Vertical assembly of few-layer graphene decorated with iron oxide nanoparticles on gold surfaces†
Abstract
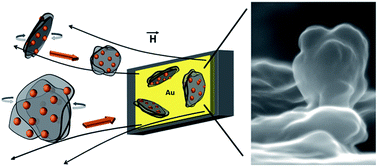
Precise assembly of exfoliated graphene sheets on electrode surfaces plays a major role for next generation electrodes to be used in sensors, batteries, fuel cells, supercapacitors and other types of energy storage devices. Here, we achieve vertical assembly of sulfonated, few-layer graphene on a metal surface. The few layer graphene flakes were extensively decorated with dopamine capped iron oxide nanoparticles utilizing electrostatic interactions between the nanoparticles and the graphene sheets. The attached nanoparticles drived the assembly of the graphene nanosheets into a vertical orientation on a gold surface under the effect of a moderate external magnetic field.


 Please wait while we load your content...
Please wait while we load your content...