Color measurement of the animal integument predicts the content of specific melanin forms†
Abstract
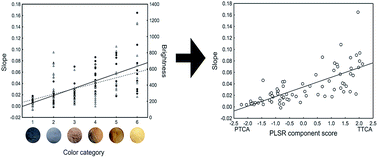
The appearance of animals largely depends on melanins present in their integument. However, it is unclear how different melanin forms create different animal color phenotypes. We used reflectance spectrophotometry to measure the color expression of feathers and hairs of 59 species of birds and 12 species of mammals, comprising a significant part of the palette of melanin-based colors, and analyzed for the first time the detailed chemical composition of melanins in the same samples by HPLC. We quantified color variation by means of the slope of percent reflectance regressed against wavelength, as this was the best predictor of a human categorization of color phenotype, increasing with the following scale: black, grey, dark brown, dark orange, light brown and light orange. Color slope variation was explained by levels of the 5,6-dihydroxyindole-2-carboxylic acid (DHICA) unit of eumelanin and the benzothiazole moiety of pheomelanin in feathers and hairs, but not by levels of the 5,6-dihydroxyindole (DHI) unit of eumelanin nor the benzothiazine moiety of pheomelanin. DHICA-eumelanin and benzothiazole-pheomelanin components explained color expression in opposite ways, decreasing and increasing, respectively, with color slope. Color slope, and also color categorization as perceived by humans, can therefore be used to infer the melanin chemical composition of feathers and hairs. Given that cytotoxic reactive oxygen species (ROS) are more abundantly formed during the synthesis of DHI than during the synthesis of DHICA in eumelanins, and in pheomelanins with higher benzothiazine/benzothiazole ratios, melanin-based colors interestingly reflect the content of the less pro-oxidant melanin forms.

 Please wait while we load your content...
Please wait while we load your content...