Fabrication of water-repellent surfaces on galvanized steel†
F. Javier Montes Ruiz-Cabello*a,
A. Amirfazlib,
M. Cabrerizo-Vílcheza and
M. A. Rodríguez-Valverdea
aBiocolloid and Fluid Physics Group, Applied Physics Department, Faculty of Sciences, University of Granada, Campus de Fuentenueva s/n, 18071, Granada, Spain. E-mail: fjmontes@ugr.es
bDepartment of Mechanical Engineering, York University, Toronto, ON M3J 1P3, Canada
First published on 22nd July 2016
Abstract
The design of durable superhydrophobic coatings for metal surfaces is a subject of interest and research. Galvanized steel is one of the most used metallic materials for components of automobiles, building structures and roofing. In spite of its wide number of applications, galvanized steel has been scarcely modified to reach superhydrophobicity. The main reason for this is that galvanized steel is essentially a zinc-coated steel surface and most of the strategies to prepare superhydrophobic coatings on metal substrates require partial removal of the surface material. For this reason, providing a non-aggressive strategy to create superhydrophobic galvanized steel (or other metal coated materials) is an important challenge. With this aim, we propose in this paper a methodology based on a two-step texturing process (sandblasting and “soft” acid-etching) followed by a fluoropolymer deposition as a non-invasive strategy to produce water repellent surfaces on this material. The roughness of the samples was analyzed by confocal microscopy and FE-SEM imaging, the surface chemical composition by EDX and the wettability properties by contact angle measurements. Our results show that both texturing methods are necessary to create hierarchically micro/nano-structured surfaces on galvanized steel. This structure induces superhydrophobic properties once the metal is subsequently Teflon-coated. Sandblasting introduces a micro-scale texture, while soft acid-etching incorporates nano-asperities.
Introduction
Fabrication of superhydrophobic (SH) materials is investigated because water repellency is interesting for certain applications in aeronautics, building facades, cookware and air-conditioning industries. Metals are high surface energy materials, so they intrinsically have a very high affinity to liquids. At times, this is undesirable, because it leads to staining, adhesion of bacteria, food, moss, algae, paints, ice, etc.1–3 Many researchers are focused on the production of non-stick, anti-icing or liquid-repellent metal-based surfaces.4,5 In many cases, a decrease of the surface energy (hydrophobization) of the material is enough for these purposes.6,7 In other cases, superhydrophobicity or superhygrophobicity is actually required.4,8 The main challenge of SH surfaces is their durability, since they are very sensitive to wear.9,10SH surfaces have two characteristic features: they are intrinsically hydrophobic (composed of low energy materials) and they have a specific surface texture. In particular, both natural SH surfaces,11,12 and through many theoretical and experimental studies,13,14 it is seen that a two-scale (micro/nano) texture is a desirable property for SH surfaces. SH surfaces exhibit very high contact angle values (typically around 150°) and very low contact angle hysteresis (CAH), defined as the difference between the advancing and receding contact angle values.15 Due to this, SH surfaces show low drag under-water and very low shedding resistance (drops roll off easily when they come into contact).16 This property is quantified by the so-called shear adhesion.17 Moreover, SH surfaces also show a very low tensile adhesion: the liquid–solid separation has a low energy cost, which is essential for release coatings, for avoiding water condensation, icing, or promoting drop rebound upon impact.
The method to produce SH surfaces on intrinsically hydrophilic materials is typically based on two strategies: the first one is the deposition of a composite coating which incorporates the texture and surface energy required.18 The second strategy is to produce texture directly on the metal surface and then the surface energy is decreased using a hydrophobic film.5,19 The first route needs coatings with high values of mechanical resistance, cohesion, and adhesion to the substrate. Unfortunately, the two latter features are barely observed for low surface energy materials. The second route can produce a more durable coating19 because the metal surface is mechanically resistant, and the roughness features can be preserved. Additionally, if the bond between surface and coating is covalent (e.g. silanization),20,21 the surface-coating adhesion is ensured and the cohesion property of the hydrophobic coating is not required. An alternative strategy has been recently proposed where the texture and the chemical composition are simultaneously modified with femto-laser patterning.22 However, this strategy is not industrially scalable.
One of the most used strategies for roughening a metal surface to reach superhydrophobicity is acid etching.5,19,23,24 Upon certain experimental conditions (acid type, acid concentration, and etching time), the etching reaction can create a double-scale roughness. To reach the final water repellency properties, a hydrophobicizing agent is applied. This strategy has been widely applied on different metal substrates such as aluminum,4,25 iron,24 steel,19 stainless steel,5 copper,5 magnesium,21 etc.
Galvanized steel (GS) is a zinc-coated steel fabricated by galvanization and is typically used in automobiles, building structures, roofing, urban materials, signs, and many other industrial structures due to their high corrosion resistance and the low-cost of production in comparison to stainless steel.26 The zinc layer prevents the oxidation and physiochemical corrosion of the underlying iron. This layer has a thickness of few tens of microns that depends on the details of the galvanization. The production of SH coatings on GS surfaces would be relevant for some of the above applications. However, since GS surface is a coated surface itself and this protective coating should not be damaged; most strategies applied on other metallic materials are very aggressive for being applied on GS. For this reason, in spite of being one of the most widely used metallic materials, GS is not usually chosen as substrate for SH coatings. To the best of our knowledge, there are no studies where GS has been used as substrate for creating SH materials.
In this paper, we present a novel strategy to make SH surfaces on GS by a combination of a two-step texturing process: sandblasting and “soft” acid-etching, followed by a fluoropolymer (Teflon) deposition. We show that this strategy can provide water repellency properties to GS surfaces without damaging the zinc layer that protects the steel substrate.
Materials and methods
Hot dip GS samples were supplied by Modulor GmbH (Germany) in sheets of 250 × 500 mm2 and 0.5 mm in thickness. These sheets were then cut into small pieces of (35 × 40) mm2. Before using, the samples were cleaned with a 5% cleaning solution of Micro 90®, and then sonicated in ethanol 96% for 5 min. Subsequently, the samples were rinsed in distillated water and dried at room temperature.To evaluate the thickness of the zinc layer on the GS samples, a Teflon tape was used to cover part of the sample and then the bare part was immersed in a 4 M solution of HCl (Sharlab, Spain). The surface reacted immediately with the etching solution and in ∼5 min the layer of zinc was completely removed, revealed by the noticeable low reactivity. Once the reaction stopped, the sample was taken from the solution, rinsed generously with Milli-Q water and the Teflon tape was unwrapped. The sample was then dried in a flow of filtered air and the surface topography was analyzed by White Light Confocal Microscopy (Plμ-Sensofar, Spain). The horizontal scan size was set to (14.28 × 0.51) mm2 and the objective magnification was 20×, giving a lateral resolution of 1.8 μm per pixel. We ensured that the scanned area was centered at the boundary between the two regions of the sample. The vertical resolution for the z-analysis was set to 0.5 μm and the total number of vertical planes was 200. We flattened the entire topography by using an area of (4.0 × 0.5) mm2 located in the part of the sample that was not in contact with the acid. From the mean heights calculated in both parts of the sample, we evaluated the loss of thickness after the acid etching, which might be attributed, in good approximation, to the initial thickness of the native zinc layer.
In this work, we used a two-step method for roughening the surface of GS to produce hierarchically structured surfaces: (1) sandblasting and (2) “soft” acid etching. Our hypothesis is that sandblasting produce a first level of roughness at micro-scale, while “soft” acid etching introduces a second level, at nano-scale.
Inspired by previous works, where sand abrasion was used to achieve superhydrophobicity on hydrophobic materials,27,28 we applied a similar strategy aimed to increase as much as possible the surface roughness of the GS without damaging the sample substantially. For this purpose we shortly sand-blasted the surfaces with the lower pressure recommended by the manufacturer (0.3 MPa).
Sandblasting
A selection of GS samples was sand-blasted. Sandblasting was done using: Sandblast Cabinet-CAT990 (MW-TOOLS). The samples were fixed to the floor of the cabinet at 30 cm from the gun and with a relative inclination angle of 45°. The pressure was set to 0.3 MPa and all samples were treated for at least 15 s. The abrasive sand was corundum of alumina WSK 46 (Munk + Schmitz), with grain size ∼300 μm. With this abrasive, the surface roughness did not change noticeably after longer periods of blasting provided that the pressure was fixed at 0.3 MPa. Both the grain size and the set pressure affect the roughness of the sandblasted sample. We found with the above set values the best balance between the higher roughness created and the lower damage induced to the galvanized coating.Roughness measurements of the sand-blasted samples confirmed that above 10 s of sandblasting, the roughness parameters did not change noticeably (see ESI†). For this reason, the blasting time was set at 15 s.
Soft acid etching
The sand-blasted samples were treated by acid etching using a low concentration solution (0.5 M) of HCl (Sharlab, Spain) in Milli-Q water. The term “soft” is referred to the low concentration of acid, in comparison to the usual values of concentration used for similar purposes.5 The samples were immersed in 80 mL of solution for different periods from 2 min up to 10 min. Subsequently, the samples were withdrawn from the acid solution, rinsed in Milli-Q water, dried in a flow of filtered compressed air and heated at 120 °C in an oven for 10 min to remove all possible traces of water. Afterwards, the samples were cooled at ambient temperature before Teflon deposition. We noted that the reaction of the sand-blasted samples was more violent than that for untreated samples. This issue will be discussed below. For comparison, a set of samples that were acid etched (using the same etching times) but with no previous texturing by sandblasting was also prepared.Hydrophobization by fluoropolymer deposition
The textured samples were sprayed with a solution of Teflon AF1600 (Dupont) in a fluorocarbon solvent FC-72 (Fluorinet) with a ratio 1/20 (v/v). A first coating was deposited and dried at ambient temperature for 1 hour. Subsequently, a second layer was deposited, and the sample was then placed in an oven at 100 °C for 10 min to remove the remaining solvent and to promote the adherence of the fluoropolymer to the substrate, as indicated by the manufacturer. The hydrophobized samples were used after they were cooled down at room temperature.Contact angle and sliding angle measurements
The advancing contact angle (ACA), the receding contact angle (RCA) and the sliding angle (SA) were measured with a tilting plate instrument described elsewhere.29,30 We used Milli-Q water as liquid and the experiments were carried out at room temperature (25 °C). The sample was fixed to the platform in horizontal position. A drop volume of 50 μL was deposited on the sample and then the platform was inclined at 5° s−1 until the drop slid down/rolled off. During this process, we acquired images at 16 fps, i.e. an image at each 0.31° of rotation was captured. For each drop image the drop profile was extracted to quantify the “uphill” and “downhill” contact angles. For this purpose, two independent elliptical fittings31 were used. ACA and RCA were estimated by averaging the downhill and uphill contact angles, respectively, using those drop images corresponding to the moment where a net displacement of each part of the contact line with respect to its initial position was observed. The sliding angle (SA) was taken as the minimum tilting angle where both points (uphill and downhill) of the contact line were moving simultaneously.Roughness and FE-SEM images
Roughness measurements were performed with the confocal microscopy (Plμ-Sensofar) using an extended topography (composed by a matrix of 20 single topographies) with a total area of (1.26 × 0.75) mm2. For this purpose, we used a lateral resolution of 0.75 μm per pixel, an objective with magnification of 50×, capturing 200 vertical planes in steps of 0.2 μm. The topographies were flattened prior to the data analysis. We computed the Ra (arithmetic mean roughness) and Rq (root-mean squared roughness).Morphology measurements were conducted with a high resolution FE-SEM (Field-Emission Scanning Electron Microscope, Auriga, Carl Zeiss SMT). Images of (3072 × 2304) pix2 were acquired at micro and nano-scale: 1953 nm per pix and 48.83 nm per pix, respectively.
Chemical analysis by EDX
The chemical composition of surfaces was analyzed by Energy-Dispersive X-ray spectroscopy (EDX). For this purpose, we used the instrument FEG-ESEM QUEMSCAN 650F coupled with a XFlash 6/30 (Bruker) operating at 20 keV and 10 keV, depending on the chemical surface composition. The analysis was performed using an area of roughly (1 × 1) μm2, located in the center of each sample. On each sample, the weight percent of those chemical elements with a presence greater than 0.5% was quantified.Results and discussion
Wettability analysis
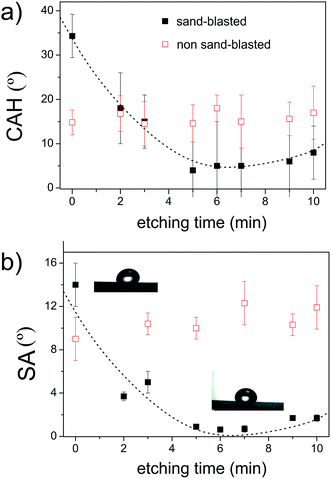
Contact angle measurements show that the two-step texturing based on sandblasting and acid etching is essential to make SH galvanized steel. Only acid etching was not enough for this purpose as it has been found for other metal surfaces.4,5 We explored a wide range of concentrations (0.5–4 M) and etching times (1–10 min) but the roughness to provide superhydrophobicity was not found. Etching the samples with HCl solutions of concentration above 1 M led to a partial or complete removal of the galvanized layer before 10 min. For these reason, the highest concentration used was 1 M. In most cases, the ACA was increased but the RCA remained constant so it was concluded that single level texturing based on acid etching, only increases the CAH value. Besides, since zinc is highly soluble in concentrated HCl solutions, long etching produced an undesired loss of the galvanized coating, as reflected by the fast oxidation observed in the samples after an intensive etching. The results for intensive acid etchings were not shown here, as they did not produce any relevant information.Results of ACA and RCA for each sample are shown in Fig. 1. The results were classified into two groups: those samples that were previously sand-blasted (black symbols) and the samples that were not sandblasted (red symbols). All the samples were acid-etched in a solution at 0.5 M of HCl for different etching times. The roughness induced by the acid etching of the smooth samples (not sand-blasted) did not produce a significant change in the wetting properties of the substrate. However, acid etching of the sandblasted sample did induce a substantial change in the ACA compared to the results of smooth samples that were etched. Unlike the smooth samples, the RCA of the sand-blasted samples changed with etching times up to values where the CAH is minimum (around 5°). After 5 min of etching, the surfaces showed water repellency properties.
These observations are reproduced in Fig. 2 where the CAH and SA in terms of etching time are plotted. From Fig. 2, it is clear that the acid-etched surfaces show very similar values of both CAH and SA, regardless of the etching time. However, for those surfaces modified with the two-step texturing process, both CAH and SA decrease substantially with the etching time down to a minimum value at 7 min. Fig. 1 and 2 confirm that the sand-blasted and acid etched surfaces are SH due to the high contact angle values and very low values of CAH and SA. As mentioned, these properties are associated to surfaces with negligible shear and tensile adhesions, i.e. SH surfaces.
The samples that were sand-blasted for only 5 s did not achieve water repellency properties after being etched for 5 min. The ACA and RCA were (139 ± 4)° and (113 ± 2)°, respectively. This suggests that a certain degree of micro roughness must be reached before etching to provide superhydrophobicity.
Roughness analysis
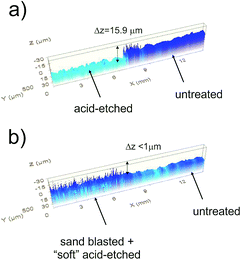
The topography obtained to evaluate the thickness of the zinc layer is shown in Fig. 3a. Here, we can observe that after an intensive etching, the height of the induced step is roughly 16 μm (note that part of the sample was protected as explained in the Experimental section).Results of the roughness parameters (Ra and Rq) measured by confocal microscopy of the samples used in this study are shown in Table 1.
| Sandblasting time (s) | Acid etching time (min) | Ra (μm) | Rq (μm) |
|---|---|---|---|
| 0 | 0 | 0.59 | 0.70 |
| 0 | 7 | 0.74 | 0.96 |
| 5 | 5 | 2.61 | 3.95 |
| 15 | 0 | 3.90 | 5.71 |
| 15 | 2 | 4.85 | 6.53 |
| 15 | 3 | 4.98 | 6.49 |
| 15 | 5 | 3.60 | 5.01 |
| 15 | 6 | 3.99 | 5.39 |
| 15 | 7 | 4.30 | 5.84 |
| 15 | 9 | 4.31 | 5.82 |
| 15 | 10 | 5.01 | 6.60 |
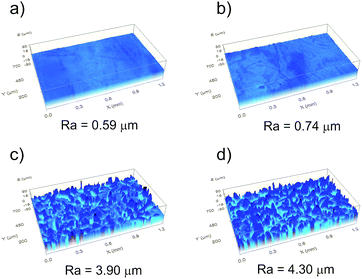
Additionally, in Fig. 4, the topographies of the most representative samples are shown: an untreated sample, an acid etched (for 7 min) sample, a sand-blasted sample, and a sample that was first sand-blasted and then acid etched (for 7 min). Last sample showed water repellent properties, if was subsequently properly covered with Teflon.
From Fig. 4 and Table 1, one can observe that for all samples that were previously sand-blasted, the “soft” acid etching did not affect the roughness parameters, since no significant difference was observed between a sand-blasted sample before etching and after (considering the 15% error bars). The differences in roughness can be attributed to the variability introduced by the sandblasting method, rather than the acid etching. Although, for those etched samples not previously sand-blasted, the roughness parameters values changed slightly after etching, but as discussed, this did not lead to meaningful changes in the wettability properties.
The effect of the sandblasting and acid-etching processes on the final texture of the samples was analyzed in more detail using the FE-SEM images of the same samples used in Fig. 4. The FE-SEM images are shown in Fig. 5. Imaging was done at two scales to analyze the microscopic and nanoscopic surface structure incorporated by each texturing process. Fig. 5a corresponds to an untreated sample. These samples reveal lower roughness at both scales of observation. Fig. 5b corresponds to an acid-etched sample and one observes that this treatment increased slightly the roughness, being more noticeable at the lowest scale, in agreement with the increase of the roughness parameters described in Table 1. Fig. 5c, compared to the two samples in Fig. 5a and b, show a substantially rougher surface at both scales. Finally, Fig. 5d shows the surface topography of a sand-blasted and acid-etched sample that is water-repellent. The topography of this sample is significantly different to the rest. In spite of the similar values of roughness parameters (micro-scale) with respect to the sand-blasted sample, here we observe that the texture is completely different, mainly due to the abundant nano-asperities, which can be observed in Fig. 5d (right). These nano-asperities are not identified in the acid-etched sample (with no sandblasting) shown in Fig. 5b (right). This confirms our hypothesis that an initial sandblasting enhances the effect of the acid etching.
Finally, to validate the non-aggressiveness of the method used to produce SH surfaces on GS, we performed a test for evaluating the thickness loss of the galvanized layer, similar to the one that was conducted to evaluate the thickness of the galvanized layer on the as-provided samples (Fig. 3a). We chose the treatment which led to the best results in terms of water-repellency. A sample was sand-blasted, but part of the sample was covered with a protective metal mask. Afterwards, the bare part was again protected by covering it with a Teflon tape. The sand-blasted part was etched in a solution of HCl 0.5 M for 7 min. This way, the part of the sample not sand-blasted was also protected from the acid etching. A topography was acquired with confocal microscopy using the same settings that in Fig. 3a aimed to evaluate the height of the step produced by both texturing processes. For comparison with the experiment performed to evaluate the thickness of the galvanized layer (shown in Fig. 3a), the topography is shown in Fig. 3b. We observed two different domains revealed by two remarkably different surface textures. Unlike the first test, we did not find a significant loss of the galvanized layer. This was also confirmed because no evidence of corrosion was observed for several weeks after the treatment. This observation disagreed for the strong acid-etched sample, where oxide deposits rapidly (hours) arose from the base steel. This test allows to validate that the procedure followed to make SH surfaces on GS produces a negligible removal of the protective zinc layer. Further, we confirmed this from the chemical analysis described in the next section.
Surface chemical composition analysis
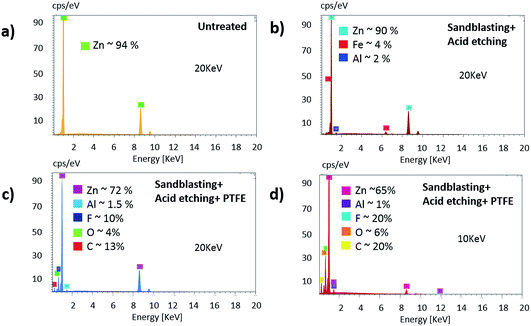
We analyzed the chemical composition of three representative samples by ESEM-EDX. In Fig. 6 the EDX spectra are shown: (a) untreated GS sample, (b) sandblasted and acid-etched (0.5 M HCl for 7 min) GS, (c) and (d) sandblasted and acid-etched (0.5 M HCl for 7 min) GS coated with Teflon. The difference between (c) and (d) is the energy applied to the detector (20 keV and 10 keV, respectively). These different settings were chosen to determine more precisely the weight percent of the lighter elements (e.g. F, O and C) which should be present in this sample. The spectrum of the untreated sample (Fig. 6a) shows a surface basically composed of zinc, with the weight percent roughly equal to 94%. The spectrum of the sandblasted and acid-etched sample (Fig. 6b) show that after both texturing processes small contents of Fe and Al are detected although the content of zinc prevails (90%). The Fe and Al detection may come from the inner steel, since the signal captured by EDX, given the penetration distance is typically of the order of few microns depending on the energy applied to the detector. The galvanized layer contains iron and its content increases with depth.26 Additionally, the absence of oxygen within the detected elements rules out the possible oxidation of the sample. After coating the GS surface with Teflon (Fig. 5c) the iron signal disappeared, while the zinc content remained still substantial. This surface also contains F (10%) and C (13%) from the PTFE molecule. The analysis performed on the same sample but using a lower excitation energy (10 keV), showed a higher weight percent of F (20%), C (20%) and lower weight percent of Zn. By reducing the activation energy, the penetration length of the detection is also reduced. The mass ratio between the elements F and C in a Teflon molecule (C2F4) is around 2. The fact that the detected weight percent for C is similar to F, and the presence of O, suggested the presence of other organic components on the surface. Finally, the presence of a strong Zn signal means that the Zn layer is preserved on the steel, so the benefit of GS is kept in this method of producing superhydrophobic surfaces.Conclusions
In this paper, we present a novel strategy to produce superhydrophobic surfaces on galvanized steel using a two-step texturing process (sandblasting followed by “soft” acid etching), and a fluoropolymer deposition. The strategy is aimed to induce, through a non-aggressive procedure, a double-scale (micro-nano) texture, which confers to the surface water repellency, once it has been properly covered by a low surface energy coating. We validated by topographic and chemical analysis (EDX) of the samples that the overall texturing process did not damage the protective layer of galvanized steel. The minimum degree of micro-roughness required is characterized by the roughness parameters Ra ∼ 3.5 μm and Rq ∼ 5 μm. The “soft” acid etching (0.5 M), led to superhydrophobicity for etching times greater than 5 min.Our results point out to that the micro-roughness of the treated samples dictates their wettability properties until the micro-roughness increases up to a certain degree (Ra above 3.5 μm in this case). Then, the wettability properties are much more sensitive to changes in the nano-roughness. To the best of our knowledge, this is the first time that superhydrophobic surfaces are fabricated on galvanized steel. The strategy developed in this work opens a new route to produce superhydrophobic surfaces on coated metal materials which may be applied to other similar surfaces such as aluminized steel or nickelled metals.
Acknowledgements
This research was supported by the projects: MAT2014-60615R funded by MICINN, the project P12-FQM-1443 funded by “Junta de Andalucía” the companies CETURSA Sierra Nevada S. A. (Spain), Doppelmayr Seilbahnen GmbH (Austria) and Siltech Corporation (Canada). F. J. Montes Ruiz-Cabello acknowledges the group FQM115 for the financial support within the program “Fortalecimiento”. Authors thank Dr Guillermo Guerrero Vacas of the University of Córdoba for their support and lab assistance.References
- X. Zhang, L. Wang and E. Levanen, RSC Adv., 2013, 3, 12003–12020 RSC.
- A. B. Tesler, P. Kim, S. Kolle, C. Howell, O. Ahanotu and J. Aizenberg, Nat. Commun., 2015, 6, 8649 CrossRef CAS PubMed.
- Y. Wang, X. Wei Liu, H. F. Zhang and Z. P. Zhou, AIP Adv., 2015, 5, 041314 CrossRef.
- C. Antonini, M. Innocenti, T. Horn, M. Marengo and A. Amirfazli, Cold Reg. Sci. Technol., 2011, 67, 58–67 CrossRef.
- L. Li, V. Breedveld and D. W. Hess, ACS Appl. Mater. Interfaces, 2012, 4, 4549–4556 CAS.
- J. W. Krumpfer and T. J. McCarthy, Faraday Discuss., 2010, 146, 103–111 RSC.
- D. L. Schmidt, C. E. Coburn, B. M. DeKoven, G. E. Potter, G. F. Meyers and D. A. Fischer, Nature, 1994, 368, 39–41 CrossRef CAS.
- Z. Guo, F. Zhou, J. Hao and W. Liu, J. Am. Chem. Soc., 2005, 127, 15670–15671 CrossRef CAS PubMed.
- G. Wang, S. Liu, S. Wei, Y. Liu, J. Lian and Q. Jiang, Sci. Rep., 2016, 6, 20933 CrossRef CAS PubMed.
- L. Boinovich, A. M. Emelyanenko and A. S. Pashinin, ACS Appl. Mater. Interfaces, 2010, 2, 1754–1758 CAS.
- W. Li and A. Amirfazli, Soft Matter, 2008, 4, 462–466 RSC.
- M. Sun, C. Luo, L. Xu, H. Ji, Q. Ouyang, D. Yu and Y. Chen, Langmuir, 2005, 21, 8978–8981 CrossRef CAS PubMed.
- Y. Xue, S. Chu, P. Lv and H. Duan, Langmuir, 2012, 28, 9440–9450 CrossRef CAS PubMed.
- S. Pechook and B. Pokroy, Soft Matter, 2013, 9, 5710–5715 RSC.
- A. Marmur, Langmuir, 2003, 19, 8343–8348 CrossRef CAS.
- A. J. B. Milne and A. Amirfazli, Langmuir, 2009, 25, 14155–14164 CrossRef CAS PubMed.
- L. Gao and T. J. McCarthy, Langmuir, 2008, 24, 9183–9188 CrossRef CAS PubMed.
- L. Zhai, F. Ç. Cebeci, R. E. Cohen and M. F. Rubner, Nano Lett., 2004, 4, 1349–1353 CrossRef CAS.
- N. Wang, D. Xiong, Y. Deng, Y. Shi and K. Wang, ACS Appl. Mater. Interfaces, 2015, 7, 6260–6272 CAS.
- H. Wang, Y. Xue, J. Ding, L. Feng, X. Wang and T. Lin, Angew. Chem., Int. Ed., 2011, 50, 11433–11436 CrossRef CAS PubMed.
- W. Xu, J. Song, J. Sun, Y. Lu and Z. Yu, ACS Appl. Mater. Interfaces, 2011, 3, 4404–4414 CAS.
- B. Wu, M. Zhou, J. Li, X. Ye, G. Li and L. Cai, Appl. Surf. Sci., 2009, 256, 61–66 CrossRef CAS.
- B. Qian and Z. Shen, Langmuir, 2005, 21, 9007–9009 CrossRef CAS PubMed.
- H. Gao, S. Lu, W. Xu, S. Szunerits and R. Boukherroub, RSC Adv., 2015, 5, 40657–40667 RSC.
- H. Zhang, L. Yin, L. Li, S. Shi, Y. Wang and X. Liu, RSC Adv., 2016, 6, 14034–14041 RSC.
- A. P. Yadav, H. Katayama, K. Noda, H. Masuda, A. Nishikata and T. Tsuru, Corros. Sci., 2007, 49, 3716–3731 CrossRef CAS.
- M. A. Nilsson, R. J. Daniello and J. P. Rothstein, J. Phys. D: Appl. Phys., 2010, 43, 045301 CrossRef.
- D. P. Datta, S. K. Garg, I. Thakur, B. Satpati, P. K. Sahoo, D. Kanjilal and T. Som, RSC Adv., 2016, 6, 48919–48926 RSC.
- E. Pierce, F. J. Carmona and A. Amirfazli, Colloids Surf., A, 2008, 323, 73–82 CrossRef CAS.
- F. J. M. Ruiz-Cabello, M. A. Rodriguez-Valverde and M. Cabrerizo-Vilchez, Soft Matter, 2011, 7, 10457–10461 RSC.
- M. Bortolotti, M. Brugnara, C. D. Volpe and S. Siboni, J. Colloid Interface Sci., 2009, 336, 285–297 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra15451d |
| This journal is © The Royal Society of Chemistry 2016 |