Solvent-polarity-tuned nanostructures assembled from modified octadecylcarbamate with an anthracen moiety†
Yimeng Zhang,
Pengyao Xing,
Minmin Yang,
Yajie Wang,
Bo Wang,
Aiyou Hao* and
Mingfang Ma
Key Laboratory of Colloid and Interface Chemistry of Ministry of Education, School of Chemistry and Chemical Engineering, Shandong University, Jinan 250100, P. R. China. E-mail: haoay@sdu.edu.cn; Fax: +86 531 88564464; Tel: +86 531 88363306
First published on 19th July 2016
Abstract
Anthracen-9-ylmethyl octadecylcarbamate (A-9-YMOC) contains an anthracen headgroup, an imide group, and a long alkyl chain, which could show π–π stacking, hydrogen-bonding, and van der Waals interactions, respectively. The capability of A-9-YMOC to form well-ordered micro/nanostructures in different solvents was explored. And the influence of solvents with various polarities that can modulate the self-assembly behavior, including H-bond-forming solvents, π-stacking-forming solvents or van der Waals-type solvents was studied. In addition, different supramolecular architectures, including nanofibers and nanoflowers were observed during the solvent-polarity-tuned process, which were found to have different molecular arrangements. This work may provide a facile method to manipulate the morphology and properties of self-assembled low-molecular-weight organic building blocks.
Introduction
Structure formation and transformation of self-assembled architectures in terms of weak intermolecular interactions are of vital importance and relate to many bio-events.1 Molecular self-assembly is a spontaneous process in the formation of ordered structures under the synergistic effect of intermolecular noncovalent interactions, including hydrogen-bonding, π–π, electrostatic, hydrophobic, and van der Waals interactions.2 Fabrication of nanostructures with tunable size, dimension, and morphology is of particular importance to make high-performance nanomaterials.3 For example, Zang and co-workers studied the relationship between structures of building block and properties of aggregates.4 Apart from molecular topology, exoteric conditions, such as assembly rate,5 environmental pH value,6 ultrasound,7 redox potentials,8 and solvents,9 have also been shown to have remarkable effects on the morphology/function of self-assembled structures ascribed to the variation of noncovalent interactions between solute–solvent molecules. In particular, solvent environment which is the second supramolecular partner of each self-assembly system, is crucial for regulating the thermodynamic assembly process. Polarity, as an important parameter of solvents, may disturb the synergistic effect of these noncovalent interactions and further affect the self-assembled nanostructures.10 Therefore, processes for tuning self-assembled structures by solvent polarity and insight into the noncovalent interactions in regulation and control morphology are of crucial importance for comprehending the organization mechanism of complex systems.Careful design of molecules to promote controllable self-assembly towards optimal supramolecular structures has become a challenging task considering the delicate balance between kinetics and thermodynamics in particular self-assembly system.11 Herein, we designed an A-9-YMOC molecule containing an anthracen headgroup, an imide group, and a long alkyl chain, which could show π–π stacking, hydrogen-bonding, and van der Waals interactions, respectively. Owing to the simple molecular structure and distinct intermolecular interactions, it could offer the possibility to study the effect of solvent on its self-assembly properties. Interestingly, diverse structures including nanofibers and nanoflowers were achieved by altering solvents. A-9-YMOC tends to form nanofibers whereas it underwent self-assembly to form long nanofibrils in polar solvents and nanoflowers in nonpolar solvents, as shown in Scheme 1. In this work, we proposed different kinds of oriented solvents and found that the interactions between the solute–solvent in various polarity solvents could subtly change the stacking of the molecules and further influence their self-assembled nanostructures. The finding of solvent-tuning self-assembly may provide more insight into nanostructure formation and transformation. This also opens up an alternative way for mediating assembly and functional integration of a range of molecules.
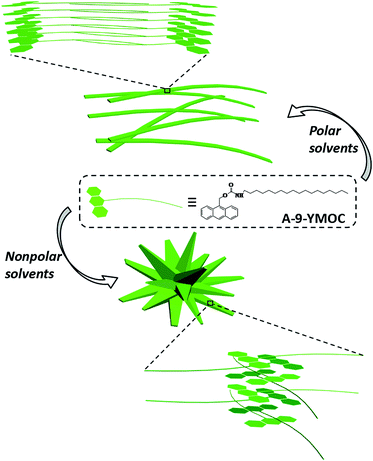 | ||
| Scheme 1 Molecular structure of A-9-YMOC and schematic representation of its self-assembled architectures in various solvents. | ||
Experimental section
Materials
Octadecyl isocyanate and 9-anthracenemethanol were purchased from Aladdin Bio-chem Technology Co. Ltd, Shanghai, China. MeOH, acetone, EtOAc, THF, phenylcarbinol, chlorobenzene, toluene, methylcyclohexane, and n-dodecane were of analytical reagent (AR) grade and were purchased from Sinopharm Chemical Reagent Co. Ltd, Shanghai, China. All other reagents were obtained from commercial chemical sources. All reagents were used without further purification. All experiments were performed with deionized water.Synthesis
The powders of 9-anthracenemethanol (0.21 g, 1 mmol) were dispersed in anhydrous toluene (40 mL). After octadecyl isocyanate (0.44 g, 1.5 mmol) were added, the mixture was stirred at 100 °C for 20 h. After the mixture was cooled to ambient temperature, faint yellow precipitates were collected by suction filtration with a sand core glass filter and washed thoroughly with ethanol. Then the target compound recrystallized in toluene and was dried under reduced pressure to yield A-9-YMOC as a light yellow power (0.33 g, 65% yield).Preparation of the samples
To prepare the samples for characterization, A-9-YMOC (10 mg) was dispersed in different solvents (1 mL) by heating until it dissolved. The solutions were sonicated in a water bath for 1 min and then deposited for another 8 h. The THF/H2O mixtures with different ratios were obtained by adding H2O into the transparent THF solution. The concentrations of all the samples were 1 wt%. The samples with other different solvents were prepared in the same way.Characterization
1H and 13C NMR spectra were recorded on a Bruker Advance DMX 400 MHz instrument using TMS as internal standard and CDCl3 as solvent. Electrospray ionization mass spectrometry was performed on a Bruker APEXII spectrometer. The SEM images were taken with an S-4800 scanning electron microscope. The supernatant of each solution was allowed to dry on a carbon-coated copper grid by solvent evaporation, followed by sputter-coating with platinum. FT-IR spectra of the samples were measured by using a TENSOR 27 FTIR spectrometer (Bruker). The dried microcrystals were pressed into KBr pellets. The solution based FTIR spectra were measured by painting on the KBr windows pressed by KBr powder. The fluorescence were recorded by a model FL-4500 spectrofluorometer (Hitachi, Tokyo, Japan), and the samples were excited at 365 nm. X-ray diffraction data were collected at room temperature with a graphite monochromatic device and a Cu filter, Cu Kα1 radiation (λ = 1.5406 Å) on an Empyrean (Panalytical, Nederland) instrument, operated in the θ:2θ mode in 0.5–10° (2θ) range and stepscan of 2θ = 0.04°. For the rheological properties, the wet gels were used directly on a Thermo Scientific HAAKE Rheo-Stress 6000 apparatus equipped with a thermostat set at a temperature of 25 °C. The elastic and viscous moduli were measured at an oscillating stress (100 Pa) and frequency (1 Hz) in the linear viscoelastic region. The optimized simulation and molecular models were drawn using the Materials Studio 5.0 software by Accelrys.Results and discussion
A-9-YMOC was synthesized via direct coupling between 9-anthracenemethanol and octadecyl isocyanate in toluene at 100 °C (Scheme S1†). The structure of A-9-YMOC was characterized using 1H NMR, 13C NMR, ESI-MS, and FT-IR (Fig. S1–S4†). There are two considerations for the design of the compound. First, anthracene chromophore has strong and well-studied π–π interaction that promotes cofacial stacking to form one-dimensional structures. Second, the long-chain unit linked at the imides node provides hydrogen-bonding and van der Waals interaction to additionally facilitate the well-ordered structure formation.Formation of aggregates in various solvents
We prepared samples (1 wt%) to gain assemble aggregates in many organic solvents, from nonpolar solvents, such as toluene, to polar solvents, such as MeOH. These A-9-YMOC solutions were clear and stable at low concentration ranges (Table 1), whereas precipitates were observed at higher concentrations. Actually, A-9-YMOC self-assembled into well-ordered and interlaced nanostructures with changeable morphology depending on the solvent polarity (Scheme 1). A diverse range of parameters have been used to describe the interactions in self-assembly, as well as a normalized empirical polarity parameter, ENT.12 In this model, the solvents are described in terms of their ability to donate (α) and accept hydrogen-bonding (β). Table 1 lists the solvent parameters and the solubility of A-9-YMOC in various organic solvents. However, we envision that the π–π stacking or hydrogen-bonding nature of a solvent can be more-precisely related to the assembled structures.| Solvent | Solubility [mg mL−1] | ENT | α | β |
|---|---|---|---|---|
| MeOH | 0.1 | 0.762 | 0.93 | 0.62 |
| Acetone | 0.5 | 0.355 | 0.06 | 0.48 |
| EtOAc | 0.5 | 0.228 | 0.00 | 0.45 |
| THF | 1.0 | 0.207 | 0.00 | 0.55 |
| Phenylcarbinol | 0.2 | 0.198 | — | 0.50 |
| Chlorobenzene | 0.5 | 0.188 | 0.00 | 0.06 |
| Toluene | 0.5 | 0.099 | 0.00 | 0.11 |
| Methylcyclohexane | 0.2 | 0.006 | 0.00 | 0.00 |
Solvent-tuned morphology of the assemblies
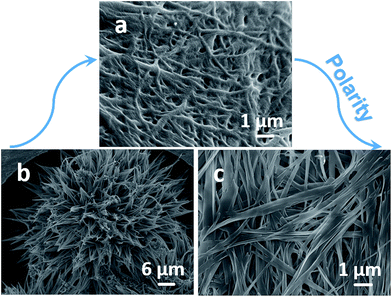
As shown in Fig. 1, the morphology of the precipitates from different kinds of solvents was observed by SEM. Generally, the polarity parameter of a solvent plays an important role in the formation of nanostructures. Remarkably different morphology forms by changing solvent environments. In polar solvents with high β and α values, such as MeOH, acetone, EtOAc, THF, and phenylcarbinol, fibrous nanostructures with high aspect ratios are observed (Fig. 1a–c and e). The width of these fibers is ranging from 200 to 300 nm, and numerous fibers are highly intertwined with each other firmly to form 3D networks. In THF, very similar fibrous structures are observed (Fig. 1d), but the width of the fibers is smaller, which may be attributed by the good solubility. When nonpolar solvents including chlorobenzene, toluene, and methylcyclohexane are used, nanoflower structures composed of short rods are obtained with polydispersity (Fig. 1e–h). The nanostructures have flower-like shapes, with a decreasing width from interior to exterior. The diameter of a nanoflower is about 30 μm. The mean width of nanorods which constitute nanoflowers is about 1 μm at one end, and narrowed down to about 300 nm at the other end (a sharp tip morphology). Stronger π–π stacking interaction may contribute to this process. In addition to solubility and π–π stacking interaction, the hydrogen-bonding nature of solvent molecules also affects the self-assembly of A-9-YMOC. Taking phenylcarbinol and toluene as examples, the obvious difference between their molecular structures is that the replacement of the hydrogen atom on the methyl H atom by a hydroxyl group. This leads to the fact that phenylcarbinol can donate a hydrogen atom to form a hydrogen-bonding site with the imide moiety of A-9-YMOC. Thus nanofiber morphology forms in phenylcarbinol while nanoflower forms in toluene. | ||
| Fig. 1 SEM images of A-9-YMOC aggregates that formed in (a) MeOH, (b) acetone, (c) EtOAc, (d) THF, (e) phenylcarbinol, (f) chlorobenzene, (g) toluene, and (h) methylcyclohexane. | ||
Fluorescence emission and FTIR spectroscopy
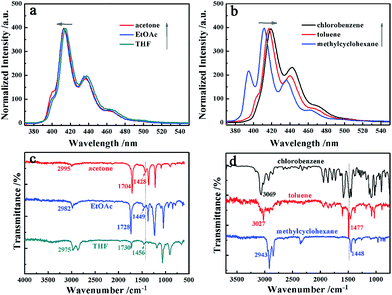
To gain further insight into these diverse nanostructures in different solvents, fluorescence emission and FTIR spectroscopy were performed, as shown in Fig. 2. Three emission bands in fluorescence spectra are ascribed to the π–π stacking interactions of the anthracene moieties (Fig. 2a and b). The amide III absorption band, which appear at 1430–1470 cm−1, suggests the hydrogen bonding between the amide groups, while the shifts of strong peaks for stretching vibrations of CH2 at 2800–3000 cm−1 demonstrate the orderly packing of alkyl chains. These all suggests that the A-9-YMOC molecules form efficient assemblies (Fig. 2c and d).13 It is interesting to find that the shifts of A-9-YMOC assemblies in emission and IR spectra are strongly depended on the solvents. Two kinds of opposite shifts are observed in different oriented solvents. We designates several kinds of oriented solvents as H-bond-forming solvents, π-stacking-forming solvents and van der Waals type-solvents due to their different polarity and molecular structures. When MeOH, acetone, EtOAc, and THF with high β and α values are used, which would provide hydrogen-bonding sites, they could form hydrogen-bonding with the nitrogen or oxygen atoms of solute molecules. We ascribed these solvents to H-bond-forming solvents. However, we attributes chlorobenzene and toluene with low β and α values to π-stacking-forming solvents because of their aromatic rings or ability to form π–π stacking interaction. Methylcyclohexane has van der Waals interaction primarily, but given the similarity of polarity, we group it into π-stacking-forming solvents. Considering phenylcarbinol's specificity as both an H-bond-forming solvent and a π-stacking-forming solvent, thus, it doesn't merit any additional discussion here.As polarity increases in H-bond-forming solvents, the emission bands of A-9-YMOC in Fig. 2a show a slight blue-shift, indicating a weak π-stacking interaction.14 The peaks for amide III in C–N stretching vibration exhibit low-wavenumber shifts in Fig. 2c, which indicates the intensive hydrogen-bonding interaction.13 While polarity increases in π-stacking-forming solvents, a red-shift reveals enhanced π–π stacking interaction observed in Fig. 2b and high-wavenumber shifts of amide III observed in Fig. 2d. These different shifts and their changes with polarity can be ascribed to different interactions between A-9-YMOC and the solvents, indicating that the hydrogen-bonding strength in π-stacking-forming solvents is weaker than that in H-bond-forming solvents but π–π stacking interaction in π-stacking-forming solvents seems to be stronger. This leads to a conclusion that the aggregates formation in H-bond-forming solvents maybe driven by strong hydrogen-bonding interactions, whereas intense π-stacking interactions are favored in assemblies formation that are obtained from π-stacking-forming solvents.
H-bond-forming solvents can affect the hydrogen-bonding of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and N–H in A-9-YMOC molecules with themselves, but this does not induce π–π stacking between molecules. The directional hydrogen-bonding promotes a long-range-ordered arrangement of A-9-YMOC molecules, preferentially along one dimension to form nanofibers or nanobelts.15 In this kind of solvents, the amide groups and the aromatic rings are packed outside the stacking arrays, similar to the case in the bulk phase. As polarity increases, the solvent molecules are suggested to form hydrogen-bonding with A-9-YMOC molecules more active but delicate changes among aromatic rings appear, owing to the alienated π–π stacking of the aromatic moiety. On the other hand, in π-stacking-forming solvents, like chlorobenzene, toluene, and methylcyclohexane with low polarity, the polar group of A-9-YMOC is buried inside the superstructures; thus, the microenvironment of the hydrogen-bonding is different to that in the bulk state. With increasing polarity, the aromatic rings of A-9-YMOC molecules are repelled from their embedment microenvironment and start to react with the solvent, which is favorable for π–π stacking interaction but weak hydrogen-bonding interaction.15
O and N–H in A-9-YMOC molecules with themselves, but this does not induce π–π stacking between molecules. The directional hydrogen-bonding promotes a long-range-ordered arrangement of A-9-YMOC molecules, preferentially along one dimension to form nanofibers or nanobelts.15 In this kind of solvents, the amide groups and the aromatic rings are packed outside the stacking arrays, similar to the case in the bulk phase. As polarity increases, the solvent molecules are suggested to form hydrogen-bonding with A-9-YMOC molecules more active but delicate changes among aromatic rings appear, owing to the alienated π–π stacking of the aromatic moiety. On the other hand, in π-stacking-forming solvents, like chlorobenzene, toluene, and methylcyclohexane with low polarity, the polar group of A-9-YMOC is buried inside the superstructures; thus, the microenvironment of the hydrogen-bonding is different to that in the bulk state. With increasing polarity, the aromatic rings of A-9-YMOC molecules are repelled from their embedment microenvironment and start to react with the solvent, which is favorable for π–π stacking interaction but weak hydrogen-bonding interaction.15
XRD patterns and possible molecule arrangement
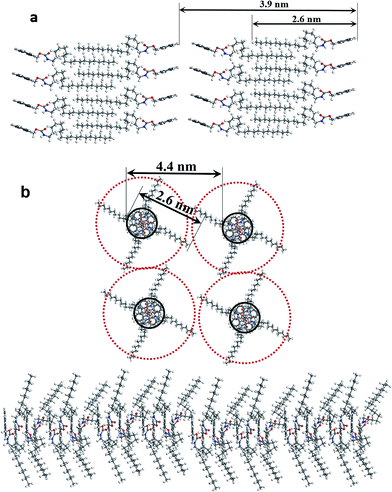
Fig. 3 shows XRD patterns of A-9-YMOC dried samples obtained from various solvents, which are also solvent-dependent. The samples selected from polar solvents, show well-defined diffraction patterns. For example, three major diffraction peaks located at low angle ranges in THF correspond to d-spacings of 3.9, 2.0 and 1.4 nm, based on Bragg's equation, which is in good consistent with the ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1/2
1/2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1/3, indicating a lamellar structure formed by parallel stacking within the fibers.16 The length of 3.9 nm calculated from the (001) peak is the length of an A-9-YMOC dimer with overlapped units, ascribed to the fact that 3.9 nm is larger than one single molecule length (ca. 2.6 nm estimated from energy minimized molecular geometry based on Material Studio 5.0 software) while less than two (ca. 5.2 nm), implying that the self-assembled structures of A-9-YMOC is made up from the interdigitated bilayer structures. Notably, for the aggregates from nonpolar solvents, such as toluene also exhibit a well-defined diffraction pattern. The d-spacings of 4.4, 3.5, and 2.1 nm, as obtained from the first three major peaks, are in agreement with a ratio of 1
1/3, indicating a lamellar structure formed by parallel stacking within the fibers.16 The length of 3.9 nm calculated from the (001) peak is the length of an A-9-YMOC dimer with overlapped units, ascribed to the fact that 3.9 nm is larger than one single molecule length (ca. 2.6 nm estimated from energy minimized molecular geometry based on Material Studio 5.0 software) while less than two (ca. 5.2 nm), implying that the self-assembled structures of A-9-YMOC is made up from the interdigitated bilayer structures. Notably, for the aggregates from nonpolar solvents, such as toluene also exhibit a well-defined diffraction pattern. The d-spacings of 4.4, 3.5, and 2.1 nm, as obtained from the first three major peaks, are in agreement with a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1/√2
1/√2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1/√3. These peaks match well the tetragonal columnar packing mode.17 The layer spacing value can be determined to be 4.4 nm according to the first peak, which is the center distance of individual columnar units. This number is in accordance with the length of double A-9-YMOC molecules with overlapped groups. Within this kind of molecular packing, an orthogonal overlap of polar heads induced by π–π stacking may occur, and the nonpolar tails would stack outside the columnar array. The interdigitization of molecules in assemblies is responsible for the emergence of the d-spacings (1.5, 1.2 in toluene and 1.4, 1.2 nm in THF) in wide angle ranges. These strong peaks are assigned to the spacing between the overlapped alkyl chains, and the π–π stacked aromatic groups.14,18 Apparently, the second diffraction peak sharpens in toluene while others have low peak intensities. This phenomenon can be easily understood, because in tetragonal packing, it is harder to show highly ordered structures with two or multiple dimensions due to their flexible orientations in π-stacking-forming solvents. Self-assembly is favored with the growth of A-9-YMOC into each dimension at a more or less relative rate. When H-bond-forming solvents are used, directional hydrogen-bonding will promote the growth of A-9-YMOC molecules in one dimension, resulting in the formation of 1D nanofibers.10
1/√3. These peaks match well the tetragonal columnar packing mode.17 The layer spacing value can be determined to be 4.4 nm according to the first peak, which is the center distance of individual columnar units. This number is in accordance with the length of double A-9-YMOC molecules with overlapped groups. Within this kind of molecular packing, an orthogonal overlap of polar heads induced by π–π stacking may occur, and the nonpolar tails would stack outside the columnar array. The interdigitization of molecules in assemblies is responsible for the emergence of the d-spacings (1.5, 1.2 in toluene and 1.4, 1.2 nm in THF) in wide angle ranges. These strong peaks are assigned to the spacing between the overlapped alkyl chains, and the π–π stacked aromatic groups.14,18 Apparently, the second diffraction peak sharpens in toluene while others have low peak intensities. This phenomenon can be easily understood, because in tetragonal packing, it is harder to show highly ordered structures with two or multiple dimensions due to their flexible orientations in π-stacking-forming solvents. Self-assembly is favored with the growth of A-9-YMOC into each dimension at a more or less relative rate. When H-bond-forming solvents are used, directional hydrogen-bonding will promote the growth of A-9-YMOC molecules in one dimension, resulting in the formation of 1D nanofibers.10
 | ||
| Fig. 3 XRD pattern of A-9-YMOC dried samples in H-bonding-forming solvent (THF) and π-stacking-forming solvent (toluene). | ||
During self-assembly process, although π–π stacking, hydrogen-bonding, and hydrophobic interaction should act synergistically, their growth preference should differ depending on the solvent. Based on these experimental observations, the behavior results from different orientations of solvents in the molecular packing and schematic models for molecular arrangements can be confirmed as shown in Scheme 2. In polar solvents, the A-9-YMOC molecules pack in such a way that the long alkyl chains pack in the interior of bilayer arrays to avoid the solvent molecules and its polar groups pack outside, so that the hydrogen-bonding of amide groups is the main driving forces for the assembly. With such well-ordered lamellar structure, the bilayer structures can extend to a longer range and favor the formation of nanofiber structures. Conversely, in nonpolar solvents, the A-9-YMOC molecule tends to form tetragonal columnar structures. However, in this case, the alkyl chains extend outside of the structure and the polar groups remain inside. The π–π stacking and van der Walls interactions are mainly attributed to this kind of self-assembly. Under ambient conditions, nuclei forms quickly due to π–π stacking interactions, and then the nuclei directly grow into flat nanofibers in an elongation process. In such a tetragonal case which is not the most tightly packed mode, the large steric hindrance of the arrangement would inhibit the stacking of the structure to a large extent. Thus, nanoflower structures are obtained.
Tuning the morphology of the nanostructures in THF solvent system
Very interestingly, the morphology of the A-9-YMOC nanostructures showed an obvious responsiveness towards component solvents system. The A-9-YMOC sample obtained from THF was taken as an example, as shown in Fig. 1d. When the aggregates were prepared in THF/water with high polarity, we observed the clear formation of nanofiber structures (Fig. 4c). The width of the nanofibers in THF/water was about 300–400 nm, which was larger than the assembly structures that were directly prepared from THF. The width of fibers in THF is ranging from 30 to 50 nm, and numerous fibers are highly intertwined with each other firmly to form 3D networks. The fibers in THF are slender and compact, but in THF/water are wide and flat, which may be attributed by the poor solubility and larger polarity. When the aggregates prepared in THF/n-dodecane with relative low polarity, nanoflower structures appeared (Fig. 4b). The nanoflower structures, with some polydispersity and sharp tips at the ends of the nanoribbon strips, had similar morphology and size to those directly obtained from nonpolar solvents.This work was also exemplified by the fluorescence emission spectra and FT-IR spectra, as shown in Fig. 5. In comparison to aggregates obtained in THF, the emission band of the anthracene moieties exhibits a red-shift in THF/n-dodecane but a blue-shift in THF/water, which indicates a strong π–π stacking interaction in polar solvents and weak one in nonpolar solvents. As for FT-IR spectra, the peaks of amide III are not distinct in some solvents, but those of amide I provide the similar information. A low-wavenumber shift of amide I observed in THF/water indicates a strong hydrogen-bonding interaction, while a high one indicates a weak interaction in nonpolar solvents.
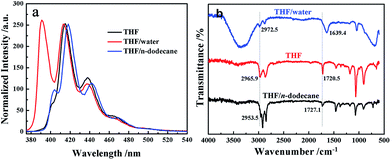 | ||
| Fig. 5 (a) Fluorescence emission spectra (b) FT-IR spectra of A-9-YMOC aggregates that were obtained in THF solvent system. | ||
This result indicates that a weak hydrogen-bonding and a strong π–π stacking form in nonpolar solvents, which means a nonpolar solvent could be a π-stacking-forming solvent even it has no aromatic ring or ability to form π–π stacking interaction.
In addition, through controlling the ratio of the THF/water solvents, gels obtained according to the “inversion of a test tube” method. Rheological characterization was conducted to study the rigidity and flow behavior of a gel at a ratio of 2/8 in Fig. S5,† which verified the gel state. Oscillatory stress–sweep provided a high yield stress and frequency-sweep also confirmed its good mechanical strength.19
All of the different solvents formed favorable interactions with A-9-YMOC molecules, which subtly affected the packing of the molecules and, subsequently, their final morphology. Thus, these nanostructures could be controlled by solvent polarity.
Conclusions
We have systematically investigated the effect of solvent on the morphology and mechanism of A-9-YMOC assemblies. It can arrange itself into nanoflowers in π-stacking-forming solvents or nanofibers in H-bond-forming solvents with different molecular stacking modes. Tuning the morphology of the nanostructures in THF solvent system verified the mechanism. Thus, by choosing appropriate solvents, we could achieve the transition of morphology from nanofibers into nanoflowers. This work provided an important clue in the design of functional assemblies by taking account of the interactions with the solvents.Acknowledgements
A. H. thanks the research foundation, Department of Science & Technology of Shandong province, China for financial support. The authors acknowledge Dr Shangyang Li, College of Science, Agricultural University of Hebei, for linguistic advice and all the members, especially Mr Rongfeng Ding of Hao laboratory for fruitful discussion and suggestions.Notes and references
- (a) M. G. Krone, L. Hua, P. Soto, R. Zhou, B. J. Berne and J.-E. Shea, J. Am. Chem. Soc., 2008, 130, 11066–11072 CrossRef PubMed; (b) T. P. Knowles, A. W. Fitzpatrick, S. Meehan, H. R. Mott, M. Vendruscolo, C. M. Dobson and M. E. Welland, Science, 2007, 318, 1900–1903 CrossRef CAS PubMed.
- (a) O. Gronwald, E. Snip and S. Shinkai, Curr. Opin. Colloid Interface Sci., 2002, 7, 148–156 CrossRef CAS; (b) M. George and R. G. Weiss, Acc. Chem. Res., 2006, 39, 489–497 CrossRef CAS PubMed; (c) G. M. Whitesides and B. Grzybowski, Science, 2002, 295, 2418–2421 CrossRef CAS PubMed.
- (a) Y. Oaki, M. Kijima and H. Imai, J. Am. Chem. Soc., 2011, 133, 8594–8599 CrossRef CAS PubMed; (b) V. C. Sundar, J. Zaumseil, V. Podzorov, E. Menard, R. L. Willett, T. Someya, M. E. Gershenson and J. A. Rogers, Science, 2004, 303, 1644–1646 CrossRef CAS PubMed; (c) L. Zang, Y. K. Che and J. S. Moore, Acc. Chem. Res., 2008, 41, 1596–1608 CrossRef CAS PubMed; (d) I. J. Li, W. P. Hu, Q. Y. Liu and D. B. Zhu, Acc. Chem. Res., 2010, 43, 529–540 CrossRef PubMed; (e) I. D. W. Samuel and G. A. Turnbull, Chem. Rev., 2007, 107, 1272–1295 CrossRef CAS PubMed; (f) M. Huang, U. Schilde, M. Kumke, M. Antonietti and H. Colfen, J. Am. Chem. Soc., 2010, 132, 3700–3707 CrossRef CAS PubMed; (g) P. Xing and Y. Zhao, Adv. Mater., 2016 DOI:10.1002/adma.201600906.
- K. Balakrishnan, A. Datar, T. Naddo, J. L. Huang, R. Oitker, M. Yen, J. C. Zhao and L. Zang, J. Am. Chem. Soc., 2006, 128, 7390–7398 CrossRef CAS PubMed.
- (a) J. Cui, A. Liu, Y. Guan, J. Zheng, Z. Shen and X. Wan, Langmuir, 2010, 26, 3615–3622 CrossRef CAS PubMed; (b) H. Cao, Q. Yuan, X. Zhu, Y. Zhao and M. Liu, Langmuir, 2012, 28, 15410–15417 CrossRef CAS PubMed.
- (a) P. Duan, L. Qin, X. Zhu and M. Liu, Chem.–Eur. J., 2011, 17, 6389–6395 CrossRef CAS PubMed; (b) M. Suzuki, M. Yumoto, H. Shirai and K. Hanabusa, Chem.–Eur. J., 2008, 14, 2133–2144 CrossRef CAS PubMed; (c) B. Verdejo, F. Rodríguez-Llansola, B. Escuder, J. F. Miravet and P. Ballester, Chem. Commun., 2011, 47, 2017–2019 RSC.
- (a) J. Wu, T. Yi, T. Shu, M. Yu, Z. Zhou, M. Xu, Y. Zhou, H. Zhang, J. Han, F. Li and C. Huang, Angew. Chem., Int. Ed., 2008, 47, 1063–1067 CrossRef CAS PubMed; (b) M. Zhang, L. Meng, X. Cao, M. Jiang and T. Yi, Soft Matter, 2012, 8, 4494–4498 RSC.
- S. Kawano, N. Fujita and S. Shinka, J. Am. Chem. Soc., 2004, 126, 8592–8593 CrossRef CAS PubMed.
- K. Murata, M. Aoki, T. Suzuki, T. Harada, H. Kawabata, T. Komori, F. Ohseto, K. Ueda and S. Shinkai, J. Am. Chem. Soc., 1994, 116, 6664–6676 CrossRef CAS.
- J. Wang, K. Liu, L. Yan, A. Wang, S. Bai and X. Yan, ACS Nano, 2016, 10, 2138–2143 CrossRef CAS PubMed.
- Y. Sun, C. He, K. Sun, Y. Li, H. Dong, Z. Wang and Z. Li, Langmuir, 2011, 27, 11364–11371 CrossRef CAS PubMed.
- (a) T. O. Mason, D. Y. Chirgadze, A. Levin, L. Adler-Abramovich, E. Gazit, T. P. J. Knowles and A. K. Buell, ACS Nano, 2014, 8, 1243–1253 CrossRef CAS PubMed; (b) M. J. Kamlet, J. L. M. Abboud, M. H. Abraham and R. W. Taft, J. Org. Chem., 1983, 48, 2877–2887 CrossRef CAS; (c) C. Reichardt, Chem. Rev., 1994, 94, 2319–2358 CrossRef CAS.
- (a) P. Xing, H. Chen, L. Bai, A. Hao and Y. Zhao, ACS Nano, 2016, 10, 2716–2727 CrossRef CAS PubMed; (b) P. Xing, X. Chu, M. Ma, S. Li and A. Hao, Chem.–Asian J., 2014, 9, 3440–3450 CrossRef CAS PubMed; (c) Y. Liu, T. Wang, Y. Huan, Z. Li, G. He and M. Liu, Adv. Mater., 2013, 25, 5875–5879 CrossRef CAS PubMed.
- P. Xing, X. Chu, G. Du, M. Ma and A. Hao, Colloid Polym. Sci., 2014, 292, 3223–3231 CAS.
- Q. Jin, L. Zhang and M. Liu, Chem.–Eur. J., 2013, 19, 9234–9241 CrossRef CAS PubMed.
- (a) Y. Zhang, S. Li, M. Ma, M. Yang, Y. Wang, A. Hao and P. Xing, New J. Chem., 2016, 40, 5568–5576 RSC; (b) P. Xing, X. Chu, S. Li, M. Ma and A. Hao, ChemPhysChem, 2014, 15, 2377–2385 CrossRef CAS PubMed.
- (a) G. Pabst, M. Rappolt, H. Amenitsch and P. Laggner, Phys. Rev. Lett., 2000, 62, 4000–4009 CAS; (b) D. Wang and J. Hao, Langmuir, 2011, 27, 1713–1717 CrossRef CAS PubMed; (c) P. Xing, X. Chu, S. Li, Y. Hou, M. Ma, J. Yang and A. Hao, RSC Adv., 2013, 3, 22087–22094 RSC; (d) P. Xing, L. Bai, H. Chen, P. H. Tham, A. Hao and Y. Zhao, ChemNanoMat, 2015, 1, 517–527 CrossRef CAS.
- (a) X. Mu, K. M. Eckes, M. M. Nguyen, L. J. Suggs and P. Ren, Biomacromolecules, 2012, 13, 3562–3571 CrossRef CAS PubMed; (b) Y. Wang, B. Erdogan, J. N. Wilson and U. H. F. Bunz, Chem. Commun., 2003, 19, 1624–1625 RSC; (c) B. Jing, X. Chen, Y. Zhao, X. Wang, F. Ma and X. Yue, J. Mater. Chem., 2009, 19, 2037–2042 RSC.
- P. Xing, X. Chu, M. Ma, S. Li and A. Hao, Phys. Chem. Chem. Phys., 2014, 16, 8346–8359 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra14563a |
| This journal is © The Royal Society of Chemistry 2016 |