) to afford pyrano[2,3-c]pyrazoles 8a–h.
General procedure for the synthesis of spiro[indoline-3,4′-pyrano[2,3-c]pyrazoles (12a–g)
The catalyst, DIPH (20 mol%), was added to a mixture of the isatins (1 mmol), malononitrile (1.2 mmol), hydrazine hydrate (2 mmol), ethylacetoacetate (1 mmol), and 1 ml of water in a 5 ml flask fitted with a reflux condenser. The resulting mixture was heated to reflux (an oil bath) for the appropriate time (see Table 2) with stirring (spin bar). After the completion of the reaction as determined by TLC (hexane–ethyl acetate, 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), the acetone (2 ml) was added and the mixture stirred for 2 min. The products and catalyst were isolated as described above and recrystallized from 96% ethanol (20 ml) to afford pyrano[2,3-c]pyrazoles 12a–g.
1), the acetone (2 ml) was added and the mixture stirred for 2 min. The products and catalyst were isolated as described above and recrystallized from 96% ethanol (20 ml) to afford pyrano[2,3-c]pyrazoles 12a–g.
Spectroscopic data of representative compounds
Acknowledgements
The authors thank the Research Council of Hakim Sabzevari University for a partial support of this work.References
- C. J. Li, Chem. Rev., 2005, 105, 3095 CrossRef CAS PubMed.
- W. S. Laitonjam, B. Thingom and S. D. Moirangthem, Org. Prep. Proced. Int., 2013, 45, 246 CrossRef CAS.
- A. Chanda and V. V. Fokin, Chem. Rev., 2009, 109, 725 CrossRef CAS PubMed.
- D. C. Rideout and R. Breslow, J. Am. Chem. Soc., 1980, 102, 7817 CrossRef.
- R. Breslow, U. Maitra and D. Rideout, Tetrahedron Lett., 1983, 24, 1901 CrossRef CAS.
- A. Lubineau, J. Org. Chem., 1986, 51, 2142 CrossRef CAS.
- A. Lubineau and E. Meyer, Tetrahedron, 1988, 44, 6065 CrossRef CAS.
- B. Maleki, M. Baghayeri, S. M. Vahdat, A. Mohammadzadeh and S. Akhoondi, RSC Adv., 2015, 5, 46545 RSC.
- S. Hatakeyama, N. Ochi, H. Numata and S. Takano, J. Chem. Soc., Chem. Commun., 1988, 1202 RSC.
- L. L. Andreani and E. Lapi, Bull. Chim. Farm., 1960, 99, 583 Search PubMed.
- M. E. A. Zaki, M. H. A. Soliman, O. A. Hiekal and A. E. Z. Rashad, Z. Naturforsch., C, 2006, 61, 1 CrossRef CAS PubMed.
- E. S. H. El-Tamany, F. A. El-Shahed and B. H. Mohamed, J. Serb. Chem. Soc., 1999, 64, 9 CAS.
- F. M. Abdelrazek, P. Metz, O. Kataeva, A. Jaeger and S. F. El-Mahrouky, Arch. Pharm., 2007, 340, 543 CrossRef CAS PubMed.
- M. Esmaeilpour, J. Javidi, F. Dehghani and F. Nowroozi Dodeji, RSC Adv., 2015, 5, 26625 RSC.
- H. Hu, F. Qiu, A. Ying, J. Yang and H. Meng, Int. J. Mol. Sci., 2014, 15, 6897 CrossRef CAS PubMed.
- M. Naglaa, A. El-Rahman and R. M. Borik, World Appl. Sci. J., 2014, 31, 1 Search PubMed.
- T. S. Jin, A. Q. Wang, Z. L. Cheng, J. S. Zhang and T. S. Li, Synth. Commun., 2005, 35, 137 CrossRef CAS.
- I. Devi and P. J. Bhuyan, Tetrahedron Lett., 2004, 45, 8625 CrossRef CAS.
- J. M. Khurana and A. Chaudhary, Green Chem. Lett. Rev., 2012, 5, 633 CrossRef CAS.
- J. Yang, S. Liu, H. Hu, S. Ren and A. Ying, Chin. J. Chem. Eng., 2015, 23, 1416 CrossRef CAS.
- H. R. Shaterian, M. Arman and F. Rigi, J. Mol. Liq., 2011, 158, 145 CrossRef CAS.
- J. M. Khurana, B. Nand and S. Kumar, Synth. Commun., 2011, 41, 405 CrossRef CAS.
- S. Balalaie, M. Sheikh-Ahmadi and M. Bararjanian, Catal. Commun., 2007, 8, 1724 CrossRef CAS.
- R. S. Bhosale, C. V. Magar, K. S. Solanke, S. B. Mane, S. S. Choudhary and R. P. Pawar, Synth. Commun., 2007, 37, 4353 CrossRef CAS.
- X. Z. Lian, Y. Huang, Y. Q. Li and W. J. Zheng, Monatsh. Chem., 2008, 139, 129 CrossRef CAS.
- R. Hekmatshoar, S. Majedi and K. Bakhtiari, Catal. Commun., 2008, 9, 307 CrossRef CAS.
- A. Mobinikhaledi and M. A. Bodaghifard, Acta Chim. Slov., 2010, 57, 931 CAS.
- L. Q. Yu, F. Liu and Q. D. You, Org. Prep. Proced. Int., 2009, 41, 77 CrossRef CAS.
- D. M. Pore, K. A. Undale, B. B. Dongare and U. V. Desai, Catal. Lett., 2009, 132, 104 CrossRef CAS.
- A. Khazaei, F. Gholami, V. Khakyzadeh, A. R. Moosavi-Zare and J. Afsar, RSC Adv., 2015, 5, 14305 RSC.
- B. Maleki and S. Sedigh Ashrafi, RSC Adv., 2014, 4, 42873 RSC.
- S. Nemouchi, R. Boulcina, B. Carboni and A. Debache, C. R. Chim., 2012, 15, 394 CrossRef CAS.
- G. Sabitha, K. Arundhathi, K. Sudhakar, B. S. Sastry and J. S. Yadav, Synth. Commun., 2009, 39, 433 CrossRef CAS.
- K. Ablajan, L. J. Wang, Z. Maimaiti and Y. T. Lu, Monatsh. Chem., 2014, 145, 491 CrossRef CAS.
- P. L. Anandgaonker, S. Jadhav, S. T. Gaikwad and A. S. Rajbhoj, J. Cluster Sci., 2014, 25, 483 CrossRef CAS.
- F. Shahbazi and K. Amani, Catal. Commun., 2014, 55, 57 CrossRef CAS.
- R. Y. Guo, Z. M. An, L. P. Mo, S. T. Yang, H. X. Liu, S. X. Wang and Z. H. Zhang, Tetrahedron, 2013, 69, 9931 CrossRef CAS.
- A. Azarifar, R. Nejat-Yami, M. Al-Kobaisi and D. Azarifar, J. Iran. Chem. Soc., 2013, 10, 439 CrossRef CAS.
- K. Tabatabaeian, H. Heidari, M. Mamaghani and N. O. Mahmoodi, Appl. Organomet. Chem., 2012, 26, 56 CrossRef CAS.
- F. Noori Sadeh, M. T. Maghsoodlou, N. Hazeri and M. Kangani, Res. Chem. Intermed., 2015, 41, 5907 CrossRef.
- B. Maleki, H. Eshghi, M. Barghamadi, N. Nasiri, A. Khojastehnezhad, S. Sedigh Ashrafi and O. Pourshiani, Res. Chem. Intermed., 2016, 44, 3071 CrossRef.
- A. Elmakssoudi, K. Abdelouahdi, M. Zahouily, J. Clark and A. Solhy, Catal. Commun., 2012, 29, 53 CrossRef CAS.
- M. Zahouily, Y. Abrouki and A. Rayadh, Tetrahedron Lett., 2002, 43, 7729 CrossRef CAS.
- A. Solhy, A. Elmakssoudi, R. Tahir, M. Karkouri, M. Larzek, M. Bousmina and M. Zahouily, Green Chem., 2010, 12, 2261 RSC.
- B. Maleki, S. Hemmati, A. Sedrpoushan, S. Sedigh Ashrafi and H. Veisi, RSC Adv., 2014, 4, 40505 RSC.
- B. Maleki, S. Babaee and R. Tayebee, Appl. Organomet. Chem., 2015, 29, 408 CrossRef CAS.
- B. Maleki, S. Barat Nam Chalaki, S. Sedigh Ashrafi, E. Rezaee Seresht, F. Moeinpour, A. Khojastehnezhad and R. Tayebee, Appl. Organomet. Chem., 2015, 29, 290 CrossRef CAS.
- B. Maleki and S. Sheikh, RSC Adv., 2015, 5, 42997 RSC.
- H. Eshghi, A. Khojastehnezhad, F. Moeinpour, S. Rezaeian, M. Bakavoli, M. Teymouri, A. Rostami and K. Haghbeen, Tetrahedron, 2015, 71, 436 CrossRef CAS.
- A. Khojastehnezhad, M. Rahimizadeh, H. Eshghi, F. Moeinpour and M. Bakavoli, Chin. J. Catal., 2014, 35, 376 CrossRef CAS.
- B. Maleki, E. Akbarzadeh and S. Babaee, Dyes Pigm., 2015, 123, 222 CrossRef CAS.
- M. Ghiaci, M. Zarghani, F. Moeinpour and A. Khojastehnezhad, Appl. Organomet. Chem., 2014, 28, 589 CrossRef CAS.
- A. Khojastehnezhad, M. Rahimizadeh, F. Moeinpour, H. Eshghi and M. Bakavoli, C. R. Chim., 2014, 17, 459 CrossRef CAS.
- B. Maleki, E. Sheikh, E. Rezaei Seresht, H. Eshghi, S. Sedigh Ashrafi, A. Khojastehnezhad and H. Veisi, Org. Prep. Proced. Int., 2016, 48, 37 CrossRef CAS.
- B. Maleki and M. Baghayeri, RSC Adv., 2015, 5, 79746 RSC.
- B. Maleki, Org. Prep. Proced. Int., 2016, 48, 303 CrossRef CAS.
- B. Maleki, Org. Prep. Proced. Int., 2016, 48, 81 CrossRef CAS.
- R. Tayebee, K. Savoji, M. K. Razi and B. Maleki, RSC Adv., 2016, 6, 20687 RSC.
- R. Tayebee, B. Maleki, F. Mohammadi Zonoz, R. M. Kakhki and T. Kunani, RSC Adv., 2016, 6, 55319 RSC.
- C. B. Cui, H. Kakeya and H. Osasa, Tetrahedron, 1996, 52, 12651 CrossRef CAS.
- M. Kitajima, T. Nakamura, N. Kogure, M. Ogawa, Y. Mitsuno, K. Ono, S. Yano, N. Aimi and H. Takayama, J. Nat. Prod., 2006, 69, 715 CrossRef CAS PubMed.
- M. Harada and Y. Ozaki, Chem. Pharm. Bull., 1978, 26, 48 CrossRef CAS PubMed.
- Y. Zou, Y. Hu, H. Liu and D. Shi, ACS Comb. Sci., 2012, 14, 38 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Including the Fig. S1–S4 for characterization of catalyst. See DOI: 10.1039/c6ra15800e |
| This journal is © The Royal Society of Chemistry 2016 |