Electronic structure and transport properties of antiferromagnetic double perovskite Y2AlCrO6†
Indrani Dasa,
Sadhan Chanda*b,
Sujoy Sahab,
Alo Duttac,
Sourish Banerjeea,
Sudipta Bandyopadhyaya and
T. P. Sinhab
aDepartment of Physics, University of Calcutta, 92, Acharya Prafulla Chandra Road, Kolkata 700 009, India
bDepartment of Physics, Bose Institute, 93/1, Acharya Prafulla Chandra Road, Kolkata 700 009, India. E-mail: sadhan.physics@gmail.com
cDepartment of Condensed Matter Physics and Material Sciences, S. N. Bose National Centre for Basic Sciences, Block-JD, Sector-III, Salt Lake, Kolkata-700098, India
First published on 17th August 2016
Abstract
The antiferromagnetic G-type magnetic ordering in Y2AlCrO6 (YAC) has been investigated by electronic band structure calculations. The material is synthesised by a sol–gel technique and the electronic structure calculations are initiated by the experimental lattice parameters, obtained from the Rietveld refinement of the X-ray diffraction data. The Rietveld refinement shows that the room-temperature crystal structure of YAC is monoclinic with the space group P21/n, and contains an ordered array of alternating AlO6 and CrO6 octahedra tilted along the three pseudocubic axes according to the Glazer notation a−a−b+. The Raman spectrum of the sample is observed for P21/n symmetry. The field cooled and zero field cooled measurements of the sample are performed at a magnetic field of 100 Oe in the temperature range from 5 to 300 K. The temperature dependent magnetization shows the anti-ferromagnetic ordering of Cr ions in YAC. The calculated magnetic moment is well matched with the experimental magnetic moment and suggests the 3+ oxidation state of Cr with the canted alignment of its spin. The octahedral co-ordination of Cr3+ ions in YAC is confirmed from the photoluminescence spectrum. The band gap obtained from the diffuse reflectance measurements shows the semiconducting nature of the material. To observe the effect of grains, grain-boundaries and electrodes in the conduction process, the dielectric relaxation of YAC has been investigated using alternating current impedance spectroscopy in the frequency range from 50 Hz to 5 MHz as a function of temperature. An electrical equivalent circuit consisting of the resistance and the constant phase element is used to explain the impedance data. The observed results are used to discuss the effect of substitution of Cr by Al in the parent compound YCrO3.
I. Introduction
The yttrium based orthoperovskite, YBO3 is one of the most intensively studied families of perovskite oxides, where yttrium orthochromate, YCrO3 (YCO) and yttrium orthoaluminate, YAlO3 (YAO) show various interesting physical properties. The crystal structure of YCO has been indexed as the orthorhombic unit cell with the Pbnm space group.1 YCO is a biferroic material having canted anti-ferromagnetic (AFM) ordering below the Neel temperature (TN) of 140 K (ref. 2), and a weak ferroelectric behaviour below the Curie temperature (TC) of 470 K.3 It is well known that the ferroelectricity in ABO3-type perovskites originates due to the formation of an electric dipole moment by the displacement of the B-cation with respect to the oxygen octahedra. Since the Pbnm space group is centrosymmetric, it should not account for the ferroelectric behaviour. Serrao et al.4 have proposed the concept of ‘local non-centrosymmetry’ to account for the observed permittivity peak and weak polarization in YCO. This local effect can lead to the creation of nanosized polar regions due to the fluctuations in local stoichiometry in perovskites.5 This in turn leads to the creation of dipoles, and a behaviour similar to the frustrated ferroelectrics is observed. The presence of only one kind of magnetic cation (Cr3+) makes YCO as a simpler promising material among the other rare-earth chromates (RCrO3) to understand the physical mechanism of multiferroicity in it.On the other hand, YAO is serving as the basic material of the laser technique, optical recording media and the substrate material for thin films of high temperature superconductors.6–8 But single crystal of YAO has a disadvantage in laser application due to its brownish colour.9 To overcome this difficulty, rare-earth or transition metals are doped at B-site of YAO. The crystals of YAO containing the rare-earth ions and transition-metal ions are known as a host material for solid-state lasers.10 According to the recent study by Zhydachevskii et al.,11 Mn doped YAO shows a high sensitivity to UV or visible light. A characteristic thermal glow from the exposed material has been observed during heating above the room temperature and thus it can be used as the thermo-luminescent screens for visualization of local heating. In general the Mn ion in YAO host lattice resides at Al-site with 4+ valency. Since the ionic radii of Mn4+ (RMn = 0.53 Å) and Al3+ (RAl = 0.535 Å) in the octahedral co-ordination are very close, the incorporation of Mn4+ ions into the octahedral positions should not give a large change in the lattice parameters and retains the orthorhombic crystal structure of the host YAO.12 But if the ionic radius of the doped transition metal such as Cr3+ (RCr = 0.615 Å) differs from Al3+, the crystal structure deviates from the perfectly orthorhombic symmetry and hence the luminescence spectra of the doped transition-metal ions may change depending on the crystal structure. The luminescence property of Cr3+ doped YAO single crystal has already been studied and it has been established that this material can be used as the solid-state laser material.13 But to the best of our knowledge no detail investigation of the magnetic and the dielectric properties of YAO:Cr3+ has been reported till date.
In this work we have investigated the magnetic and dielectric properties of Cr3+ doped YAO. Since the polycrystalline materials offer significant advantage over the single crystals related to the ease and cost effective fabrication, we have used the sol–gel citrate method to prepare the polycrystalline material Y2AlCrO6 (YAC) where 50% of Al3+ is replaced by the Cr3+ ions in YAC. The ground state electronic band structure calculations of YAC have been performed using the density functional theory (DFT) to investigate its AFM behaviour.
II. Experimental
YAC in powder form was synthesized by the sol–gel citrate method. At first, stoichiometric quantities of the reagent grade Y(NO3)3·6H2O (Alfa Aesar), Al(NO3)2·9H2O (Alfa Aesar) and Cr(NO3)2·9H2O (Alfa Aesar) were taken and dissolved separately in de-ionized water by stirring with a magnetic stirrer. The obtained clear solutions were then mixed together. Citric acid (CA) and ethylene glycol (EG) were added to this solution drop wise according to the molar ratio of {Y3+}![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) {CA}
{CA}![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) {EG} = 1
{EG} = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 to form a polymeric-metal cation network. The role of CA and EG to form the polymeric-metal cation network has been explained in details in our previous work.14 The solution was stirred at 348 K using a magnetic stirrer for 4 h to get a homogeneous mixture and then the solution was dried at 393 K to obtain the gel precursor. After combustion of the gel, a fluffy powder of the material was collected. The powder was calcined at 1473 K in the air for 12 h and cooled down to room temperature (RT ∼ 300 K) at a cooling rate of 1 K min−1. The discs of thickness = 1.5 mm and diameter = 8 mm were prepared by the calcined sample using polyvinyl alcohol as the binder. Finally, the discs were sintered at 1523 K and cooled down to RT by cooling at the rate of 1 K min−1.
4 to form a polymeric-metal cation network. The role of CA and EG to form the polymeric-metal cation network has been explained in details in our previous work.14 The solution was stirred at 348 K using a magnetic stirrer for 4 h to get a homogeneous mixture and then the solution was dried at 393 K to obtain the gel precursor. After combustion of the gel, a fluffy powder of the material was collected. The powder was calcined at 1473 K in the air for 12 h and cooled down to room temperature (RT ∼ 300 K) at a cooling rate of 1 K min−1. The discs of thickness = 1.5 mm and diameter = 8 mm were prepared by the calcined sample using polyvinyl alcohol as the binder. Finally, the discs were sintered at 1523 K and cooled down to RT by cooling at the rate of 1 K min−1.
The crystal-structure of YAC was studied using a X-ray powder diffractometer (Rigaku Miniflex II) having Cu-Kα radiation in the 2θ range of 15–90° by scanning at 0.02° per step at RT. The refinement of the X-ray diffraction (XRD) pattern was performed by the Rietveld method with the Full-prof program.15 In the refinement process of the XRD profile, the background was fitted with the 6-coefficients polynomial function, and the peak shapes were described by the pseudo-Voigt profiles. Throughout the refinement, the scale factor, lattice parameters, positional coordinates (x, y, z) and thermal parameters were varied and the occupancy parameters of all the ions were kept fixed. To find out the various vibrational modes of the crystal structure, the micro-Raman scattering spectrum of the sample was collected at RT by Jobin-Yvon LABRAM-HR spectrometer (objective 50×) using back-scattering the geometry. The 488 nm line of an Ar-ion laser (effective power of 200 mW) was used as the exciting line. The diffuse reflectance (DR) spectrum of the sample was obtained in the range of 200–800 nm using a Shimadzu UV-visible spectrometer. The photoluminescence (PL) spectra of the sample were obtained at RT using spectrofluorometer (FP-8500-Jasco) at the excitation wavelength of 620 nm.
Magnetization vs. temperature data were collected in the zero field-cooled (ZFC) and the field cooled (FC) modes at a magnetic field of 100 Oe in the temperature range from 5 K to 300 K using a small piece of sintered pellet by a Quantum design SQUID magnetometer. The magnetic hysteresis curve between the magnetic field and the magnetization were measured at 50 K and 300 K.
To study the electrical properties, both the flat surfaces of the sintered pellet were electroded with the fine silver paint and kept at 573 K for 2 h prior to performing the experiment. The impedance (Z), conductance (G) and phase angle (δ) were measured using an LCR meter (HIOKI) in the frequency range from 50 Hz to 1 MHz at the oscillation voltage of 1.0 V. The measurements were performed over the temperature range from 303 K to 593 K using an inbuilt cooling–heating system. The temperature was controlled by an Eurotherm 818P programmable temperature controller connected with the oven. Each measured temperature was kept constant with an accuracy of ±1 K. The complex dielectric constant ε* (=1/iωCoZ*) was obtained from the temperature dependence of the real (Z′) and imaginary (Z′′) parts of the complex impedance Z* (=Z′ − iZ′′), where ω is the angular frequency (ω = 2πν) and  . Co = εoA/d is the empty cell capacitance, where A is the sample area and d is the sample thickness. The ac electrical conductivity σ (=Gd/A) was calculated from the conductance.
. Co = εoA/d is the empty cell capacitance, where A is the sample area and d is the sample thickness. The ac electrical conductivity σ (=Gd/A) was calculated from the conductance.
III. Computational details
The DFT calculations were performed by the projector augmented wave (PAW) method as implemented in VASP16 to get the ground state electronic structure of YAC. The generalized gradient approximation (GGA) as parameterized by Perdew et al.17 was used for the exchange–correlation (XC) functional. The 5s state of Y with the core radius of 3.43 a.u., and 3p and 4s states of Cr with the core radius of 2.5 a.u. were taken as the valence states, whereas the standard PBE potentials for Al and O having the core radius of 2.65 a.u. and 1.55 a.u. respectively were applied. For the self-consistent calculation with the plane wave basis, 4 × 4 × 4 Monkhorst–Pack18 k-point mesh was used. Initially the lattice parameters and the atomic positions obtained from the Rietveld refinement were used as the input for the calculations and then the structure was relaxed in such a way that the Hellmann–Feynman forces on each atom were reduced to within 0.05 eV Å−1. Further calculations were performed with this relaxed structure. The plane-wave energy cutoff of 500 eV was chosen to obtain the well converged results. For Cr, the correlation effect of the localized d electrons was considered and the GGA + U19 calculations were performed with the effective Coulomb potential (UCr-3d = 4 eV and J = 1 eV).IV. Results and discussion
A X-ray diffraction
Fig. 1 shows the XRD pattern of YAC where the symbol represents the experimental data and the solid line represents the best fit to the diffraction profile obtained by the Rietveld refinement. The vertical bar symbols denote the Bragg-positions and the solid curve at the bottom represents the difference between the experimental and the calculated patterns. It has been inferred that YAC may crystallize in a perovskite structure with either orthorhombic Pbnm or monoclinic P21/n space group. The former space group corresponds to the random distribution of the B site ions over the octahedral sites, while the latter one indicates the ordered stacking of the B site ions. It is also difficult to simply distinguish Pbnm and P21/n space groups by the X-ray diffraction technique due to the small difference in the mean scattering factors of Cr and Al especially if the ordering is incomplete and β is very close to 90°. The presence of the super-lattice diffraction peak (101) at 2θ ≈ 20° in the XRD pattern indicates in phase tilting of the octahedra with B-site cation ordering. Hence the centrosymmetric space group P21/n, which permits B-sites ordering is adopted here to refine the crystal structure of YAC. The refined lattice parameters are found to be a = 5.215(3) Å, b = 5.429(3) Å and c = 7.455(4) Å and β = 90.01°. A schematic presentation of the YAC unit cell is shown in the inset of Fig. 1 with the distribution of ions at crystallographic positions 4e for Y3+ ions, 2d for Al3+ ions, 2c for Cr3+ ions, and 4e for O2− ions as given in Table ESI1.† Each of Al3+ and Cr3+ ions is surrounded by six O2− ions, constituting AlO6 and CrO6 octahedra respectively which are arranged alternatively. The final structure parameters along with the bond lengths and the bond angles associated with AlO6 and CrO6 octahedra are listed in Table ESI1.†B Raman spectroscopy
The room temperature Raman spectrum of YAC is displayed in Fig. 2. A multiple splitting of the Raman peaks at low wavenumbers may support the monoclinic structure of the material as obtained by the XRD analysis. The zone-center vibrational modes of YAC can be represented by C2h point group due to its P21/n symmetry, where Cr and Al ions occupy the 2a and 2c Wyckoff sites of Ci symmetry and Y and O ions are at the 4e sites of general C1 symmetry. Since the monoclinic crystal structure is centrosymmetric subgroup of the prototype Fm3m cubic structure having two octahedra per primitive cell, only the Raman active (gerade) modes of the octahedron should be considered. In the irreducible representation of the C2h point group as obtained from the site-group method, the Raman active modes at the Brillouin zone centre is given by| Γ = 6T(3Ag + 3Bg) + 6L(3Ag + 3Bg) + 2υ1(Ag + Bg) +4υ2(2Ag + 2Bg) + 6υ5(3Ag + 3Bg) | (1) |
Here, T and L stand for translational and librational modes respectively and they depend upon the O–B–O angles of the material. The υ1, υ2 and υ5 modes correspond respectively to the totally symmetric stretching, anti-symmetric stretching and symmetric bending of CrO6 and AlO6 octahedra. The Raman spectrum of YAC is well fitted with the sum of 24 Lorentzian peaks and shown in two parts (Fig. 2) for the clarity of the data. The narrow line width of the Raman peaks suggests the B-site ordering in YAC. The peak positions of the Raman bands are listed in Table ESI2.† As discussed by Ayala et al.,20 the higher frequency modes are assigned as υ1 and υ2 with υ1 > υ2 and correspond to the vibration of the BO6 octahedron having strongest B–O bond. Since Al–O bonds are the strongest bond in YAC, υ1 and υ2 modes can be assigned for the vibration of AlO6 octahedra. The υ5 mode of YAC falling in the range from 340 to 430 cm−1 splits into four bands due to the tilting of BO6 octahedra. On the other hand T and L modes are present in the lower frequency range. The assignment of the different modes is given in Table ESI2.†
C Magnetic properties
Fig. 3(a) shows the temperature dependence of the magnetization of YAC for ZFC and FC measurements. The high temperature paramagnetic region of the inverse susceptibility curve is shown in the inset of Fig. 3(a), which is fitted to the Curie–Weiss law, χ = C/(T + θ), where C is the Curie constant and θ is the paramagnetic Curie–Weiss temperature. It is observed that the fitted line cut the negative X-axis, indicating the dominant role of the AFM interactions in YAC and the Neel temperature is found to be 81 K. The linear curve fitting gives an effective magnetization (μeff) of 4.57 μB/f.u., and a Weiss temperature of 207 K. The theoretical effective magnetic moment of Cr3+ can be calculated aswhere, the total spin (S) of Cr3+ is 3/2 and the Lande's splitting factor (g) is 2. But, Tiwari et al.21 have estimated the value of experimental magnetic moment of Cr3+ in YCrO3 as 4.7 μB/f.u. In YAC, the Al is doped at Cr site, hence the value of the magnetic moment is decreased to 4.57 μB/f.u. The magnetic hysteresis curves of the sample at 50 K and 300 K are presented in Fig. 3(b). The hysteresis loop at 300 K shows the pure paramagnetic behaviour. On the other hand the loop at 50 K exhibits lossy loop with a coercive field of 300 Oe. This indicates the typical canted AFM ordering in the sample. This kind of magnetization loop is attributed to the existence of weak ferromagnetism where dc magnetization increases linearly in the larger magnetic field region and the obtained magnetization can be estimated as M(H) = χAFH + Ms; where χAFH is the antiferromagnetic contribution and Ms is the saturation magnetization of weak ferromagnetism. Inset of Fig. 3(b) shows the extracted ferromagnetic contribution at the temperature 5 K. The weak ferromagnetic saturation magnetizations is found to be 0.01 μB/f.u./Cr in YAC. The weak ferromagnetism is attributed to the observed canting of Cr3+ (t32g e0g) spin magnetic moments which is followed by the tilting of two adjacent CrO6 corner sharing octahedral with a 〈Cr–O–Cr〉 bond angle of 150° far from the ideal 180° in distorted orthorhombic perovskite-like materials. Thus, the deviation of μeff from the theoretical value is due to its weak ferromagnetic nature.21
It is observed that TN of YAC is less than that of YCO. The decrease of TN is due to the 50% substitution of Cr by non-magnetic Al ions in the YCO matrix. The CrO6 and AlO6 octahedra are arranged alternately in YAC. Since Al is non-magnetic cation, the substitution of Cr by Al destabilizes the network of Cr ions by breaking some bonds which increases the interaction path length between the nearest Cr ions.
As a result the magnetic interaction strength between Cr ions decreases which may decrease TN in YAC with respect to YCO.
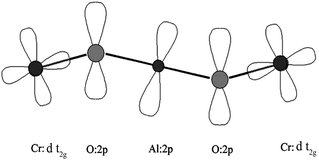
It is to be mentioned that the magnetic coupling in YAC may arise from π-superexchange mediated by the overlap of Cr-dt2g and O-p and Al p-orbitals as shown in Fig. 4, where the Cr-cations and Al-cations interact with each other through O-anion giving the super-exchange path as Cr–O–Al–O–Cr. These pathways are pure AFM provided that the orbitals are approximately coplanar, as has been found in various undistorted double perovskites.22–24 Moreover, the spins of Cr atoms are not perfectly anti-parallel to each other; it has two components, one along the c-axis and the other in ab-plane. The c-axis components of spins of the two nearest Cr ions are anti-parallel whereas the ab-plane components are parallel. This type of spin alignment gives rise to the canted AFM configuration of YAC. Since the Cr–Cr distance (obtained from the crystal structure) along the c-axis is small with respect to the ab-plane, the anti-parallel spin alignment (c-axis) dominates over the parallel alignment (ab-plane) and gives rise to a strong AFM effect in YAC though it is canted.
D Electronic structure and antiferromagnetism
To investigate the exact nature of the magnetic ordering in YAC, we have carried out the electronic band structure calculations in four different magnetic orderings of Cr atoms, which are ferromagnetic (FM), A-AFM, C-AFM and G-AFM. The calculations are performed assuming the collinear spin alignment. In this strongly correlated electron system having half-filled d-band (Cr3+:3d3), a strong electron correlation effect is expected. Hence in our calculations we have incorporated the on-site Coulomb energy U of 4 eV and exchange interaction energy J of 1 eV for the localized d-orbitals of Cr. In FM and G-AFM, the spins of all the nearest neighbour Cr atoms are aligned parallel and anti-parallel respectively. For A-AFM and C-AFM configurations, the spin of nearest neighbour Cr atoms in the ab-plane are parallel and anti-parallel respectively whereas along the c-axis they are anti-parallel and parallel respectively. The ground state energy values for YAC in these four magnetic orderings are listed in Table 1. It is observed that G-AFM configuration has the lowest energy value. Thus it can be concluded that the ground state of YAC has G-AFM configuration.| Configuration | Energy | Magnetic moment at Cr-site |
|---|---|---|
| FM | 7.7 | 4.12 |
| AFM-G | 0 | ±4.14 |
| AFM-A | 7.6 | ±4.14 |
| AFM-C | 7.6 | ±4.14 |
The spin polarized total and partial density of states (DOS) of Y-d, Cr-d, Al-p and O-p for YAC per formula unit in the G-AFM state are shown in Fig. 5. The ground state of YAC is observed with a finite band gap energy of 1.75 eV at the Fermi level (set at 0 eV) in the both up and down spin channels. It is observed that the valence bands in both spin channels extended from −2 to −7 eV mainly consist of O-2p states. The p-states of Al interact with O-2p states in the bottom region of valence band extended around −5.5 eV. The crystal field produced by the oxygen octahedra lifts the degeneracy of the Cr-d states and spits it into t2g and eg states with the eg state of higher energy with respect to the t2g state.
 | ||
| Fig. 5 The spin-polarized total and partial density of states calculated within GGA + U for G-AFM configuration. | ||
In the up spin channel, Cr-dt2g states are localized between the O 2p band and the Fermi level and Cr-deg states are in the conduction band, whereas in the down spin channel, both Cr-dt2g and Cr-deg states are in the conduction band. This indicates that in the up spin channel, the t2g state is completely filled and the eg state is completely empty. On the other-hand, in the down spin channel, both eg and t2g states are completely empty. The above nature of Cr-d states implies the high spin (d3; t3↑2g e0g) configuration in YAC. The exchange splitting between the eg up and the eg down states for Cr is 1.6 eV, whereas the exchange splitting between the t2g up and the t2g down states is 2.57 eV. It is observed from the partial DOS that the electronic structure of YAC involves a strong Cr–O covalency. The AFM coupling between any two Cr-ions leads to a zero total magnetic moment however the calculated magnetic moment at each Cr-site is found to be 4.16 μB which is in good agreement with the experimental value.
E Optical properties
To verify the semiconducting ground state of YAC experimentally, the optical band gap of YAC is calculated from DR spectrum using Kubelka–Munk (KM) function defined as25
 | (2) |
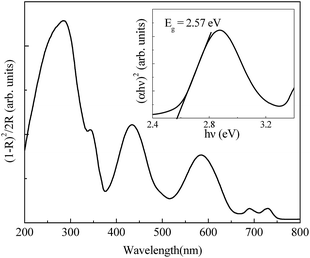 | ||
| Fig. 6 Kubelka–Munk function as a function of the wavelength. Inset shows UV-visible absorption spectrum for direct transition (αhν)2 vs. hν. | ||
Broad absorption bands at around 433 nm and 585 nm are the characteristic bands for Cr3+ ions in octahedral coordination. These bands correspond to the d–d transition from the ground state 4A2g to 4T1g and 4T2g states of Cr3+ respectively.27,28 The highest intensity band at around 285 nm is the characteristic absorption band for YAO. Two low intensity bands in the range of 680–730 nm are due to the spin-forbidden transitions from 2T1g and 2Eg. Since, the scattering coefficient is only weakly dependent on the energy, F(R∞) can be assumed to be proportional to the absorption coefficient within the narrow energy range of the absorption edge29 and hence the energy dependence of α near the absorption edge can be expressed as:30
 | (3) |
Fig. 7 shows the photoluminescence excitation and emission spectra of YAC. The excitation spectrum exhibits two broad absorption bands at around 500 and 630 nm. The emission spectrum taken at an excitation wavelength of 630 nm shows a characteristic peak at 725 nm of YAC. Sugiyama et al.31 have studied the photoluminescence properties of YAO doped with Cr. The photoluminescence property retains in YAC when the % doping of Cr is increased to 50%. It has been observed that the excitation spectrum of our material is blue shifted nearly by 100 nm with respect to the single-crystalline YAO:Cr.31
F Dielectric relaxation and ac conductivity
The angular frequency dependence of dielectric constant (ε′) and loss tangent (tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ) of YAC at various temperatures is shown in Fig. 8. The presence of three well resolved relaxation peaks in Fig. 8(b) confirms the existence of at least three types of relaxation process in YAC. In the higher frequency range (>104 Hz) the relaxation process corresponds to the grain effect, in the intermediate frequency range (103 to 104 Hz) it originates due to the grain boundary contribution, and in the lower frequency side (<102 Hz) the electrode effect is more dominant. To explain the relaxation phenomenon in each frequency region, we have considered the Debye model. According to this model, below the relaxation frequency of each relaxation process all the dipoles follow the applied field and fully contribute to the relaxation process. With the increase of the applied field frequency, the dipoles begin to lag behind the applied field and at the relaxation frequency a sudden drop of the ε′ is evident. For further increase of the applied frequency, most of the dipoles do not respond and ε′ becomes nearly independent of the frequency. But this feature is not clearly observed in Fig. 8(a), may be due to the overlapping of the frequency range associated with more than one relaxation process. It is observed from Fig. 8 that the dispersions in ε′ and the corresponding relaxation peaks in tan
δ) of YAC at various temperatures is shown in Fig. 8. The presence of three well resolved relaxation peaks in Fig. 8(b) confirms the existence of at least three types of relaxation process in YAC. In the higher frequency range (>104 Hz) the relaxation process corresponds to the grain effect, in the intermediate frequency range (103 to 104 Hz) it originates due to the grain boundary contribution, and in the lower frequency side (<102 Hz) the electrode effect is more dominant. To explain the relaxation phenomenon in each frequency region, we have considered the Debye model. According to this model, below the relaxation frequency of each relaxation process all the dipoles follow the applied field and fully contribute to the relaxation process. With the increase of the applied field frequency, the dipoles begin to lag behind the applied field and at the relaxation frequency a sudden drop of the ε′ is evident. For further increase of the applied frequency, most of the dipoles do not respond and ε′ becomes nearly independent of the frequency. But this feature is not clearly observed in Fig. 8(a), may be due to the overlapping of the frequency range associated with more than one relaxation process. It is observed from Fig. 8 that the dispersions in ε′ and the corresponding relaxation peaks in tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ move towards the higher frequencies with the increase of temperature which suggests the thermally activated nature of the relaxation process.
δ move towards the higher frequencies with the increase of temperature which suggests the thermally activated nature of the relaxation process.
Since the peak in the tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ depends on the mobility and the temperature, the mobility of the thermally activated charge carriers increases with the increase of the temperature, and they start to relax at the higher frequency thereby shifting the loss peak towards the higher frequency side. It is observed from Fig. 8(b) that the value of tan
δ depends on the mobility and the temperature, the mobility of the thermally activated charge carriers increases with the increase of the temperature, and they start to relax at the higher frequency thereby shifting the loss peak towards the higher frequency side. It is observed from Fig. 8(b) that the value of tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ in the grain region is smallest (<0.3) with respect to the grain-boundary (>0.32) and electrode (∼0.5) regions. Since the increase in the value of ε′ with the increase of the temperature is more pronounced in the lower frequencies, the observed high value of ε′ and tan
δ in the grain region is smallest (<0.3) with respect to the grain-boundary (>0.32) and electrode (∼0.5) regions. Since the increase in the value of ε′ with the increase of the temperature is more pronounced in the lower frequencies, the observed high value of ε′ and tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ at the lower frequency side can be attributed to the presence of the electrode–semiconductor interface, which results in the Maxwell–Wagner type polarization.32 The Maxwell–Wagner type polarization may be originated due to the presence of heterogeneous components in the material, which have different interfaces with different conductivity. The surface charge accumulation is taken place when the current is passed through these interfaces which gives a Debye like relaxation under the application of an ac voltage. At the electrode–semiconductor contact, a high capacitance is formed due to the Schottky-type barrier layer which may be originated due to different work functions of the charge carriers at the electrode and in the materials.33 This results in the high dielectric constant at the lower frequency and at the higher temperature in YAC. The transport mechanism in YAC can be assessed by the hopping of charge carriers, and the frequency dependent tan
δ at the lower frequency side can be attributed to the presence of the electrode–semiconductor interface, which results in the Maxwell–Wagner type polarization.32 The Maxwell–Wagner type polarization may be originated due to the presence of heterogeneous components in the material, which have different interfaces with different conductivity. The surface charge accumulation is taken place when the current is passed through these interfaces which gives a Debye like relaxation under the application of an ac voltage. At the electrode–semiconductor contact, a high capacitance is formed due to the Schottky-type barrier layer which may be originated due to different work functions of the charge carriers at the electrode and in the materials.33 This results in the high dielectric constant at the lower frequency and at the higher temperature in YAC. The transport mechanism in YAC can be assessed by the hopping of charge carriers, and the frequency dependent tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ is a requisite part of the charge carriers hopping transport process. Hence, we have studied the temperature dependence of the most probable relaxation frequency (ωm) corresponding to the peak position in tan
δ is a requisite part of the charge carriers hopping transport process. Hence, we have studied the temperature dependence of the most probable relaxation frequency (ωm) corresponding to the peak position in tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ vs. log
δ vs. log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ω plot. It is observed that the temperature dependence of log
ω plot. It is observed that the temperature dependence of log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ωm for all three (grain, grain-boundary and electrode) relaxation processes follows the Arrhenius law as shown in Fig. 9 with the activation energy of 0.31, 0.39, and 0.44 eV respectively. These values of activation energy support inhomogeneous character of the sample having low resistive grains separated by more resistive grain-boundaries.34
ωm for all three (grain, grain-boundary and electrode) relaxation processes follows the Arrhenius law as shown in Fig. 9 with the activation energy of 0.31, 0.39, and 0.44 eV respectively. These values of activation energy support inhomogeneous character of the sample having low resistive grains separated by more resistive grain-boundaries.34
The temperature dependence of ε′ of YAC measured at various frequencies is shown in Fig. 10. At each frequency ε′(T) shows a step like nature and a temperature independent region of ε′ is observed. Such behaviour of ε′(T) is typical of the Maxwell–Wagner relaxation in the material. If compared with YCO4 the value of ε′ is nearly 10 times smaller in YAC. This decrease of ε′ can be correlated with the band gap values, because in perovskite oxides dielectric relaxation is basically a conduction process and the transport of charge carriers plays the major role in relaxation phenomenon. The wider band gap of YAC (∼2.57 eV) with respect to YCO (∼1.4 eV)35 may hinder the charge transport and hence decreases the conductivity. Similar dependency of conductivity and ε′ on the band gap value has been observed in Mn doped YCO.35 It is also observed from Fig. 9 that there is no peak in ε′(T) in the entire measuring temperature range. Whereas Serrao et al.4 have got a broad peak in ε′(T) plot for YCO which provides the evidence of ferroelectricity. They predicted that the origin of ferroelectric properties in YCO is its non-centrosymmetric crystal structure P21. On the other hand YAC has a centrosymmetric P21/n space group which may be responsible for the absence of peak in ε′(T) in Fig. 10.
In order to understand the origin of the different relaxation processes in YAC, we have studied its complex impedance plane plot (Z-plot) as shown in Fig. 11. The presence of three semi-circular arcs in Fig. 11 confirms that three different types of relaxation process are involved in the charge transport of YAC. The inset of Fig. 11 shows the high frequency data for the clarity of the grain and grain-boundary regions. Usually the Z-plot is fitted with an electrical equivalent circuit consisting of three parallel resistance–capacitance (RC) circuits connected in series. One parallel branch is associated with the grain effect and the other two represent the grain-boundary and the electrode effects. Due to the non-ideal behaviour of the capacitance, sometimes both grain and grain-boundary and/or grain-boundary and electrode contributions, though small, are present in the same frequency range, which may give rise to the depressed arcs or even only a spike-like nature in the low-frequency region with a small arc in the high-frequency region of the Z-plot. For such cases, the capacitance term in the RC-equivalent circuit is replaced by a constant phase element (CPE). The capacitance of CPE can be expressed as CCPE = Q1/kR(1−k)/k, where k estimates the non-ideal behaviour and Q is the CPE component. The value of k is zero for the ideal resistance and 1 for the ideal capacitance. The solid lines in Fig. 11 represent the fitting to the electrical equivalent circuit and the fitted parameters are found to be 2650 Ω, 52 kΩ, 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 kΩ, 1.22 nF, 2.138 nF, 2.04 nF, 0.60, 0.41, and 0.51 for Rg, Rgb, Re, Cg, Cgb, Ce, kg, kgb, and ke respectively.
000 kΩ, 1.22 nF, 2.138 nF, 2.04 nF, 0.60, 0.41, and 0.51 for Rg, Rgb, Re, Cg, Cgb, Ce, kg, kgb, and ke respectively.
The frequency dependent log–log plots of the ac conductivity of YAC are shown in Fig. 12(a) at a various temperatures. A strong frequency dependent and a very week temperature dependent effect are observed for the ac conductivity in YAC. Due to the effect of grain, grain-boundary and electrode, one gets three plateaus and three dispersion regions in the frequency dependent ac conductivity plots as shown by I, II and III.36 In the region I, the low frequency plateau represents the total conductivity followed by a dispersion region in which the electrode contribution relaxes. In the region II i.e., mid frequency region the plateau represents the grain boundary contribution to the total conductivity. The grain-boundary contribution relaxes in the dispersion region after this plateau. In the region III, the highest frequency plateau represents the contribution of grains to the total conductivity. The dispersion region followed by this plateau represents the frequency dependence of the bulk conductivity. At the higher temperature, the low frequency plateau in region I becomes nearly independent of frequency and its value at ν → 0 gives the value of dc conductivity at that temperature. This is due to the fact that the intrinsic conductivity is dominant in the low frequency region at the higher temperatures. The increase of the conductivity with the increase of the frequency at a particular temperature indicates that the ac conductivity follows the power law. Since three plateaus are present in the ac conductivity we have used the power law equation having three frequency dependent parts defined as follows:
| σ(ω) = σo + A1ωn1 + A2ωn2 + A3ωn3 | (4) |
 | ||
| Fig. 12 Frequency (angular) dependence of the ac conductivity (σ) at various temperatures. The solid lines are the fitting of the experimental data with the power law. | ||
| Temperature (K) | A1 (×10−6) | n1 | A2 (×10−4) | n2 | A3 (×10−4) | n3 |
|---|---|---|---|---|---|---|
| 413 | 2.58 | 1.98 | 2.85 | 0.99 | 5.38 | 0.7 |
| 453 | 59.4 | 1.96 | 1.11 | 0.98 | 45.4 | 0.7 |
V. Conclusions
The double perovskite oxide Y2CrAlO6 (YAC) has been synthesized by the sol–gel technique. The Rietveld refinement of the XRD profile at the room temperature shows the monoclinic P21/n crystal symmetry of the system which is supported by the Raman spectrum. The crystal structure of YAC differs from its two end materials YCrO3 and YAlO3. The magnetic measurements as well as the ground state electronic structure calculations suggest the G-type AFM ordering of Cr ions in YAC. The magnetic ordering temperature, TN is found to be 81 K and below this temperature magnetic hysteresis loop shows lossy nature due to the canted spin alignment of Cr ions. The characteristic absorption and emission bands of Cr3+ ions in octahedral co-ordination are obtained in the absorption and photoluminescence spectra. The effect of grain, grain-boundary and electrode in the relaxation process is explained from the frequency dependent dielectric constant and loss tangent. An electrical equivalent circuit consisting of resistance and constant phase element is used to explain the complex impedance plane plot. The frequency dependent ac conductivity spectra follow the power law equation having three frequency dependent parts for the effect of grain, grain-boundary and electrode.Acknowledgements
Sujoy Saha acknowledges the financial support provided by the UGC New Delhi in the form of SRF. Alo Dutta thanks to Department of Science and Technology of India for providing the financial support through DST Fast Track Project under grant no. SB/FTP/PS-175/2013.References
- S. Geller and E. A. Good, Acta Crystallogr., 1967, 1, 948 Search PubMed.
- T. Morishita and K. Tsushima, Phys. Rev. B: Condens. Matter Mater. Phys., 1981, 24, 341 CrossRef CAS.
- C. V. Colin, A. G. Pérez, P. Bordet, C. Goujon and C. Darie, Phys. Rev. B: Condens. Matter Mater. Phys., 2012, 85, 224103 CrossRef.
- C. R. Serrao, A. K. Kundu, S. B. Krupanidhi, U. V. Waghmare and C. N. R. Rao, Phys. Rev. B: Condens. Matter Mater. Phys., 2005, 72, 220101 CrossRef.
- R. Ranjan, R. Hackl, A. Chandra, E. S. D. Trots and H. Boysen, Phys. Rev. B: Condens. Matter Mater. Phys., 2007, 76, 224109 CrossRef.
- I. F. Elder and M. J. Payne, Opt. Commun., 1998, 148, 265 CrossRef CAS.
- A. J. Wojtowicz, J. Glodo, A. Lempicki and C. Brecher, J. Phys.: Condens. Matter, 1998, 10, 8401 CrossRef CAS.
- M. A. Noginov, N. Noginova, M. Curley, N. Kukhtarev, H. J. Caulfield, P. Venkateswarlu and G. B. Loutts, J. Opt. Soc. Am. B, 1998, 15, 1463 CrossRef CAS.
- P. Korczak and C. B. Staff, J. Cryst. Growth, 1973, 20, 71 CrossRef CAS.
- A. A. Kaminskii, Laser Crysrals, Springer, Berlin, 1990 Search PubMed.
- Y. Zhydachevskii, A. Durygin, V. Drozd, A. Suchocki, D. Sugak and J. Wrobel, J. Phys.: Condens. Matter, 2008, 20, 095204 CrossRef.
- R. Diehl and G. Brandt, Mater. Res. Bull., 1975, 10, 85 CrossRef CAS.
- M. Yamagat, H. Takeuchii, T. P. J. Hans and B. Henderson, Phys. Rev. B: Condens. Matter Mater. Phys., 1993, 5, 8097 CrossRef.
- S. Saha, S. Chanda, A. Dutta and T. P. Sinha, J. Sol-Gel Sci. Technol., 2014, 69, 553 CrossRef CAS.
- C. J. Rodriguez, Phys. B, 1993, 192, 55 CrossRef.
- G. Kresse and J. Furthmuller, Phys. Rev. B: Condens. Matter Mater. Phys., 1996, 54, 11169 CrossRef CAS.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865 CrossRef CAS PubMed.
- H. J. Monkhorst and J. D. Pack, Phys. Rev. B: Solid State, 1976, 13, 5188 CrossRef.
- V. I. Anisimov, I. V. Solovyev, M. A. Korotin, M. T. Czyzyk and G. A. Sawatzky, Phys. Rev. B: Condens. Matter Mater. Phys., 1993, 48, 16929 CrossRef CAS.
- A. P. Ayala, I. Guedes, E. N. Silva, A. S. Augsburger, M. C. Viola and J. C. Pedregosa, J. Appl. Phys., 2007, 101, 123511 CrossRef.
- B. Tiwari, M. K. Surendra and M. S. R. Rao, J. Phys.: Condens. Matter, 2013, 25, 216004 CrossRef PubMed.
- J. B. Goodenough, Phys. Rev. B, 1955, 100, 564 CrossRef CAS.
- M. Retuerto, M. García-Hernández, M. J. Martínez-Lope, M. T. Fernández-Díaz, J. P. Attfieldc and J. A. Alonso, J. Mater. Chem., 2007, 17, 3555 RSC.
- H. T. Jeng and G. Y. Guo, Phys. Rev. B: Condens. Matter Mater. Phys., 2003, 67, 094438 CrossRef.
- G. Kortüm, Reflectance Spectroscopy: Principles, Methods, Applications, Springer, New York, 1969 Search PubMed.
- M. Ardit, M. Dondi, G. Cruciani and F. Matteucci, Mater. Res. Bull., 2009, 44, 666 CrossRef CAS.
- M. J. Weber and T. E. Varitimos, J. Appl. Phys., 1974, 45, 810 CrossRef CAS.
- A. B. P. Lever, Inorganic Electronic Spectroscopy, Elsevier, Amsterdam, 2nd edn, 1984 Search PubMed.
- D. G. Barton, M. Shtein, R. D. Wilson, S. L. Soled and E. Iglesia, J. Phys. Chem. B, 1990, 103, 630 CrossRef.
- E. A. Davis and N. F. Mott, Philos. Mag., 1970, 22, 903 CrossRef CAS.
- M. Sugiyama, T. Yanagida, D. Totsuka, Y. Yokota, Y. Futami, Y. Fujimoto and A. Yoshikawa, J. Cryst. Growth, 2013, 362, 157 CrossRef CAS.
- J. Liu, C. Duan, W. N. Mei, R. W. Smith and J. R. Hardy, J. Appl. Phys., 2005, 98, 093703 CrossRef.
- A. Karamaker, S. Majumdar, A. K. Sing and S. Giri, J. Phys. D: Appl. Phys., 2009, 42, 092004 CrossRef.
- J. R. Macdonald, Impedance Spectroscopy, Wiley, New York, 1987 Search PubMed.
- R. Sinha, S. Basu and A. K. Meikap, Phys. E, 2015, 69, 47 CrossRef CAS.
- R. Waser, J. Am. Ceram. Soc., 1991, 74, 1934 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra15355k |
| This journal is © The Royal Society of Chemistry 2016 |