DABCO catalyzed unusual formal [4 + 2] cycloaddition of 3-acyl(or alkoxycarbonyl)-1,4-enediones with 2,3-butadienoates: effective access to highly functionalized pyrans†
Xiang-Suo Menga,
Shan Jianga,
Xiao-Yun Xua,
Qiang-Xian Wua,
Yu-Cheng Gub and
De-Qing Shi*a
aKey Laboratory of Pesticide and Chemical Biology of Ministry of Education, College of Chemistry, Central China Normal University, 152 Luoyu Road, Wuhan 430079, P. R. China. E-mail: chshidq@mail.ccnu.edu.cn; Fax: +86-27-67862041
bSyngenta Jealott's Hill International Research Centre, Bracknell, Berkshire RG42 6EY, UK
First published on 5th July 2016
Abstract
A convenient and efficient DABCO-catalyzed formal [4 + 2] cycloaddition of 3-acyl(or alkoxycarbonyl)-1,4-enediones with 2,3-butadienoates is presented. This transformation takes advantage of mild conditions, wide substrate scope and significant functional group tolerance as well as excellent regioselectivity, which makes this method powerful for one-pot synthesis of highly functionalized pyrans in moderate to excellent yields.
Multi-substituted pyran rings are important frameworks which can be found in biologically active compounds and natural products.1 Despite many strategies being developed to synthesize substituted pyrans,2 the development of novel methodologies for the convenient and effective synthesis of high functionalized pyrans remains highly desirable.
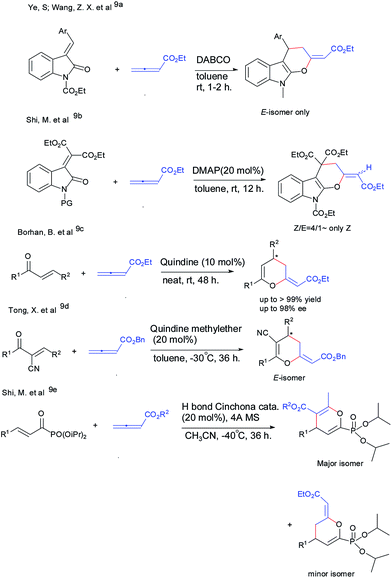
In the past few decades, Lewis base catalyzed reactions of allenoates have attracted much attention for constructing molecular diversity and complexity.3 Since 1995, Lu et al. first published phosphine-catalyzed [3 + 2] cycloaddition reaction of allenoates with alkene;4 in 2003, Kwon et al. reported a novel [4 + 2] annulation of α-alkylallenoates with imines;5 In addition, several other reactions, such as Kwon's [3 + 3] annulations with aziridines6 and Tong's [4 + n], [3 + n] annulations were well established.7 Compared to phosphines, the corresponding amine analogues usually display markedly different reaction modes in these types of transformations. However, the cycloaddition of allenoates catalyzed by amine is relatively rare8 and only a few [4 + 2] cycloadditions have been developed (Fig. 1).9 As shown in Fig. 1, in most cases, when amine was used as the catalyst, allenoate acted as a surrogate of a “1,2-dipole” and underwent β,γ-addition, exocyclic double bond adducts were formed.9a–e However, when H-bond bifunctional organocatalyst derived from Cinchona alkaloid was used, the mixture of two regioisomers was yielded, the major isomer was the α,β-adduct.9f
On the other hand, the 1,4-enedione is an important structural motif that is not only widely presented in natural products and medicinal molecules,10 but also served as versatile building blocks in a variety of transformations, such as Diels–Alder cyclization and Michael addition11 as well as precursors for the preparation of many heterocyclic compounds such as furans, thiophenes, pyrroles etc.12
Recently, we reported the Ph3P and 1,4-diazabicyclo[2.2.2]octane (DABCO) catalyzed cycloaddition of 2,3-butadienoates with 3-acyl-2H-chromen-ones for the convenient synthesis of dihydropyran-fused and cyclopenten-fused chromen-2-ones with high regio- and stereo-selectivities.13 As part of our continuing interest in cycloaddition involving 2,3-butadienoate and investigating its regioselectivity, herein, we report a DABCO-catalyzed unusual formal [4 + 2] cycloaddition of 2,3-butadienoates with 3-acyl(or alkoxycarbonyl)-1,4-enediones, the unexpected α,β-adduct regioisomer – highly functionalized pyrans 3 were generated (Scheme 1).
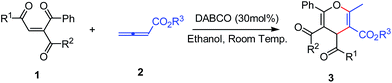 | ||
| Scheme 1 DABCO-catalyzed unusual formal [4 + 2] cycloaddition of 2,3-butadienoates with 3-acyl(or alkoxycarbonyl)-1,4-enediones. | ||
Initially, the cycloaddition of 3-benzoyl-1,4-biphenyl-1,4-enedione (1a) with ethyl 2,3-butadienoate (2b) was used as the model substrate to screen for experimental conditions. When 1,4-enedione 1a was treated with 1.5 equiv. of ethyl 2,3-butadienoate 2a in the presence of 20 mol% of DABCO in toluene (4.0 mL) at room temperature, the reaction afforded the desired multi-substituted pyran 3a in 57% yield (Table 1, entry 1). Encouraged by this result, different amine catalysts such as DMAP, DBU, TMEDA and Et3N were examined (Table 1, entries 1–5), DABCO gave the highest yield (Table 1, entry 1). However, a variety of phosphines (Ph3P, n-Bu3P and Me3P) cannot trigger the reactions (Table 1, entries 6–8). The effect of solvents was investigated, and EtOH was shown to be the optimal solvent for this reaction (Table 1, entries 9–14).
| Entry | Catalyst (mol%) | Solvent | Temp (°C) | Time (h) | Yieldb (%) |
|---|---|---|---|---|---|
| a Reaction conditions: 1a (0.50 mmol), 2a (0.75 mmol), catalyst in solvent (4.0 mL), under N2 atmosphere.b Isolated yield. | |||||
| 1 | DABCO (20) | Toluene | Room temp. | 24 | 57 |
| 2 | DMAP (20) | Toluene | Room temp. | 24 | Trace |
| 3 | DBU (20) | Toluene | Room temp. | 24 | Trace |
| 4 | TMEDA (20) | Toluene | Room temp. | 24 | 40 |
| 5 | Et3N (20) | Toluene | Room temp. | 24 | 35 |
| 6 | Ph3P (20) | Toluene | Room temp. | 36 | Trace |
| 7 | n-Bu3P (20) | Toluene | Room temp. | 24 | Trace |
| 8 | Me3P (20) | Toluene | Room temp. | 24 | Trace |
| 9 | DABCO (20) | CH2Cl2 | Room temp. | 24 | 50 |
| 10 | DABCO (20) | CH3CN | Room temp. | 24 | 73 |
| 11 | DABCO (20) | THF | Room temp. | 24 | 60 |
| 12 | DABCO (20) | C2H5OH | Room temp. | 12 | 87 |
| 13 | DABCO (20) | DMF | Room temp. | 24 | 63 |
| 14 | DABCO (20) | DMSO | Room temp. | 24 | 71 |
| 15 | DABCO (20) | C2H5OH | −5 | 40 | 75 |
| 16 | DABCO (20) | C2H5OH | 0 | 36 | 85 |
| 17 | DABCO (20) | C2H5OH | 40 | 12 | 79 |
| 18 | DABCO (20) | C2H5OH | 50 | 12 | 81 |
| 19 | DABCO (30) | C2H5OH | Room temp. | 10 | 90 |
Subsequently, the effect of reaction temperature was also explored (Table 1, entries 15–18). Increasing or decreasing the temperature did not affect the reaction yield remarkably. The amount of catalyst was also evaluated, and the results showed that a loading of 30 mol% DABCO gave the best result, led to the desired product 3a in 90% yield (Table 1, entry 19).
With the optimized conditions in hand, initially, a variety of 3-acyl(or alkoxycarbonyl)-1,4-enediones 1 were subjected to the above mentioned optimal conditions to examine the generality of this methodology (Table 2). The 1-substituted phenyl(or heteroaryl) substituted 1,4-enediones bearing electron-donating (CH3, OCH3) or electron-withdrawing groups (F, Cl, NO2) or different substituted pattern on the phenyl ring were found to be suitable substrates for this reaction, gave the desired products 3a–3j in a range of 78–91% yields. More importantly, 1-furyl or thienyl substituted 1,4-enediones 1k and 1l also proceeded smoothly to give the desired products 3k–3l with satisfactory results (75% and 71% yield, respectively). It was noted that 3-ethoxycarbonyl substituted 1,4-enediones can be also employed in this transformation and provide the desired products 3m and 3n in 85% and 68% yield, respectively, and it was found that the 3-substituents has no significant influence on the reaction (3a, 3m–3n). However, when trans-1,4-diphenyl-2-butene-1,4-dione was used as the substrate, the formal [3 + 2] product 3r was obtained as a light yellow crystal in 76% yield (please see the ESI†), which displayed different reaction mode from the 3-acyl(or alkoxycarbonyl)-1,4-enedione substrates.
Then, we evaluated the scope of 2,3-butadienoates 2. Methyl or benzyl 2,3-butadienoates (2b, 2c) are also suitable candidates for this transformation, affording the desired products 3o–3q in excellent yields (86–96% yield). Unfortunately, when α- or γ-alkyl substituted allenoates (for example, α-methyl or benzyl substituted or γ-methyl substituted 2,3-butadienoates) were used as the substrates, no reaction occurred under these above conditions (the results are not listed).
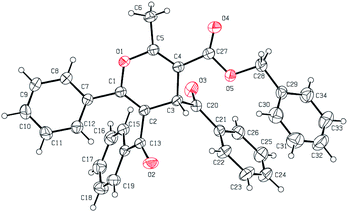
The structures and configurations of the formal [4 + 2] cycloadducts 3 were assigned via 1H-NMR, 13C-NMR, MS, elemental analysis (see the ESI†). Moreover, the structure of 3q was unambiguously determined by X-ray crystallographic analysis (Fig. 2, CCDC: 1483768†).
Based on our investigations and the reported literature,9a,9b,9f a possible mechanism is suggested as shown in Scheme 2. First of all, DABCO attacks the β-carbon of ethyl 2,3-butadienoate 2a to give intermediate A, which can isomerize to the more stable intermediate B, because both the ammonium ion and ester group can stabilize the β-carbanion of B. Subsequently, compound B undergoes 1,4-addition with 1,4-enedione 1a to generate intermediate C, which follows by the enolization and 1,3-proton transfer and converts to intermediate E. Finally, the formal [4 + 2] cycloadduct 3a is formed by the intramolecular nucleophilic attacking and then the release of DABCO.
 | ||
| Scheme 2 Possible mechanism proposed for the formal [4 + 2] cycloadditions of 3-acyl(or alkoxycarbonyl)-1,4-enediones with 2,3-butadienoates catalyzed by DABCO. | ||
Conclusions
In conclusion, we developed a convenient and efficient DABCO-catalyzed formal [4 + 2] cycloaddition of 3-acyl(or alkoxycarbonyl)-1,4-enediones with 2,3-butadienoates. This methodology possesses mild condition, wide substrate scope and significant functional group tolerance as well as excellent regioselectivity, which provides a convenient and effective pathway for the preparation of highly functionalized pyrans. Further investigation of the chiral amine-catalyzed formal [4 + 2] annulation of 3-acyl(or alkoxycarbonyl)-1,4-enediones with 2,3-butadienoates is currently underway and will be reported in due course.Acknowledgements
We are grateful to the National Natural Science Foundation of China (No. 21342004 and 51573066), and the Syngenta Ph.D. (postgraduate) program for support of this work.Notes and references
- (a) W. P. D. Goldring and G. Pattenden, Acc. Chem. Res., 2006, 39, 354 CrossRef CAS PubMed; (b) E. J. Kang and E. Lee, Chem. Rev., 2005, 105, 4348 CrossRef CAS PubMed; (c) K.-S. Yeung and I. Paterson, Chem. Rev., 2005, 105, 4237 CrossRef CAS PubMed; (d) R. H. Liu, W. D. Zhang, Z. B. Gu, C. Zhang, J. Su and X. K. Xu, Nat. Prod. Res., 2006, 20, 866 CrossRef CAS PubMed; (e) A. A. L. Gunatilaka, J. Nat. Prod., 2006, 69, 509 CrossRef CAS PubMed.
- For reviews, see: (a) K. C. Nicolaou and S. A. Synder, Classics in Total Synthesis, Wiley-VCH, Weinhein, 2003 Search PubMed; (b) A. B. Smith III, R. J. Fox and T. M. Razler, Acc. Chem. Res., 2008, 41, 675 CrossRef PubMed; (c) P. A. Clarke and S. Santos, Eur. J. Org. Chem., 2006, 2045 CrossRef CAS.
- For a review, see: (a) X. Lu, C. Zhang and Z. Xu, Acc. Chem. Res., 2001, 34, 535 CrossRef CAS PubMed; (b) B. J. Cowen and S. J. Miller, Chem. Soc. Rev., 2009, 38, 3102 RSC; (c) J. L. Methot and W. R. Roush, Adv. Synth. Catal., 2004, 346, 1035 CrossRef CAS; (d) Y. Wei and M. Shi, Acc. Chem. Res., 2010, 43, 1005 CrossRef CAS PubMed; (e) C. Nising and S. Bräse, Chem. Soc. Rev., 2008, 37, 1218 RSC; (f) L.-W. Ye, J. Zhou and Y. Tang, Chem. Soc. Rev., 2008, 37, 1140 RSC.
- (a) C. Zhang and X. Lu, J. Org. Chem., 1995, 60, 2906 CrossRef CAS; (b) Y. Du, X. Lu and Y. Yu, J. Org. Chem., 2002, 67, 8901 CrossRef CAS PubMed; (c) Y. Du and X. Lu, J. Org. Chem., 2003, 68, 6463 CrossRef CAS PubMed.
- (a) X. F. Zhu, J. Lan and O. Kwon, J. Am. Chem. Soc., 2003, 125, 4716 CrossRef CAS PubMed; (b) R. P. Wurz and G. C. Fu, J. Am. Chem. Soc., 2005, 127, 12234 CrossRef CAS PubMed.
- H. Guo, Q. Xu and O. Kwon, J. Am. Chem. Soc., 2009, 131, 6318 CrossRef CAS PubMed.
- (a) Q. Zhang, L. Yang and X. Tong, J. Am. Chem. Soc., 2010, 132, 2550 CrossRef CAS PubMed; (b) C. Li, Q. Zhang and X. Tong, Chem. Commun., 2010, 46, 7828 RSC; (c) K. Kumar, R. Kapoor, A. Kapur and M. P. S. Ishar, Org. Lett., 2000, 2, 2023 CrossRef CAS PubMed.
- Selected papers on the nitrogen containing Lewis base catalyzed cyclization of allenoates: (a) G.-L. Zhao, J.-W. Huang and M. Shi, Org. Lett., 2003, 5, 4737 CrossRef CAS PubMed; (b) Y.-L. Shi and M. Shi, Org. Lett., 2005, 7, 3057 CrossRef CAS PubMed; (c) G.-L. Zhao, Y.-L. Shi and M. Shi, Org. Lett., 2005, 7, 4527 CrossRef CAS PubMed; (d) B. J. Cowen, L. B. Saunders and S. J. Miller, J. Am. Chem. Soc., 2009, 131, 6105 CrossRef CAS PubMed; (e) C. A. Evans and S. J. Miller, J. Am. Chem. Soc., 2003, 125, 12394 CrossRef CAS PubMed; (f) C. A. Evans, B. J. Cowen and S. J. Miller, Tetrahedron, 2005, 61, 6309 CrossRef CAS; (g) L.-Z. Dai, Y.-L. Shi, G.-L. Zhao and M. Shi, Chem.–Eur. J., 2007, 13, 3701 CrossRef CAS PubMed; (h) M. Shi, L.-Z. Dai, Y.-L. Shi and G.-L. Zhao, Adv. Synth. Catal., 2006, 348, 967 CrossRef CAS; (i) X.-Y. Guan, Y. Wei and M. Shi, J. Org. Chem., 2009, 74, 6343 CrossRef CAS PubMed.
- (a) X.-Y. Chen, M.-W. Wen, S. Ye and Z.-X. Wang, Org. Lett., 2011, 13, 1138 CrossRef CAS PubMed; (b) X.-C. Zhang, S.-H. Cao, Y. Wei and M. Shi, Org. Lett., 2011, 13, 1142 CrossRef CAS PubMed; (c) K. D. Ashtekar, R. J. Staples and B. Borhan, Org. Lett., 2011, 13, 5732 CrossRef CAS PubMed; (d) X. Wang, T. Fang and X. Tong, Angew. Chem., Int. Ed., 2011, 50, 5361 CrossRef CAS PubMed; (e) C.-K. Pei, Y. Jiang and M. Shi, Org. Biomol. Chem., 2012, 10, 4355 RSC; (f) C.-K. Pei, Y. Jiang, Y. Wei and M. Shi, Angew. Chem., Int. Ed., 2012, 51, 11328 CrossRef CAS PubMed.
- (a) J. Salvá and D. J. Faulkner, J. Org. Chem., 1990, 55, 1941 CrossRef; (b) C. Nguyen, J. L. Teo, A. Matsuda, M. Eguchi, E. Y. Chi, W. R. Henderson and M. Kahn, Proc. Natl. Acad. Sci. U. S. A., 2003, 100, 1169 CrossRef CAS PubMed; (c) J. Q. Yu and E. J. Corey, J. Am. Chem. Soc., 2003, 125, 3232 CrossRef CAS PubMed; (d) M. Fouad, R. A. Edrada, R. Ebel, V. Wray, W. E. G. Müller, W. H. Lin and P. Proksch, J. Nat. Prod., 2006, 69, 211 CrossRef CAS PubMed.
- (a) S. Danishefsky and M. Kahn, Tetrahedron Lett., 1981, 22, 489 CrossRef CAS; (b) R. Ballini and G. Bosica, Tetrahedron, 1995, 51, 4213 CrossRef CAS; (c) J. G. Allen and S. J. Danishefsky, J. Am. Chem. Soc., 2001, 123, 351 CrossRef CAS PubMed; (d) M. P. DeMartino, K. Chen and P. S. Baran, J. Am. Chem. Soc., 2008, 130, 11546 CrossRef CAS PubMed.
- (a) T. Eicher, S. Hauptmann and A. Speicher, The Chemistry of Heterocycles, Wiley-VCH, Weinheim, Germany, 2003 CrossRef; (b) G. D. Yin, Z. H. Wang, A. H. Chen, M. Gao, A. X. Wu and Y. J. Pan, J. Org. Chem., 2008, 73, 3377 CrossRef CAS PubMed; (c) Y. Yang, M. Gao, L. M. Wu, C. Deng, D. X. Zhang, Y. Gao, Y. P. Zhu and A. X. Wu, Tetrahedron, 2011, 67, 5142 CrossRef CAS.
- Y. Wang, Z.-H. Yu, H.-F. Zheng and D.-Q. Shi, Org. Biomol. Chem., 2012, 10, 7739 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, copies of 1H and 13C NMR spectra. CCDC 1483768. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6ra15117e |
| This journal is © The Royal Society of Chemistry 2016 |