The progress of single-band upconversion nanomaterials
Lei Leiab,
JunJie Zhanga and
Shiqing Xu*a
aCollege of Materials Science and Engineering, China Jiliang University, Hangzhou 310018, China. E-mail: shiqingxu75@163.com; Fax: +86 57186875607; Tel: +86 57186875607
bState Key Laboratory of Structural Chemistry, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, Fuzhou, Fujian 350002, PR China
First published on 29th July 2016
Abstract
Upconversion (UC) nanomaterials (NMs) with single-band emission properties provoke widespread interest in the bio-medical field due to their advantages over those with multi-band emissions such as for high resolution in situ multiplexed molecular mapping. Several methods have been applied in recent years to realize single-band emission with different colors. In this feature article, for the first time, we provide an overview of the recent progresses in realizing single-band UC emission through different methods and the related mechanisms. Three types of strategies, including choosing an appropriate matrix, a doping ion with suitable energy level and a coating organic dye with a certain absorption wavelength, are discussed in detail. Finally, the challenges and future perspectives for these novel NMs with single-band UC emission are stated.
1 Introduction
UCNMs have been widely researched in recent years due to their unique properties that enable the conversion of low-energy photons (infrared or near infrared photons (NIR)) into high-energy photons (visible (VIS) to ultraviolet (UV) photons) via multi-photon processes.1–15 Benefitting from this feature, lanthanide (Ln) doped UCNMs possess low background auto-fluorescence, deep light penetration depth, and minimal photo-damage to bio-tissue.16–20 In addition, these UCNMs offer sharp emission peaks, large anti-Stokes shifts, long-lived excited electronic states, and high resistance to photo-bleaching.21–27 Considering all of these excellent characteristics, Ln3+-doped UCNMs are expected to be an alternative to organic fluorescent dyes or quantum dots for various bio-medical applications. For some special fields, single-band optical properties are required, e.g., in in situ multiplexed molecular mapping, accurate molecular profiling can only be achieved with single-band emission nanoprobes by excluding crosstalk between different labelling signals.28Ln3+ ions have a 4fn5s25p6 electron configuration with n = 0–14. The partly-filled 4f shell is responsible for the unique optical and magnetic properties. For n electrons in 14 available orbitals, there are 14 over n possible configurations and all configurations can have different energies. This gives rise to a rich energy level structure in the NIR, VIS and UV spectral range (Table 1). Owing to the long lifetime of the excited states, an excited Ln3+ ion can sequentially absorb a second photon of suitable energy and reach an ever-higher excited state,29,30 and then various emission bands can be obtained when those electrons in different excited states relax to the ground state. The emission wavelengths are independent of host materials due to the transitions between different 4fn states being parity forbidden (no change in dipole moment); however, the ratio of different emission bands can be tuned. Actually, several methods, such as changing the possibilities of cross-relaxation (CR) processes between different energy levels or using an organic dye with a high molar absorption coefficient to absorb the unwanted emission bands, have been used to realize single-band UC luminescence.28,31,32 To the best of our knowledge, there is no review reported about single-band UC emission to date.
| Activators | Major emissions (nm) | Energy transitions |
|---|---|---|
| Er3+ | 411, 523, 542, 656 | 2H9/2 → 4I15/2, 2H11/2 → 4I15/2, 4S3/2 → 4I15/2, 4F9/2 → 4I15/2 |
| Ho3+ | 542, 645, 658 | 5S2/5F4 → 5I8, 5F5 → 5I8 |
| Tm3+ | 294, 345, 368, 450, 475, 650, 700, 800 | 1I6 → 3H6, 1I6 → 3F4, 1D2 → 3H6, 1D2 → 3F4, 1G4 → 3H6, 1G4 → 3F4, 3F3 → 3H6, 3H4 → 3H6 |
| Tb3+ | 490, 540, 580, 615 | 5D4 → 7F6, 5D4 → 7F5, 5D4 → 7F4, 5D4 → 7F3 |
| Eu3+ | 590, 615, 690 | 5D0 → 7F1, 5D0 → 7F2, 5D0 → 7F4 |
| Sm3+ | 555, 590 | 4G5/2 → 6H5/2, 4G5/2 → 6H7/2 |
In this review, we systematically summarize the progress of different methods to actualize single-band UC emission in various systems and the related mechanisms, which is useful for understanding single-band optical properties and promoting their further development. For better comparison, some basic knowledge about UC with multi-band emission is first discussed in Section 2. Finally, the challenges and future outlook of this novel single-band UC emission property are pointed out.
2 UC with multi-band emission and the related mechanism
Ln3+-doped UCNMs generally comprise an inorganic host matrix co-doped with sensitizer and activator. The host lattice determines the relative spatial distribution of the dopant ions, the coordination numbers and the type of anions surrounding the dopant. The properties of the host lattice and its interaction with the dopant ions have a strong influence on UC processes. Moreover, the host lattice with low phonon energies can minimize nonradiative losses and maximize radiative emission. For example, fluorides, which are widely chosen as matrixes due to the relatively low phonon energies (ca. 350 cm−1) of their crystal lattices, generally have higher luminescent efficiency than that of oxides (ca. 600 cm−1).33–35 Although UC can be actualized in principle from most lanthanide-doped crystalline host materials, efficient UC only occurs when appropriate dopants (including activators and sensitizers) are selected. To actualize multicolor emissions, different Ln3+ ions (such as Er3+, Ho3+ and Tm3+) with ladder-like arranged energy levels are widely chosen as activators and doped into different matrixes. However, most Ln3+ activators exhibit low absorption cross-section, leading to low pump efficiency and resulting relatively low UC efficiency for the singly-doped NMs. To improve the UC emission intensity, the sensitizer, which have large absorption cross-section in the excitation wavelength region, is used to absorb the photon energy and then transfer to the activators, e.g., Yb3+ ions possesses large absorption cross-section (1.2 × 10−20 cm2) at around 980 nm due to the 2F7/2 → 2F5/2 transition; Nd3+ ions have a larger absorption cross-section (1.2 × 10−19 cm2) at around 800 nm due to the 4F5/2/2H9/2 → 4I9/2 transition.36–39For example, Yb/Er and Yb/Tm co-doped NaYF4 nanoparticles show strong yellow and blue emissions attributed to 2H11/2/4S3/2 → 4I15/2 (∼539 nm), 4F9/2 → 4I15/2 (∼650 nm) transitions of Er3+ and 1D2 → 3F4/1G4 → 3H6 (∼470 nm) transitions of Tm3+, respectively (Fig. 1a and b).40–46 The yellow color of Er3+ ions results from two major constituent emissions, which can be separately observed with the aid of green and red color filters. Nd3+ is also used as a sensitizer to actualize green UC emission in Yb/Er co-doped NMs under excitation with 980 nm laser.38,39 Yb/Ho:NaGdF4 is another luminescent material that exhibits multi-band emission with yellow light.47,48 Actually, several other Ln ions, including Tb3+, Eu3+, Dy3+ and Sm3+, without long-lived intermediary energy states, also serve as the activators to generate multicolor UC emission by controlling gadolinium sublattice-mediated energy migration through a well defined core/shell structure (Fig. 1c and d).49,50 The main mechanism in these systems is energy transfer upconversion (ETU). In an ETU process, taking Yb/Er:NaYF4 as an example, an initial energy transfer from an Yb3+ ion in the 2F5/2 state to an Er3+ ion populates the 4I11/2 level. A second 980 nm photon or energy transfer from an Yb3+ ion can then populate the 4F7/2 level of the Er3+ ion. The Er3+ ion can then relax nonradiatively (without emission of photons) to the 2H11/2 and 4S3/2 levels, and the green 2H11/2 → 4I15/2 and 4S3/2 → 4I15/2 emissions occur. Alternatively, the ion can further relax and populate the 4F9/2 level leading to the red 4F9/2 → 4I15/2 emission. The 4F9/2 level may also be populated from the 4I13/2 level of the Er3+ ion by absorption of a 980 nm photon or energy transfer from an Yb3+ ion with the 4I13/2 state being initially populated via the nonradiative 4I11/2 → 4I13/2 relaxation.
 | ||
| Fig. 1 (a) Proposed energy transfer mechanisms exhibiting the upconversion processes in Er3+, Tm3+, and Yb3+ doped crystals under 980 nm excitation. (b) Luminescence emission spectra of 1 wt% colloidal solutions of nanocrystals in toluene excited with a 980 nm laser diode (power density = 100 W cm2): NaYF4:2% Er3+,20% Yb3+ and NaYF4:2% Tm3+,20% Yb3+. Inset: total upconversion luminescence of NaYF4:2% Er3+,20% Yb3+ solution and isolation of green and red luminescence using appropriate filters; total upconversion luminescence of NaYF4:2% Tm3+,20% Yb3+ solution. (c) Proposed energy transfer mechanisms in the core–shell–shell nanoparticles. Note that only partial energy levels of Tm3+, Gd3+, and A3+ (A = Dy, Sm, Tb, and Eu) are shown for clarity. The optical emissions from higher-lying energy levels of Tb3+ and Eu3+ are highlighted with colored arrows. (d) Luminescence images of representative samples in cyclohexane solution (2 mg mL−1) under irradiation of a 980 nm laser.40,50 | ||
3 UC with single-band emission and the related mechanism
Ln3+ activators generally have abundant meta-stable excited states enabling them to emit photons covering from the ultraviolet to infrared spectral regions in appropriate hosts, and the emission profiles are various with different electron transition or relaxation pathways. For example, the 4F7/2 and 4F9/2 levels of Er3+ are the origin of the green and red emissions, respectively;51 if the population of 4F7/2 level is suppressed or the electrons in the 4F7/2 state relax nonradiatively to the 4F9/2 state, the green emission will disappear and the single red one will be achieved.Compared with multi-band UC emission, the single-band emission has much higher chromatic purity, resulting in better sensitivity and spatial resolution in bio-imaging applications, e.g., KMnF3:Yb/Er with strong pure red emission can afford deeper tissue penetration than NaYF4:Yb/Er with both green and red emission, and the red emission intensity of the KMnF3:Yb/Er NCs is found to be substantially higher than that of the NaYF4:Yb/Er nanocrystals of similar particle size irrespective of the pump power.52 In addition, for in situ multiplexed molecular mapping, nanoprobes with single-band emission, which can exclude crosstalk between different labeling signals, is the prerequisite for accurate molecular profiling.28 Recently, a variety of novel techniques, including choosing the appropriate matrix, a doping ion with suitable energy level and coating with an organic dye with a certain absorption wavelength, are applied to realize single-band UC emission.
3.1 Choosing an appropriate matrix
The selection of an appropriate matrix is essential for obtaining Ln3+-doped materials with favorable optical properties and controllable emission profiles. Normally, the matrix requires similar lattice parameters with dopant ions for high solubility and low lattice phonon energy to minimize nonradiative loss and maximize the radiative emission. To actualize single-band UC emission, the matrix needs to benefit a certain CR or the depopulation of one metastable energy level so as to suppress the other emissions.In fluoride materials, long lifetimes of the excited states are commonly observed because of the low phonon energies (ca. 350 cm−1) of the crystal lattice, whereas conventional oxygen-based systems often exhibit large phonon energies above 500 cm−1, so fluoride materials are better for obtaining high UC efficiency in comparison with oxides. However, the normally adopted fluorides, such as CaF2 and NaYF4, only exhibit cubic and hexagonal phases, whereas lanthanide oxyfluorides present more than three crystal phases, which is better for tuning the emission wavelengths.
Wang et al. reported the single red (∼660 nm) and NIR emission (∼800 nm) observed, respectively, in Yb/Er and Yb/Tm co-doped Na3MF7 (M = Zr, Hf) nanocrystals (NCs) with tetragonal phase, and the red upconversion intensity of the Yb/Er:Na3ZrF7 nanocrystals is about 5 times as high as that of the hexagonal Yb/Er:NaYF4 ones with a similar crystal size.32 The authors propose that the Ln3+ clusters, which can shorten the distances between Ln3+ and then assist or enhance the CR (4F7/2 + 4I11/2 → 2 4F9/2) of Er3+/(1G4 + 3F4 → 3H4 + 3F2) of Tm3+ ions, play a key role to induce this intrinsic single-band emission (Fig. 2). Yan et al. reported that the single-band red UC luminescence was achieved in Yb/Er co-doped K3MF7 NCs.56 Their study suggest that the UC luminescence feature of Yb/Er:K3MF7 NCs is independent of the doping levels of Yb3+/Er3+ and the pump power of incident light, and the high-energy vibrational oscillators on the NCs surfaces are likely to result in a striking increase in the population of the 4F9/2 level of Er3+, accounting for the spectrally pure emission. A similar result was reported in perovskite Yb/Er:KMgF3 NCs as well by Zhang et al.57 Actually, the reasons for the single-band emission in those matrixes given in the related papers need more strong evidence, such as the ion distribution in the crystal structure and the real-time electron population in different energy levels, which are significant for realizing this novel optical property in other hosts.
 | ||
| Fig. 2 (a) TEM micrograph and (b) SAED pattern of Yb/Er (20/0.5 mol%):Na3ZrF7 NCs; the inset of (a) shows a histogram of the particle size distribution, (c) HRTEM image of an individual NC and its FFT pattern, and (d) EDS spectrum of the product, showing the existence of Na, F, Zr and Yb signals (Cu signals come from the copper grid, and an Er signal is not detected owing to the low doping content), (e) and (f) are the UC emission spectra of Yb/Er(Tm) (20/0.5 mol%):Na3ZrF7 NCs under 980 nm laser excitation, (g) energy level diagrams of Er3+, Yb3+ and Tm3+ ions, showing the possible energy transfer mechanisms for the single-band red (NIR) UC emission of Er3+(Tm3+) activators in Na3ZrF7 host, under 980 nm laser excitation.32 | ||
In addition, although the Mn2+-containing matrix, such as KMnF3,52 can also give rise to single-band UC emission of Er3+/Ho3+/Tm3+, the mechanisms are quite different, which will be discussed in Section 3.2, because the optical properties and mechanism are similar to the Mn2+-doped situation.
Actually, for the widely investigated NaYF4 matrix, a few methods were applied by several groups to tailor the emission profile of the doped Ln3+ activators approximate to single-band. Yan et al. reported that the red emission of Er3+ was greatly enhanced through engineering the local structure of Ln3+ activators in NaxYF3+x.58 The local structure engineering was achieved through precisely tuning the composition of NCs, with different [Na]/[RE] ([F]/[RE]) ratios, and a significant difference in the red to green emission ratio, which varied from 1.9 to 71 and 1.6 to 116, was observed for Yb/Er:NaxYF3+x and Yb/Er:NaxGdF3+x NCs, respectively (Fig. 3). It can also be realized through doping Gd3+ ions at Sc3+ sites and attaching gold NCs at the surface in NaSc0.8Er0.02Yb0.18F4 NCs, which was reported by Rai et al.31 The intensity of NIR emission (∼802 nm) in Yb/Tm:NaYF4 NCs with ultrasmall size (7–10 nm) was demonstrated to increase by up to 43 times and approach to single-band, along with an increase in the relative content of Yb3+ ions from 20% to 100% and a corresponding decrease in the Y3+ content from 80% to 0%, which was reported by Prasas et al.59
 | ||
| Fig. 3 Schematic of NaxYF3+x and the corresponding UC emission spectra upon 980 nm excitation.58 | ||
![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m, tetragonal PbFCl-type P4/nmm and cubic fluorite-like Fm
m, tetragonal PbFCl-type P4/nmm and cubic fluorite-like Fm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m) and ever-present low symmetry of Ln3+ due to the distortion in their crystal lattices.60 Hence, excellent and novel UC performance of activators was observed in LnOF matrixes such as Er:YbOF,60 Yb/Er:Lu5O4F7,61,62 Yb/Er(Tm):ScOF63 and Yb/Er:YOF@YOF64 NMs.
m) and ever-present low symmetry of Ln3+ due to the distortion in their crystal lattices.60 Hence, excellent and novel UC performance of activators was observed in LnOF matrixes such as Er:YbOF,60 Yb/Er:Lu5O4F7,61,62 Yb/Er(Tm):ScOF63 and Yb/Er:YOF@YOF64 NMs.Wang et al. reported that in Er3+ (2–12 mol%)-doped Vernier phase ytterbium oxyfluoride (V-YbOF) host, single-band red emission centered at 660 nm (4F9/2 → 4I15/2) was observed and the maximum UC output was achieved when the Er3+ doping concentration is about 4 mol% (Fig. 4).60 The high concentration of Yb3+ in the host lattice greatly facilitated the energy-back-transfer (EBT) process from Er3+ to Yb3+ [4F7/2 (Er3+) + 2F7/2 (Yb3+) → 4I11/2 (Er3+) + 2F5/2 (Yb3+)] and the photon excitation to higher 2H9/2, 2H11/2 and 4S3/2 levels of Er3+ was suppressed, which results in the absence of blue (2H9/2 → 4I15/2) and green (2H11/2, 4S3/2 → 4I15/2) emissions. Sun et al. and Chen et al. reported that the UC spectrum of Lu5O4F7:Er3+/Yb3+ nanoparticles can also be tailored into red single-band due to a large amount of nonradiative (4I11/2 → 4I13/2), which benefits red emission.61,62 Gao et al. observed strong red single-band emission in Er3+/Yb3+:YOF@YOF NCs with a core@shell structure. Their study suggests that the YOF shell enhanced the red emission at ∼669 nm (∼18 times) and suppressed the green emission of Er3+ at ∼530 nm.64
 | ||
| Fig. 4 Schematic of the orthorhombic Vernier phase structure of Yb6O5F8 and the possible sites for Ln3+ doping: (a) the a × nb × c unit cell with n = 6 along the b-axis and (b) the assorted array of O2− and F− ions offers four different types of Yb3+ cation sites. Note that the O2− and F− anions located at 8e sites shown as green balls are of disordered distribution; (c) TEM image of Ln3+ doped V-YbOF nanoparticles. Inset shows the high resolution TEM image of an individual V-YbOF particle; (d) UC emission spectra of Er3+ doped V-YbOF samples under 980 nm excitation (∼3 W cm−2) and the emission intensity variations of Er3+-doped V-YbOF samples with the increase of Er3+ doping concentration (inset).60 | ||
The UC emissions of Er3+ ions normally contain green (2H11/2, 4S3/2 → 4I15/2) and red (4F9/2 → 4I15/2) wavelengths (Fig. 1b); the ratio of green to red depends on the competition between 4F7/2 → 2H11/2/4S3/2 and 4S3/2 → 4F9/2, 4I13/2 → 4I11/2 or 4F7/2 + 4I11/2 → 2 4F9/2. It is relatively easier to transfer most of the high energy photons to the lower ones at a certain condition and the single red emission can be realized; however, the green one originates from two different excited-state levels (2H11/2, 4S3/2), and it is unlikely to suppress all the CR and depopulation for the red emission under strong green luminescence, so the single-band green emission is difficult to be realized. However, pure green light containing ∼525 nm and ∼545 nm was achieved in Yb/Er:LaVO4 (ref. 69) and Yb/Er(Ho):NaCe(MoO4)2 (ref. 70) phosphors. The CR 4F7/2 + 4I11/2 → 2 4F9/2 of Er3+ and 5F3 + 5I8 → 5F5 + 5I7 of Ho3+, which contribute to the red population, were suppressed due to the boundary effect of nanorods in Yb/Er:LaVO4 and the different band edge absorption by adding EDTA into the Yb/Er(Ho):NaCe(MoO4)2 reaction system.
3.2 Doping ion with suitable energy level
It is well known that ion doping is an important route for imparting new and useful properties for numerous functional NMs. For luminescent materials, doping ions with suitable energy level can be used to realize single-band emission through changing the energy transfer processes or enhancing one certain CR possibility.Mn2+ ions can be used as an exchange-energy transfer medium in Yb/Er-, Yb/Ho- and Yb/Tm-containing systems and it then actualizes single-band UC emission.71–74 Zhao et al. reported that Yb/Er:NaYF4 phase changed from hexagonal nanorods to small nanocubes with cubic phase after doping 30% Mn2+ ions.71 The emission profile altered from multi-band to single band and the emission color changed from green to red, which was ascribed to nonradiative energy transfer from the 2H9/2 and 4S3/2 levels of Er3+ to the 4T1 level of Mn2+, followed by energy-back-transfer to the 4F9/2 level of Er3+ (Fig. 5). Several Mn2+-containing matrixes, such as Yb/Er:MnF2,75 Yb/Er(Ho,Tm):KMnF3 (ref. 52 and 76) and Yb/Er:NaMnF3,77,78 were also applied to realize single-band UC emission.
 | ||
| Fig. 5 (a) XRD patterns of NaYF4 doped with 0, 3, 5 and 30 mol% Mn2+ ions. TEM micrographs of NaYF4 NCs (b) without Mn2+ dopants and (c) doped with 30 mol% Mn2+ ions. (d) UC emission spectra of Yb/Er (18/2 mol%):NaYF4 NCs doped with 0, 5 and 30 mol% Mn2+ ions; insets are luminescent images of the corresponding samples. (e) Schematic energy level diagram showing the possible UC mechanism of Mn2+-doped Yb/Er:NaYF4 NCs. (f) Luminescence time traces of the 30 mol% Mn2+-doped Yb/Er:NaYF4 NCs acquired with 200 ms time bins under continuous 980 nm laser illumination for more than 3 h, suggesting the durable photostability of the UC NCs. (g) Luminescent image of 30 mol% Mn2+-doped UC NCs dispersed in hexane.71 | ||
Ce3+ ions have appropriate energy levels to facilitate CR with Ho3+ ions, which was used to modify the emission profile of Ho3+ ions. Chen et al. reported the single-band red luminescence of Ho3+ upon excitation of 808 nm based on the elaborate combination of Nd3+ sensitization, Ce3+-assisted CR and the active-core@active-shell structure design.79 They found that increasing Ce3+ dopants results in monotonic enhancement of red to green UC intensity ratio of Ho3+ and the CR processes involving Ce3+ and Ho3+, i.e., CR1: Ho3+: 5I6 + Ce3+: 2F5/2 → Ho3+: 5I7 + Ce3+: 2F7/2, CR2: Ho3+: 5S2/5F4 + Ce3+: 2F5/2 → Ho3+: 5F5 + Ce3+: 2F7/2 and CR3: Ho3+: 5F5 + Ce3+: 2F5/2 → Ho3+: 5I4 + Ce3+: 2F7/2 were the main reason for the observed optical phenomena (Fig. 6). Hao et al. reported that a tunable UC multicolor output from green/yellow to red was achieved in a fixed Yb3+/Ho3+ composition in BaGdF5 by doping Ce3+ under the excitation of 980 nm, and the emission spectrum approached single-band with Ce3+ concentration increased to 30%.80
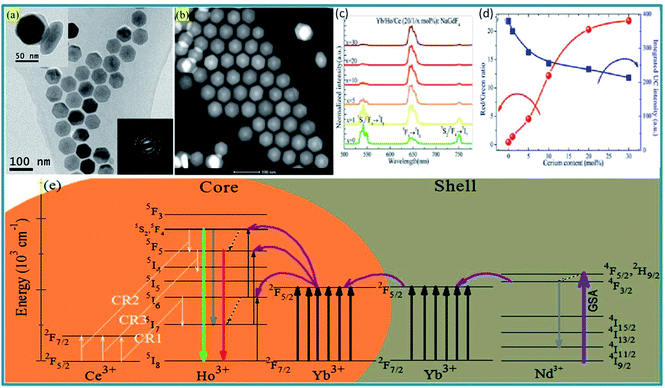 | ||
| Fig. 6 (a) TEM and (b) HAADF-STEM micrographs of (a) 20Yb/1Ho/30Ce:NaGdF4@20Yb/20Nd:NaYF4 core–shell NCs. Insets of (a) show the SAED pattern of these core–shell NCs (bottom) and the enlarged core–shell NCs (top); (c) Ce3+ content-dependent UC emission spectra for Yb/Ho/Ce:NaGdF4 core NCs; (d) the integrated UC intensity as well as the ratio of red to green emission versus Ce3+ content; (e) energy level diagrams of Ce3+, Ho3+, Yb3+, and Nd3+ as well as the proposed mechanisms for the achievement of pure red UC luminescence under 980 or 808 nm laser excitation in Yb/Ho/Ce:NaGdF4@Yb/Nd:NaYF4 core–shell NCs.79 | ||
3.3 Coating organic dye with a certain absorption wavelength
Coating material with high reabsorption ability at a certain wavelength outside UCNMs to eliminate unwanted emission bands is also an efficient method to actualize single-band UC emission. Zhang et al. achieved single-band UC emission with different colors by coating the UCNCs with a screen layer containing an organic dye with a high molar absorption coefficient as a nanofilter to filter the unwanted emission bands.28 To obtain green and blue single-band emission, nickel(II)phthalocyanine-tetrasulfonic acid tetrasodium salt (NPTAT), organic dyes with a maximum absorption wavelength of 657 nm, were added with tetraethyl orthosilicate (TEOS) to form NPTAT-doped silica nanofilters on the 20Yb/2Er:β-NaGdF4@NaGdF4@SiO2 and 20Yb/0.2Tm:β-NaGdF4@NaGdF4@SiO2 NCs to filter red emission bands, respectively (Fig. 7a–h) and to obtain the red single-band emission, coating pure SiO2 layers and rhodamine B isothiocyanate outside 10Er:α-NaYbF4@NaYF4 NCs with strong red to green UC emission to filter the green emission (Fig. 7i–l). We believe that amounts of other absorbents will be applied in various Ln3+-doped systems to actualize single-band emission with different colors in the future. | ||
| Fig. 7 TEM images and size distributions of (a) 20Yb/2Er:β-NaGdF4@NaGdF4 NCs, (b) green emission sb-UCNPs (20Yb/2Er:β-NaGdF4@NaGdF4@SiO2@NPTAT-doped SiO2), (e) 20Yb/0.2Tm:β-NaGdF4@NaGdF4 NCs, (f) blue emission sb-UCNPs (20Yb/0.2Tm:β-NaGdF4@NaGdF4@SiO2@NPTAT-doped SiO2), (i) a-NaYbF4:10% Er@NaYF4 NCs and (j) red emission sb-UCNPs (NaYbF4:10% Er@NaYF4@SiO2@rhodamine B isothiocyanate-doped SiO2). UC photoluminescence spectra of the green, blue and red sb-UCNPs (d, h, and l) and original UCNCs measured in water and cyclohexane, respectively (c, g, and k) (insets: corresponding photoluminescent images of the colloidal solutions under continuous wavelength 980 nm laser excitation). Scale bars, 100 mm.28 | ||
4 Conclusions and outlook
We summarized the research progress of single-band UC emission with different colors, including various routes and related mechanisms. To obtain spectra with single-band, several strategies have been investigated, such as choosing an appropriate matrix, a doping ion with suitable energy level and coating an organic dye with a fixed absorption wavelength. In general, the matrix accelerates a certain CR, the ion with suitable energy level alters the energy transfer processes and the organic dye absorbs unwanted emission bands. These materials with single-band emission have been widely employed as a platform for bio-imaging and therapeutic applications. Among the abovementioned three approaches, we believe choosing the appropriate matrix exhibits the highest UC efficiency, because a doping ion with suitable energy level will introduce extra energy transfer paths and coating with an organic dye with a fixed absorption wavelength will waste part of the absorbed energy. Although tremendous achievements have been made, there still exist many fundamental and practical issues to be solved in this field, which will provide great opportunities and challenges for related applications in the future.First, more different single-band emission wavelengths with various output colors should be investigated in the future. Different wavelengths have different advantages in various applications. To date, most of the single-band emissions are obtained from Er3+ (∼540 nm, ∼650 nm), Ho3+ (∼650 nm) or Tm3+ (∼479 nm, ∼800 nm). The activators may be extended to other Ln3+ ions (such as Tb3+, Eu3+, Dy3+ and Sm3+) or transition metal ions (such as Mn2+ and Cr3+). Second, the single-band UC emission efficiency is very low for practical application. The existing methods for actualizing single-band emission are mainly through CR, different energy transfer processes or reabsorption to suppress the unwanted emission, which will result in an amount of energy loss. It is important to develop some superior methods for realizing single-band UC emission. Third, the mechanisms for single-band UC emission are still unclear. Although several single-band emissions are observed in different systems, the related mechanisms lack strong evidence, particularly concerning the energy transfer processes. More experiments and characterizations need to be carried out systematically.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (No. 51472225 and 51372235) and the Zhejiang Provincial Natural Science Foundation of China (LR14E020003).References
- F. Wang, Y. Han, C. S. Lim, Y. H. Lu, J. Wang, J. Xu, H. Y. Chen, C. Zhang, M. H. Hong and X. G. Liu, Nature, 2010, 463, 1061 CrossRef CAS PubMed.
- J. B. Zhao, D. Y. Jin, E. P. Schartner, Y. Q. Lu, Y. J. Liu, A. V. Zvyagin, L. X. Zhang, J. M. Dawes, P. Xi, J. A. Piper, E. M. Goldys and T. M. Monro, Nat. Nanotechnol., 2013, 8, 729 CrossRef CAS PubMed.
- M. Haase and H. Schäfer, Angew. Chem., Int. Ed., 2011, 50, 5808 CrossRef CAS PubMed.
- R. R. Deng, X. J. Xie, M. Vendrell, Y. T. Chang and X. G. Liu, J. Am. Chem. Soc., 2011, 133, 20168 CrossRef CAS PubMed.
- G. Y. Chen, T. Y. Ohulchanskyy, A. Kachynski, H. Ågren and P. N. Prasad, ACS Nano, 2011, 5, 4981 CrossRef CAS PubMed.
- D. Q. Chen, Z. Y. Wan, Y. Zhou, W. D. Xiang, J. S. Zhong, M. Y. Ding, H. Yu and Z. G. Ji, J. Mater. Chem. C, 2015, 3, 3141 RSC.
- T. Y. Cao, Y. Yang, Y. Gao, J. Zhou, Z. Q. Li and F. Y. Li, Biomaterials, 2011, 32, 2959 CrossRef CAS PubMed.
- G. F. Wang, Q. Peng and Y. D. Li, J. Am. Chem. Soc., 2009, 131, 14200 CrossRef CAS PubMed.
- Y. P. Du, Y. W. Zhang, Z. G. Yan, L. D. Sun and C. H. Yan, J. Am. Chem. Soc., 2009, 131, 16364 CrossRef CAS PubMed.
- Y. P. Du, Y. W. Zhang, L. D. Sun and C. H. Yan, J. Am. Chem. Soc., 2009, 131, 3162 CrossRef CAS PubMed.
- Y. L. Dai, H. H. Xiao, J. H. Liu, Q. H. Yuan, P. A. Ma, D. M. Yang, C. X. Li, Z. Y. Cheng, Z. Y. Hou, P. P. Yang and J. Lin, J. Am. Chem. Soc., 2013, 135, 18920 CrossRef CAS PubMed.
- Q. Liu, Y. Sun, T. S. Yang, W. Feng, C. G. Li and Y. F. Li, J. Am. Chem. Soc., 2011, 133, 17122 CrossRef CAS PubMed.
- N. Niu, P. P. Yang, F. He, X. Zhang, S. L. Gai, C. X. Li and J. Lin, J. Mater. Chem., 2012, 22, 10889 RSC.
- Y. S. Liu, D. T. Tu, H. M. Zhu, R. F. Li, W. Q. Luo and X. Y. Chen, Adv. Mater., 2010, 22, 3266 CrossRef CAS PubMed.
- P. Huang, W. Zheng, S. Y. Zhou, D. T. Tu, Z. Chen, H. M. Zhu, R. F. Li, E. Ma, M. D. Huang and X. Y. Chen, Angew. Chem., Int. Ed., 2014, 53, 1252 CrossRef CAS PubMed.
- K. Kuningas, T. Ukonaho, H. Päkkilä, T. Rantanen, J. Rosenberg, T. Lövgren and T. Soukka, Anal. Chem., 2006, 78, 4690 CrossRef CAS PubMed.
- P. L. A. M. Corstjens, M. Zuiderwijk, H. J. Tanke, J. J. van der Ploegvan Schip, T. H. M. Ottenhoff and A. Geluk, Clin. Biochem., 2008, 41, 440 CrossRef CAS PubMed.
- O. Ehlert, R. Thomann, M. Darbandi and T. Nann, ACS Nano, 2008, 2, 120 CrossRef CAS PubMed.
- H. M. Kim and B. R. Cho, Acc. Chem. Res., 2009, 42, 863 CrossRef CAS PubMed.
- D. K. Chatterjee, M. K. Gnanasammandhan and Y. Zhang, Small, 2010, 52, 3584 Search PubMed.
- F. Auzel, Chem. Rev., 2004, 104, 139 CrossRef CAS PubMed.
- B. M. van der Ende, L. Aarts and A. Meijerink, Phys. Chem. Chem. Phys., 2009, 11, 11081 RSC.
- F. Wang and X. G. Liu, Chem. Soc. Rev., 2009, 38, 976 RSC.
- J. C. Zhou, Z. L. Yang, W. Dong, R. J. Tang, L. D. Sun and C. H. Yan, Biomaterials, 2011, 32, 9059 CrossRef CAS PubMed.
- J. Zhou, Z. Liu and F. Y. Li, Chem. Soc. Rev., 2012, 41, 1323 RSC.
- Y. S. Liu, D. T. Tu, H. M. Zhu and X. Y. Chen, Chem. Soc. Rev., 2013, 42, 6942 Search PubMed.
- L. D. Sun, Y. F. Wang and C. H. Yan, Acc. Chem. Res., 2014, 47, 1001 CrossRef CAS PubMed.
- L. Zhou, R. Wang, C. Yao, X. M. Li, C. L. Wang, X. Y. Zhang, C. J. Xu, A. J. Zeng, D. Y. Zhao and F. Zhang, Nat. Commun., 2015, 6, 6938 CrossRef CAS PubMed.
- X. M. Li, F. Zhang and D. Y. Zhao, Nano Today, 2013, 8, 643 CrossRef CAS.
- G. Y. Chen, C. H. Yang and P. N. Prasad, Acc. Chem. Res., 2013, 46, 1474 CrossRef CAS PubMed.
- M. Rai, S. K. Singh, A. K. Singh, R. Prasad, B. Koch, K. Mishra and S. B. Rai, ACS Appl. Mater. Interfaces, 2015, 7, 15339 CAS.
- D. Q. Chen, L. Lei, R. Zhang, A. P. Yang, J. Xu and Y. S. Wang, Chem. Commun., 2012, 48, 10630 RSC.
- H. X. Mai, Y. W. Zhang, R. Si, Z. G. Yan, L. D. Sun, L. P. You and C. H. Yan, J. Am. Chem. Soc., 2006, 128, 6426 CrossRef CAS PubMed.
- F. Wang, D. Banerjee, Y. S. Liu, X. Y. Chen and X. G. Liu, Analyst, 2010, 135, 1839 RSC.
- L. Lei, D. Q. Chen, P. Huang, J. Xu, R. Zhang and Y. S. Wang, Nanoscale, 2013, 5, 11298 RSC.
- Y. T. Zhong, G. Tian, Z. J. Gu, Y. J. Yang, L. Gu, Y. L. Zhang, Y. Ma and J. L. Yao, Adv. Mater., 2013, 26, 2831 CrossRef PubMed.
- Y. F. Wang, G. Y. Liu, L. D. Sun, J. W. Xiao, J. C. Zhou and C. H. Yan, ACS Nano, 2013, 7, 7200 CrossRef CAS PubMed.
- X. J. Xie, N. Y. Gao, R. R. Deng, Q. Sun, Q. H. Xu and X. G. Liu, J. Am. Chem. Soc., 2013, 135, 12608 CrossRef CAS PubMed.
- F. He, G. X. Yang, P. P. Yang, Y. X. Yu, R. C. Lv, C. X. Li, Y. L. Dai, S. L. Gai and J. Lin, Adv. Funct. Mater., 2015, 25, 3966 CrossRef CAS.
- J. C. Boyer, L. A. Cuccia and J. A. Capobianco, Nano Lett., 2007, 7, 847 CrossRef CAS PubMed.
- D. M. Yang, Y. L. Dai, P. A. Ma, X. J. Kang, M. M. Shang, Z. Y. Cheng, C. X. Li and J. Lin, J. Mater. Chem., 2012, 22, 20618 RSC.
- F. Wang and X. G. Liu, J. Am. Chem. Soc., 2008, 130, 5642 CrossRef CAS PubMed.
- W. Zheng, S. Y. Zhou, Z. Chen, P. Hu, Y. S. Liu, D. T. Tu, H. M. Zhu, R. F. Li, M. D. Huang and X. Y. Chen, Angew. Chem., Int. Ed., 2013, 52, 1 CrossRef.
- J. A. Damasco, G. Y. Chen, W. Shao, H. Agren, H. Y. Huang, W. T. Song, J. F. Lovell and P. N. Prasad, ACS Appl. Mater. Interfaces, 2014, 6, 13884 CAS.
- P. Li, Q. Peng and Y. D. Li, Adv. Mater., 2009, 21, 1945 CrossRef CAS.
- Y. Wu, D. S. Wang and Y. D. Li, Chem. Soc. Rev., 2014, 43, 2112 RSC.
- G. Z. Ren, S. J. Zeng and J. H. Hao, J. Phys. Chem. C, 2011, 115, 20141 CAS.
- Y. F. Wang, L. D. Sun, J. W. Xiao, W. Feng, J. C. Zhou, J. Shen and C. H. Yan, Chem.–Eur. J., 2012, 18, 5558 CrossRef CAS PubMed.
- F. Wang, R. R. Deng, J. Wang, Q. X. Wang, Y. Han, H. M. Zhu, X. Y. Chen and X. G. Liu, Nat. Mater., 2011, 10, 968 CrossRef CAS PubMed.
- Q. Q. Su, S. Y. Han, X. J. Xie, H. M. Zhu, H. Y. Chen, C. K. Chen, R. S. X. Y. Chen, F. Wang and X. G. Liu, J. Am. Chem. Soc., 2012, 134, 20849 CrossRef CAS PubMed.
- Q. B. Xiao, H. M. Zhu, D. T. Tu, E. Ma and X. Y. Chen, J. Phys. Chem. C, 2013, 117, 10834 CAS.
- J. Wang, F. Wang, C. Wang, Z. Liu and X. G. Liu, Angew. Chem., Int. Ed., 2011, 50, 1 CrossRef.
- S. Lu, D. T. Tu, P. Huang, J. Xu, R. F. Li, M. Wang, Z. Chen, M. D. Huang and X. Y. Chen, Angew. Chem., Int. Ed., 2015, 54, 7915 CrossRef CAS PubMed.
- X. R. Deng, Y. L. Dai, J. H. Liu, Y. Zhou, P. A. Ma, Z. Y. Cheng, Y. Y. Chen, K. R. Deng, X. J. Li, Z. Y. Hou, C. X. Li and J. Lin, Biomaterials, 2015, 50, 154 CrossRef CAS PubMed.
- T. S. Yang, Y. Sun, Q. Liu, W. Feng, P. Y. Yang and F. Y. Li, Biomaterials, 2012, 33, 3733 CrossRef CAS PubMed.
- X. H. He and B. Yan, CrystEngComm, 2015, 17, 7169 RSC.
- M. Wu, E. H. Song, Z. T. Chen, S. Ding, S. Ye, J. J. Zhou, S. Q. Xu and Q. Y. Zhang, J. Mater. Chem. C, 2016, 4, 1675 RSC.
- H. Dong, L. D. Sun, Y. H. Wang, J. Ke, R. Si, J. W. Xiao, G. M. Lyu, S. Shi and C. H. Yan, J. Am. Chem. Soc., 2015, 137, 6569 CrossRef CAS PubMed.
- G. Y. Chen, T. Y. Ohulchanskyy, R. Kumar, H. Agren and P. N. Prasad, ACS Nano, 2010, 4, 3163 CrossRef CAS PubMed.
- T. Wen, Y. N. Zhou, Y. Z. Guo, C. M. Zhao, B. C. Yang and Y. G. Wang, J. Mater. Chem. C, 2016, 4, 684 RSC.
- Z. F. Wang, S. S. Zeng, J. F. Yu, X. M. Ji, H. D. Zeng, S. Y. Xin, Y. H. Wang and L. Y. Sun, Nanoscale, 2015, 7, 9552 RSC.
- J. Xu, S. Y. Zhou, D. T. Tu, W. Zheng, P. Huang, R. F. Li, Z. Chen, M. D. Huang and X. Y. Chen, Chem. Sci., 2016, 7, 2572 RSC.
- Y. G. Wang, T. Wen, H. N. Zhang, J. Sun, M. Zhang, Y. Z. Guo, W. J. Luo, M. J. Xia, Y. X. Wang and B. C. Yang, J. Phys. Chem. C, 2014, 118, 10314 CAS.
- G. S. Yi, Y. F. Peng and Z. Q. Gao, Chem. Mater., 2011, 23, 2729 CrossRef CAS.
- P. P. Lei, P. Zhang, Q. H. Yuan, Z. Wang, L. L. Dong, S. Y. Song, X. Xu, X. L. Liu, J. Feng and H. J. Zhang, ACS Appl. Mater. Interfaces, 2015, 7, 26346 CAS.
- L. Liu, Y. X. Wang, Y. F. Bai, X. R. Zhang, K. Yang, C. H. Huang and Y. L. Song, Opt. Commun., 2012, 285, 1528 CrossRef CAS.
- Y. X. Liu, Q. B. Yang and C. F. Xu, J. Appl. Phys., 2008, 104, 064701 CrossRef.
- C. G. Ming, F. Song, J. Hou, Y. Yu, G. Zhang, H. Yu, T. Q. Sun and J. G. Tian, Opt. Commun., 2011, 284, 3304 CrossRef CAS.
- F. Zhang, G. Q. Li, W. F. Zhang and Y. L. Yan, Inorg. Chem., 2015, 54, 7325 CrossRef CAS PubMed.
- X. X. Yang, Z. L. Fu, G. F. Liu, C. P. Zhang, Y. L. Wei, Z. J. Wu and T. Q. Sheng, RSC Adv., 2015, 5, 70220 RSC.
- G. Tian, Z. J. Gu, L. J. Zhou, W. Y. Yin, X. X. Liu, L. Yan, S. Jin, W. L. Ren, G. M. Xing, S. J. Li and Y. L. Zhao, Adv. Mater., 2012, 24, 1226 CrossRef CAS PubMed.
- H. B. Wang, W. Lu, Z. G. Yi, L. Rao, S. J. Zeng and Z. Li, J. Alloys Compd., 2015, 618, 776 CrossRef CAS.
- X. Xu, Z. Wang, P. P. Lei, X. L. Liu, Y. Su, L. L. Dong, S. Yao, L. Zhou, S. Y. Song, J. Feng and H. J. Zhang, Dalton Trans., 2015, 44, 17286 RSC.
- Z. Z. Chen, E. H. Song, M. Wu, S. Ding, S. Ye and Q. Y. Zhang, J. Alloys Compd., 2016, 667, 134 CrossRef CAS.
- Z. H. Bai, H. Lin, J. Johnson, S. C. R. Gui, K. Imakita, R. Montazami, M. Fujii and N. Hashemi, J. Mater. Chem. C, 2014, 2, 1736 RSC.
- J. H. Zeng, T. Xie, Z. H. Li and Y. D. Li, Cryst. Growth Des., 2007, 7, 2774 CAS.
- Y. Zhang, J. D. Lin, V. Vijayaragavan, K. K. Bhakoo and T. T. Y. Tan, Chem. Commun., 2012, 48, 10322 RSC.
- Z. H. Bai, H. Lin, K. Imakita, R. Montazami, M. Fujii and N. Hashemi, RSC Adv., 2014, 4, 61891 RSC.
- D. Q. Chen, L. Liu, P. Huang, M. Y. Ding, J. S. Zhong and Z. G. Ji, J. Phys. Chem. Lett., 2015, 6, 2833 CrossRef CAS PubMed.
- Z. G. Yi, T. M. Zeng, Y. R. Xu, W. Lu, C. Qian, H. R. Liu, S. J. Zeng and J. H. Hao, Nanotechnology, 2015, 26, 385702 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2016 |
