Tungsten addenda mixed heteropolymolybdates supported on functionalized graphene for high-performance aqueous supercapacitors†
Abstract
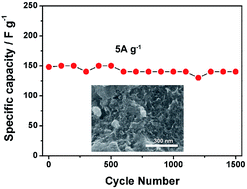
Polyoxometalates (POMs) based inorganic–organic composite materials have attracted considerable interest for energy storage and conversion applications. Modification of substrate materials is an effective method to achieve ideal electrochemical performance. Herein, poly(dimethyl diallyl ammonium chloride) (PDDA) functionalized graphene (PDDA–RGO) was used as the conductive matrix to support tungsten addenda mixed heteropolymolybdate, PMo12−xWxO403− (PMoW), with a facile, straightforward one-pot method. The cationic polyelectrolyte, PDDA, was employed as a linker to adsorb both RGO and POMs through electrostatic interactions. The resulting PMoW–PDDA–RGO composite exhibited a homogeneous honeycomb-like porous structure leading to fast ion transport and short ion diffusion pathways. In a typical two-electrode symmetric system, the supercapacitor exhibited a high rate specific capacitance of 140 F g−1 even at a high current density of 10 A g−1 and an energy density of 6.15 W h kg−1 at a power density of 250 W kg−1. After 1700 cycles at a fast discharge speed of 5 A g−1, the capacitance remained 94.6% of its initial capacitance.

 Please wait while we load your content...
Please wait while we load your content...