On the molecular dynamics in long-acting calcium channel blocker lacidipine: solid-state NMR, neutron scattering and periodic DFT study
Aleksandra Pajzderska*a,
Kacper Drużbickiab,
Anna Kiwilszaac,
Miguel Angel Gonzalezd,
Jacek Jenczykc,
Stefan Jurgac,
Jadwiga Mielcareke and
Jan Wąsickiac
aDepartment of Radiospectroscopy, Faculty of Physics, Adam Mickiewicz University, Umultowska 85, 61-614, Poznan, Poland. E-mail: aleksandra.pajzderska@amu.edu.pl
bFrank Laboratory of Neutron Physics, Joint Institute for Nuclear Research, 141980, Dubna, Russian Federation
cNanoBioMedical Center, Adam Mickiewicz University, Umultowska 85, 61-614, Poznan, Poland
dInstitute Laue Langevin, B.P. 156x, 38042 Grenoble Cedex 9, France
eDepartment of Inorganics and Analytical Chemistry, Poznan Univeristy of Medical Sciences, Grunwaldzka 6, 60-780 Poznan, Poland
First published on 6th July 2016
Abstract
Molecular and vibrational dynamics of a new-generation lipophilic calcium channel blocker lacidipine (LCDP) are thoroughly explored by combining solid-state nuclear magnetic resonance (NMR) with high-flux quasi-elastic (QENS) and inelastic neutron scattering (INS) experiments. Contrary to the dynamically averaged 13C CP/MAS NMR response, neutron vibrational spectroscopy confirms our previous findings on the thermodynamically stable structure. High-resolution low-wavenumber INS spectrum is reported and fully interpreted based on periodic density functional theory (DFT) calculations in the quasi-harmonic approximation, staying in excellent agreement with the experiment. The INS spectrum was found to be clearly dominated by CH3 torsional features, widely spread over the range of 5–35 meV. 1H NMR relaxation indicates a molecular reorientation with different correlation times. The NMR relaxometry was further combined with an extended QENS study, providing a quantitative description of the intramolecular motions in terms of their activation barriers and correlation times, while their assignment was fully supported by theoretical analysis. While the internal dynamics of side-chain methyl groups can be described by rotation about the threefold-axes, the high-resolution QENS measurements give evidence of rotational tunneling of 2,6-methyl groups at low temperature. The vibrational analysis suggests that strong coupling of methyl librations with lattice modes promotes such an intriguing quantum effect.
1. Introduction
Dihydropyridine (DHP) L-type calcium channel antagonists play a well-recognized role in the long-term treatment of hypertension, either as first-line therapy agents or in combination with other anti-hypertensive drugs. Lacidipine (LCDP, Caldine®, Lacimen®, Lacipil®, Midotens®, Motens®) is a third-generation, hydrophobic, membrane-soluble calcium channel blocker that was developed to provide a slow onset of activity so as to avoid the sympathetic activation and reflex tachycardia associated with the first-generation, rapid-acting dihydropyridine calcium antagonists, such as nifedipine or felodipine. Due to a prolonged duration of action and improved vascular selectivity, greater than that of most other dihydropyridine calcium antagonists, LCDP effectively controls blood pressure in patients with hypertension without any clinically significant effects on cardiac conduction.1Although LCDP has a well-established position on the pharmaceutical market, there had been a lack of reports on its molecular or bulk structure, partially because of photo-instability issues.2 Eventually, we have recently filled this gap by presenting high-resolution crystallographic analysis on pure LCDP.3 Our research reveals that the structure of bulk LCDP can be described within the framework of a highly-ordered orthorhombic space group, Ama2 (see Fig. 1), although the thermodynamic structural equilibrium corresponds to the face-center, Cc, symmetry. It has been proven that substantially large vibrational atomic displacements give raise to the mirror-plane symmetry of the molecular framework. The molecular flexibility was further explored by means of optical vibrational spectroscopy, going toward terahertz frequency range.3 In the present work the structural analysis was supplemented with 13C CP/MAS NMR study, while the vibrational dynamics was further explored with the help of inelastic neutron scattering (INS), revealing the spectral features inaccessible to optical vibrational spectroscopy.
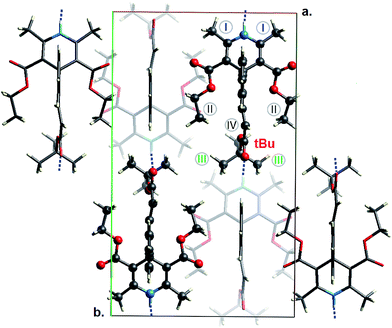 | ||
| Fig. 1 Unit cell of LCDP fully-optimized within dispersion-corrected periodic DFT (PBE-TS). The methyl groups treated as equivalent are denoted as I–IV, while the tert-butyl moiety is denoted as tBu. | ||
LCDP is an interesting system due to the presence of a large number of flickering molecular fragments, including seven methyl rotors undergoing stochastic dynamics at a different time scale. This work undertakes the challenge to provide a sound description of complex molecular dynamics in bulk LCDP by combining neutron scattering experiments in the quasi-elastic regime (QENS) with computationally supported solid-state nuclear magnetic resonance (NMR) relaxometry. Particularly, we want to answer the question if there is any potential relation between the molecular dynamics of LCDP and its crystal structure with all the earlier revealed peculiarities in the crystal packing and molecular flexibility. Gathering of such information paves the way to deduce other more relevant information from pharmaceutical purposes such as the compound solubility, receptor binding or membrane solubility. These aspects can only be explored with the help of further molecular dynamics simulations, which call for accurate values of the reorientation barriers and correlation times. This is not only to provide the bulk reference, but to provide the data required for the force fields tuning. With this goal in mind, the first important step (provided with this study) is to understand the stochastic dynamics in a well-defined crystal environment.
2. Experimental and computational details
2.1 Sample
The high-purity LCDP sample was provided by BIOFARM pharmaceutical company, Poznan, Poland. X-ray diffraction characterisation of the sample used throughout this work has been described in details in ref. 3.2.2 Experimental methods
13C CP/MAS NMR spectrum was acquired using an Agilent spectrometer operating at the Larmor frequency of 400 MHz for protons. The sample was placed within a 4 mm diameter zirconia rotor and spun at the 5 kHz frequency. The cross-polarization contact time was set to 800 μs. Two-pulse phase-modulated decoupling was utilized during the acquisition period. 8k transients were accumulated as to achieve a satisfactory S/N ratio. Total suppression of spinning sidebands (TOSS) sequence was used to detect solely isotropic signals.4 The 13C chemical shifts were referenced to the 38.3 ppm signal of adamantane.The 1H NMR relaxation measurements were performed for a powder sample degassed and sealed off in a glass ampoule. The measurements of the second moment of proton 1H NMR line were carried out as a function of temperature, with a continuous wave spectrometer, operating at the frequency of 28 MHz (El-Lab Tel-atomic). 1H NMR measurements of the spin–lattice relaxation time T1 were performed using a pulsed NMR spectrometer working at 25 MHz (El-Lab Tel-atomic). T1 times were determined using the saturation – recovery sequence. The proton relaxation time in the rotating frame T1ρ (with the magnetic field B1 = 18 G) were measured by pulse spectrometer working at 58.9 MHz. All spectrometers were equipped with cryostats: a nitrogen cryostat with an accuracy of 0.1 K (28 MHz, 58.9 MHz) and helium–nitrogen setup with an accuracy of 0.01 K (25.0 MHz). Temperature stabilization was set at the level of 1 degree and 0.1 degrees, respectively.
The sample for QENS measurements was prepared by placing 0.315 g of LCDP in an aluminium flat container (30.0 mm × 40.0 mm) with a thickness of 0.3 mm as to get a transmission of about 0.9. The experiment was performed at the time-of-flight IN5 spectrometer (Institute Laue–Langevin, Grenoble, France) working with a neutron wavelength of λ = 6 Å and an energy resolution of 35 μeV (FWHM). This setup allows one to examine the Q range of 0.2–1.9 Å−1. The measurements were done in the range of 50–200 K. The second part of the experiment was performed with the IN16 backscattering spectrometer using an incident neutron wavelength of λ = 6.27 Å, providing an energy resolution of 0.9 μeV (FWHM), Q range from 0.19 Å−1 to 1.92 Å−1 and energy transfer range of ±14.5 μeV. The measurements were done at the following temperatures: 1.5 K, 10 K, 20 K, 160 K and 210 K. For both set-ups the angle between the incident neutron beam and the sample was fixed to 135° and the temperature was stabilized by a helium–nitrogen cryostat with an accuracy of ±0.1 K. The empty cell along with a 1.0 mm thick vanadium sample were also measured to obtain the instrument background and resolution, respectively.
The raw experimental data were treated with LAMP software (ILL, Grenoble, France) including: the subtraction of background from an empty cell, the correction with the detector efficiency, the normalization to the vanadium spectrum and the correction for the absorption. Additionally, the detectors influenced by Bragg peaks were removed from further analysis of the recorded spectra. The neutron vibrational density of states was estimated using the approximation given by Egelstaff and Schofield5 with a cutoff of 1.5 Å−1 and neglecting multiphonon corrections.
2.3 Computational details
Theoretical calculations in periodic boundary conditions (PBC) were carried out using a plane-wave/pseudopotential formulation of density functional theory (PW-DFT) as implemented in CASTEP.6,7 Both, mirror-plane symmetry, Ama2, and face-centered, Cc, structures were considered. Exchange and correlation were defined in the framework of the generalized gradient approximation (GGA) with ‘standard’ Perdew–Burke–Ernzerhof (PBE) exchange–correlation functional.8 In addition we have employed its ‘hard’ revision introduced by Hammer (rPBE),9 along with the ‘soft’, solid-state oriented alternative, PBEsol.10 The calculations were performed both in the fixed-cell methodology as well as with unconstrained relaxation at the atmospheric pressure. Due to the well-known limitation of semi-local DFT to describe van der Waals (vdW) interactions, the calculations in full-optimization scheme were performed with use of Tkatchenko & Scheffler (TS) dispersion corrections11 as well as using standard, Grimme's D2 scheme.12Core electrons were represented by norm-conserving pseudopotentials, while the electronic wave functions were expanded using a PW basis set with a kinetic-energy cutoff of 1050 eV. The Monkhorst–Pack grid was kept to maintain the k-spacing of 0.07 Å−1. The convergence criteria in variation of total energy, maximum force, external stress, maximum displacement and SCF iterations were defined as: 1 × 10−10 eV per atom, 1 × 10−5 eV Å−1, 0.0001 GPa, 1 × 10−6 Å and 1 × 10−12 eV per atom, respectively.
The phonon frequencies were obtained by diagonalization of dynamical matrices computed using density functional perturbation theory (DFPT),7,13,14 while the inelastic neutron scattering intensities were predicted with the help of aClimax program, based on the computed eigenfrequencies and eigenvectors.15
The 13C NMR calculations were performed with the gauge-including projector-augmented wave (GIPAW) method.16 The all-electron information, needed for the calculation of the absolute NMR chemical shielding tensors, was reconstructed using on-the-fly generated scalar relativistic ultrasoft pseudopotentials (USPP),17 with the same numerical settings as quoted above. The isotropic shift of 171 ppm was used as an effective reference for presented computations as adopted from ref. 18.
The analysis of the second moment of 1H NMR line was based on the Monte Carlo iterative scheme, according to the procedure described in detail earlier in ref. 19. The calculations were performed for the {3 × 3 × 3} supercell. The activation barriers for each recognized motion were further estimated with use of solid-state DFT, through the set of relaxed scans of total energy as a function of each dihedral angle.
3. Results
3.1 13C CP/MAS NMR
The structural and spectral properties of LCDP have been surprisingly concealed in the literature, although it has being widely used in therapy starting from the early 1990s. Contrary to the first-generation of dihydropyridine calcium antagonists, there is still no report on solid-state spectroscopy of this important active pharmaceutical ingredient (API). Solid-state 13C NMR has been found as a valuable tool to characterize the first-generation calcium antagonists and the polymorphism therein.20–22 In order to provide a valuable support for polymorph screening and drug control, the high-resolution solid-state 13C NMR spectrum of LCDP has been recorded and is presented in Fig. 2, along with the results of theoretical GIPAW calculations. The full assignment of the recorded resonances is given in Table 1.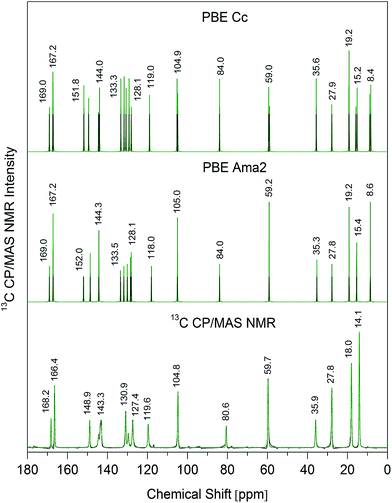 | ||
| Fig. 2 Room-temperature 13C CP/MAS NMR spectrum of LCDP along with theoretical GIPAW (PBE) spectra calculated for both Cc and Ama2 crystallographic structures. | ||
| 13C chemical shift δ [ppm] | ||||
|---|---|---|---|---|
| Lacidipine | ||||
| δexp | Ama2 | Cc | Νο. | Group |
| 168.2 | 169.0 | 169.0 | C(13) | C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯H O⋯H |
| 166.4 | 167.2 | 167.2/167.3 | C(17) | C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O O |
| 148.9 | 152.0 | 151.8 | C(5) | Ph-DHP |
| 144.4 | 148.6 | 149.4 | C(11) | HC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH CH |
| 143.3 | 144.3 | 144.0/144.5 | C(2) | –N– |
| 130.9 | 133.5 | 133.3 | C(6) | -ortho |
| 131.8 | 131.7 | C(7) | -meta | |
| 129.6 | 130.0 | 130.7 | C(10) | -ortho |
| 127.4 | 128.5 | 129.2 | C(9) | -meta |
| 128.1 | 128.1 | C(8) | -para | |
| 119.6 | 118.0 | 119.0 | C(12) | HC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH CH |
| 104.8 | 105.0 | 104.9/105.2 | C(3) | C–COOC2H5 |
| 80.6 | 84.0 | 84.0 | C(14) | t-Butyl |
| 59.7 | 59.2 | 59.0/59.4 | C(18) | CH2 |
| 35.9 | 35.3 | 35.6 | C(4) | C(4) |
| 27.8 | 27.8 | 27.9 | C(15) | CH3 (IV) |
| 19.2 | 19.2/19.3 | C(16) | CH3 (III) | |
| 18.0 | 15.4 | 15.2/15.8 | C(1) | CH3 (I) |
| 14.0 | 8.6 | 8.4/8.9 | C(19) | CH3 (II) |
The number of expected resonances in the 13C NMR spectrum depends on the number of inequivalent carbon atoms in the crystal phase. There are four molecules equivalent by symmetry in the conventional unit cell of both Cc and Ama2 structures. The asymmetric unit of the Cc structure consists of full LCDP molecule, while in the case of the Ama2 structure, the Cs symmetry molecule is lying on a crystallographic mirror.3 However the theoretical calculations illustrate, that similarly to X-ray diffraction,3 solid-state 13C NMR cannot discern between both symmetries as the loss of the signal degeneracy results in spectral differences smaller than 1 ppm. The spectrum, however, also suggests, that some of the signals are observed as time-averaged, indicating existence of prominent internal dynamics in the molecular ensemble.
By inspection of both Fig. 2 and Table 1, one can find that the 13C NMR reflects the signals common for calcium channel blockers, which share the same 4-aryl-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate unit.3 Furthermore, one can refer also to the liquid 13C NMR spectrum of LCDP, reported by Prasada Raju,23 and being quite similar to the solid-state spectrum. This suggests that LCDP molecules have a considerable freedom of motion in the solid-state. Indeed, the previously reported intermolecular-interactions analysis3 revealed that the most-attracting forces come from N–H⋯O hydrogen bondings, propagated infinitely through one of the carbonyl groups, that is via (tert-butoxycarbonyl)vinyl chain. The trail of these forces makes the carbonyl groups inequivalent, giving the signals at 168 and 166 ppm, respectively. According to our previous findings on the first-generation DHPs,22 166 ppm can be treated as the lower-limit for the unbind ester chain and any upper shift from this value reflects the hydrogen-bond strength, and it shows that LCDP forms moderate-strength N–H⋯O chains (similar to the ones in nitrendipine).22
The intermolecular interactions analysis also revealed a noticeable influence of the side-chain interactions CH2⋯π, which seems to be confirmed by a slight difference between the solid-state and the liquid spectrum in the range 131–127 ppm. The remaining forces were identified to be rather non-specific, with a large-dispersive contribution to the stabilization energy.
However, analysis of the theoretical and experimental data suggests a noticeable difference in the CH3 range. While theory clearly suggests the presence of four signals, only three resonances are found. This intriguing difference may suggest that some of the signals are dynamically averaged within the time-scale of the NMR experiment. In line with our previous findings on DHPs,22 the ethyl and 2,6-CH3 groups give the resonances beneath 20 ppm, while the resonances from iso-propyl or tert-butyl methyls are expected around 25 ppm. Hence, the assignment of the single-resonance from 28 ppm to the methyl groups no. III and IV, lead us to the conclusion that tert-butyl group may undergo reorientation.
3.2 Vibrational dynamics
Even at the cryogenic conditions, vibrational dynamics contributes noticeable to the non-rigidicity of any crystal lattice. Therefore, understanding the vibrational response is important for any relaxation experiment. An initial structural study on LCDP revealed an intriguing molecular flexibility, raising the crystal-phase symmetry up to the orthorhombic space group Ama2.3 A considerable vibrational disorder has been evidenced with the help of time-domain terahertz spectroscopy (THz-TDs), suggesting large-amplitude oscillations in the (tert-butoxycarbonyl)vinyl moiety. While THz-TDs is generally focused on vibrations inducing charge fluctuations, neutron spectroscopy has the great advantage of being directly related to nuclear motion. Contrary to optical vibrational spectroscopy, the observed spectral intensity depends in principle only on the amplitude of motion of a nucleus associated to a particular vibration and its incoherent scattering cross section. The latter factor is an isotope-specific property, which is independent of the molecular environment. Due to large incoherent scattering cross section of protium, INS has an outstanding sensitivity to hydrogen motion and can reveal vibrations that do not affect the dipole moment nor the polarizability and therefore are inaccessible to any optical vibrational technique, e.g. methyl torsional modes. Therefore, INS is a perfect tool for exploring low-wavenumber structural distorsions in molecular crystals, which are easily promoted by the temperature.Representative low-wavenumber G(ω) spectrum of LCDP, recorded with the direct-geometry time-of-flight spectrometer IN5 at 125 K are shown in Fig. 3a, along with the results of lattice-dynamics calculations in the quasi-harmonic approximation. The spectrum was fully interpreted with use of PBE calculations as to keep the consistency with the previously reported vibrational analysis.3 The G(ω) spectrum was calculated for both mirror-plane Ama2 symmetry and the Cc structures, while the latter model provides a striking good match to the experimental spectrum. In addition to the zone-center calculations (Γ), the theoretical spectrum for the Cc model was also simulated by inclusion of phonon dispersion thorough the Brillouin-Zone (BZ), which are projected in Fig. 3b.
In line with the THz-TDs study, INS directly supports the Cc structure with the presence of several bands as well as with a better match to the intensity distribution, which could not be properly reproduced in the frame of the mirror-plane symmetry. The most prominent spectral differences refer to the lattice-mode range below 20 meV, where phonon-dispersion is of larger importance (see bottom Fig. 3b). The full interpretation of the INS spectrum is given in Table 2, while the partial G(ω) contributions from different molecular fragments are projected in Fig. 3b (see upper panels).
| INS (125 K) | CASTEP PBE (Cc) | |||
|---|---|---|---|---|
| ν [meV] (cm−1) | Symmetry | Int. [arb.u.] | Band assignment | |
| 39.4 (317.8) | 36.6; 36.4 | [A′′]; [A′] | 0.49; 0.44 | N–C–CH3 (I); δO–CH2–CH3 (II); δC–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O O |
| 35.7 (287.9) | 33.3; 33.2 | [A′′]; [A′] | 1.44; 2.20 | τCH3 (IV; III); C–C–CH3 (I) |
| 34.0 (274.2) | 31.3; 31.3 | [A′]; [A′′] | 1.52; 1.50 | τCH3 (II) |
| 32.4 (261.3) | 29.9; 29.8 | [A′]; [A′′] | 1.55; 1.79 | τCH3 (III); δC–O–CH2 |
| 29.0 (233.9) | 26.4; 26.4 | [A′′]; [A′] | 2.05; 1.85 | τCH3 (III; IV) |
| 25.6 (206.5) | 23.5; 23.2 | [A′′]; [A′] | 0.89; 0.93 | τ(Ο![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C)–(HC C)–(HC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH); τCH3 (II) CH); τCH3 (II) |
| 21.0 (169.4) | 20.1; 20.0 | [A′′]; [A′] | 0.84; 0.94 | δO–CH2–CH3 (II) |
| 18.4 (148.4) | 17.4; 17.1 | [A′′]; [A′] | 2.00; 1.67 | τCH3 (I) |
| 17.1 (137.9) | 16.2; 15.9 | [A′′]; [A′] | 1.17; 1.79 | τ(HC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH)–(COO); τCH3 (I); δO–CH2–CH3 (II) CH)–(COO); τCH3 (I); δO–CH2–CH3 (II) |
| 13.8 (111.3) | 12.7 | [A′] | 1.16 | τCH2CH3; γDHP; τCH3 (I) |
| 12.2 | [A′] | 1.23 | τCH2–CH3; τDHP; τCH3 (II) | |
| 9.9 (79.8) | 9.8 | [A′′] | 1.52 | τCH2H3 |
| 9.8 | [A′] | 1.13 | τ(t-Bu); τCH2–CH3 | |
| 7.8 (62.9) | 7.6; 7.4 | [A′]; [A′′] | 0.68; 0.60 | τC–(C2H2–COO–t-Bu); τ(t-Bu); τCH3 (I) |
| 5.4 (43.6) | 5.7 | [A′] | 0.71 | τDHP; τCH3 (I) |
| 5.7 | [A′′] | 0.65 | τCH3 (I); τ(DHP)–(COOC2H5) | |
| 3.5 (28.2) | 4.0 | [A′] | 0.76 | τLCDP ∠ a → b; C–C → a (‘crankshaft’) |
| 2.0 (16.1) | 2.8; 2.6 | [A′]; [A′′] | 0.44; 0.60 | LCDP shearing modes |
From inspection of these data one can clearly see that the Cc model clearly reveals the presence of weak features at 3.5 meV (28 cm−1) and 7.8 meV (63 cm−1), which were earlier detected with THz-TDs at 28 and 57 cm−1, respectively, as corresponding to the (tert-butoxycarbonyl)vinyl motion. It also describes correctly the relative intensity about 9.9 meV (80 cm−1), which corresponds to the most-intense feature in the earlier studied TDs-THz spectrum, due to tert-butyl and ethyl torsions. Since the INS intensity depends directly on the amplitude of vibrational displacements, these modes reflect a substantial motion of the quoted moieties.
The INS spectrum is dominated by methyl-group vibrations, which are spread over the range 5–35 meV. As deduced from Fig. 3b, there is only a small-gap between the lattice and internal modes, between 20 and 25 cm−1. The lowest features in the internal range correspond to the isolated torsional modes of CH3. These modes are due to methyl groups no. II; III and IV, which suggests that the quoted rotors may undergo classical rotational dynamics. Thermal excitation of these modes results in lowering of their activation barriers measured in the relaxation experiments described later on. In contrary, τ(CH3) modes of 2,6-methyls (no. I), give an intense feature at 17–18 meV and are also spread in the low-energy lattice range down to 5 meV, being strongly coupled to other types of vibrations. Such a low excitation-energy may suggest that these modes can eventually undergo quantum rotational tunneling.
Finally, from inspection of Table 2, one can note that there is also a considerable contribution from the torsional, τ, and bending, δ, modes of the side OCH2CH3 fragments, which also tend to enter the lattice-range of vibrations.
Therefore one can expect that vibrational dynamics of (tert-butoxycarbonyl)vinyl; methyl and propionate moieties accompany the temperature-induced molecular dynamics of LCDP. To check this point, 1H NMR relaxometry has been combined with quasi-elastic neutron scattering in order to explore the dynamics of LCDP both qualitatively and quantitatively across a wide temperature-range.
3.3 Molecular dynamics
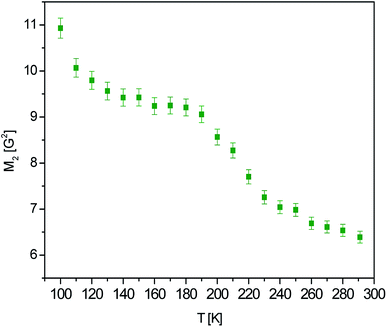
Such a model, however, does not elucidate further rapid-decrease of M2 from 9.2 G2 (at 190 K) down to 7.0 G2 (at 240 K), suggesting that another additional type of motion is observed. A possible phase transition has been dismissed in our previous work.3 The further inclusion of the tert-butyl group reorientation results in a reduction of the estimated value down to a reasonable value of 8.8 G2. Since the reduction of M2 is observed at about 190 K, one can tell that the frequency of this reorientation is at the order 105 s−1.25 This also means that the frequency of the reorientation of methyl groups (responsible of the reduction of M2 already below 100 K) is several orders faster than the reorientation of the tert-butyl group.
A further slow ongoing decrease of M2 on the experimental curve can be clearly associated with the increasing amplitude of the oscillations in the aliphatic fragments. One should keep in mind that according to the intermolecular-interactions analysis,3 the formation of hydrogen bonds restrains the dynamics of the (tert-butoxycarbonyl)vinyl chain, since the hydrogen bonding leads to the presence of antiparallel molecular chains, distributed toward the crystallographic axis-b. However, the vibrational energy is clearly dissipated over the ‘crankshaft’ motion, earlier disclosed with terahertz spectroscopy.
Furthermore, as has been previously presented in ref. 3, there are prominent stabilizing interactions from the –OCH2CH3⋯π contacts (toward a-axis) as well as attracting forces stabilizing the crystal towards the c-axis, including anti-parallel attractions of the whole propionate fragments. All these factors constrain the side-chains and suggest that large-amplitude CH2CH3 oscillations may be coupled to the above described ‘crankshaft’ deformations, which may release their motion as being in close contact.
In order to shed more light on the relaxation processes, further NMR measurements were performed. The results of the spin–lattice (T1) relaxation time measurements in the laboratory frame are presented in Fig. 5, along with the results of the relaxation time measurements in the rotating frame, T1ρ. From inspection of these data one can resolve two minima on the T1 curve, namely the low-temperature one at 37 K (the value of 92.9 ms) and the high-temperature one 154 K (the value of 48.9 ms). The temperature dependence of T1ρ revealed a single minimum at 224 K (the value of 2.56 ms). The T1 minima found at different temperatures support the assumption on the occurrence of several types of reorientations with different frequencies.
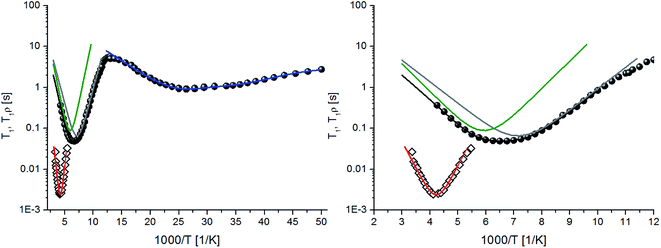 | ||
| Fig. 5 Spin–lattice relaxation time T1 (●) and relaxation time in the rotating frame T1ρ (◊) versus inverse temperature and the best fit (solid lines). | ||
The temperature dependence of the T1 relaxation time was approximated by the Haupt's model:26–28
 | (1) |
The temperature dependence of the correlation time can be described by the formula:
τc−1 = (τ′0)−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−E′A/RT) + (τ′′0)−1 exp(−E′A/RT) + (τ′′0)−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−E′′A/RT) exp(−E′′A/RT)
| (2) |
 | (3) |
For the reorientations observed at higher temperatures eqn (1) reduces to the classical BPP formula:29
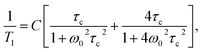 | (4) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(EA/RT)), with τ0 standing for pre-exponential factor and EA denoting the activation barrier), ω0 stands for the resonant frequency, and C is a constant.
exp(EA/RT)), with τ0 standing for pre-exponential factor and EA denoting the activation barrier), ω0 stands for the resonant frequency, and C is a constant.
The temperature dependence of T1ρ according to Andrew et al.30 takes the form:
 | (5) |
The activation parameters derived from the 1H NMR experiments are collected in Table 3. The temperature-dependence of T1 relaxation was fitted by assuming one process to describe the low-temperature minimum (eqn (1)) and (2) processes to describe the high-temperature regime and avoid over-parametrization (eqn (4)). The T1ρ minimum was also described by a single event (eqn (5)). The low-temperature process is therefore characterised by a small activation energy of 2.6 kJ mol−1, the high-temperature processes by the activation energies varying from 10.01–12.88 kJ mol−1, and the slow process reflecting in the T1ρ minimum by an activation energy of 25.82 kJ mol−1. In consistence with the M2 results, this last process can be related to the reorientation of the tert-butyl group, while the remaining motions involve reorientation of methyl groups. This also implies that the frequency of the tert-butyl group reorientation is much smaller than the reorientation of the methyl groups making it, which is not always the case as e.g. in the compounds 2-methyl-2-propanol and 2-methyl-3-propanethiol,31,32 which show a rate of tert-butyl group reorientation higher than that of the methyl groups composing it. The resulting values of both τ0 and C parameters also suggest that the low-temperature minimum (LT) corresponds to the reorientation of two methyl groups, while the high-temperature one (HT) to the reorientation of five methyl groups.
| BPP model | |||
|---|---|---|---|
| HT | τ0 [s] | EA [kJ mol−1] | C [s−2] |
| 4.04 × 10−13 | 12.9 ± 0.5 | 1.22 × 109 | |
| 7.27 × 10−13 | 10.0 ± 0.4 | 1.67 × 109 | |
| T1ρ | 2.76 × 10−12 | 25.8 ± 0.6 | 0.55 × 109 |
| Haupt's model | |||||||
|---|---|---|---|---|---|---|---|
| LT | τ′0 [s] | τ′′0 [s] | E′A [kJ mol−1] | E′′A [kJ mol−1] | CAE [s−2] | CEE [s−2] | ω0t [s] |
| 0.29 × 10−11 | 82.8 × 10−11 | 2.6 ± 0.1 | 0.6 ± 0.1 | 0.04 × 109 | 0.11 × 109 | 5.8 × 109 | |
3.4 Quasi-elastic neutron scattering
In order to better understand the analyzed motions the QENS measurements were performed in a wide range of temperature and frequency. Two spectrometers of different resolution, IN5 and IN16, were employed to cover the broad time scale of the analysed events. At the lowest temperatures, 1.5–20 K, the inelastic peaks at an energy transfer ±3.75 μV were observed with IN16 (Fig. 6), which gives an unambiguous evidence that methyl groups undergo rotational tunnelling.33–35 The observed tunnelling frequency of 3.75 μeV stay in good agreement with the one obtained from the Haupt's model fitted to NMR data, ω0t = 5.8 × 109 s−1 (= 3.8 μeV). It is worth to mention that the positions of the tunnelling bands as well as their widths are practically temperature independent, which is easy to understand according to eqn (3) and the parameters collected in Table 3.Representative spectra recorded at higher temperatures with IN16 and IN5 are shown in Fig. 7. These spectra were fitted by the standard equation (convoluted with the resolution function R(Q,ω)):
| S(Q,ω) = A0(Q)δ(ω) + (1 − A0(Q))L(ω) + B(Q,ω), | (6) |
At each temperature, the best fit was obtained with a single Lorentzian function fitting of the quasielastic broadening (as in eqn (6)). The width of the Lorentzian line (within the uncertainty limits) does not depend on Q, giving strong support to the model of jumps between three minima.
This phenomenological fit permits to extract a model-independent EISF A0(Q) and the correlation time (which is inversely proportional to Γ). The latter can be further compared with the correlation times obtained from NMR measurements, while A0(Q) provides information about the geometry of motion.
In a methyl group rotation, a given hydrogen jumps between three equidistant equivalent sites with the distance between protons in CH3 defined as d1, so the corresponding elastic incoherent structure factor can be written as37
 | (7) |
For the jumps of tert-butyl groups between three equidistant sites38 (assuming that CH3 are fixed), the EISF takes the form:
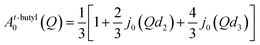 | (8) |
According to the NMR results, the reorientations of methyl groups and the reorientation of the tert-butyl group are visibly separated in frequency. However, we can also write the EISF for the case when they have comparable frequencies and thus correlation times:
 | (9) |
While all the atoms contribute to the EISF, the contribution from the hydrogen atoms represents more than 90% of the total scattering, so the contribution from other atoms (nitrogen, carbon, oxygen) can be neglected. Furthermore in the crystal structure of LCDP we can assume that only the motion of the methyl groups and/or tert-butyl group can take place within the time scale given by the finite resolution of the spectrometer, which in the case of our experiment in IN5 is of the order of several hundred ps, while for IN16 is several ns.
Since LCDP consists of seven methyl groups and three of them build the tert-butyl moiety, the measured elastic incoherent structure factor (EISFmeas) can be written as:
(a) For reorientation of methyl groups only (fixed tert-butyl):
 | (10) |
(b) For reorientation of t-butyl groups only (while all methyl groups are fixed):
| EISF_meas = ct + (1 − ct)At-butyl0(Q) | (11) |
(c) For reorientation of methyl groups and t-butyl (while methyl groups in tert-butyl moiety are fixed)
 | (12) |
(d) For simultaneous reorientation of all methyl groups and t-butyl (implying also the rotation of the methyl groups in the tert-butyl group)
 | (13) |
Fig. 8a presents a few EISF dependencies on Q as calculated from the formulae (8)–(11) for different numbers of reorienting methyl groups and/or t-butyl reorientation. All the calculated EISF taking into account the reorientation of t-butyl exhibit a minimum around 1.25 Å−1. However the experimentally measured EISF decreases with increasing Q and does not show any minimum, suggesting that in the explored temperature and frequency range the reorientation of t-butyl group is not observed.
 | ||
| Fig. 8 (left) EISF_meas calculated from formulae (9)–(12), (a) eqn (9), 2 rotating CH3 groups, (b) eqn (10) – tert-butyl group reorientation, (c) eqn (11) – 2 CH3 fixed, 2 CH3 rotating, tert-butyl reorientation (d) eqn (12) – 2 CH3 fixed, 2 CH3 rotating, reorientation of tert-butyl group and CH3 belonging to tert-butyl group. (right) Experimental (points) elastic incoherent structure factor. Solid lines show the theoretical expectations assuming reorientations of different number of methyl groups (lines) n = 1, 2, …, 7 (eqn (9)). | ||
The measured EISFs obtained from the fit of the QENS spectra using eqn (6) are projected in Fig. 8b, together with the theoretical lines corresponding to eqn (9) for n = 1–7 methyl groups. It is crucial to note that the experimental points from IN5 at 75 K are most properly described by a model involving reorientations of two methyl groups. For IN16, the EISF measured at 160 K reflects the reorientation of three groups. Finally, the EISF observed with IN16 at 210 K reflects the dynamics of five methyl groups, indicating that with increasing temperature their frequencies of motion enter into the accessible time-scale of IN16. In that way all methyl groups were observed with IN6 and IN16. By inspection of Fig. 9 it is also clear that calculations assuming the reorientation of t-butyl do not agree with experimental results (see the lines b–d in the left panel). Therefore we conclude that in the time window of the two spectrometers we only observe the reorientations of methyl groups and that the reorientation of tert-butyl is much slower as suggested by NMR data.
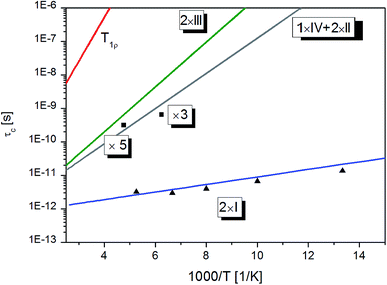 | ||
| Fig. 9 Correlation times obtained from QENS (▲) IN5, (■) IN16 and NMR measurements (solid lines). Arabic numbers denote the number of rotating methyl groups and Roman numbers their type. | ||
As mentioned, the half-width Γ obtained from the fitting of the QENS spectra is related to the correlation time τc by Γ = 3ℏ/(2τc).37 The correlation time established from QENS and NMR experiments (calculated using the parameters from Table 3 and the Arrhenius relation from eqn (4)), is given in Fig. 9. One can find that the correlation time corresponding to the reorientation of tert-butyl is much slower w.r.t. the methyl groups and cannot be detected with QENS. In the range of 75–200 K, the correlation time observed with IN5 coincides with the solid line obtained from the low-temperature T1 minimum, which can be clearly assigned to the reorientation of the two methyl groups undergoing quantum tunnelling.
On the other hand, the correlation times observed with IN16 match well the values derived from the high-temperature T1 minimum fitting. The EISF obtained on the basis of QENS data at 160 K describes the reorientations of three methyl groups, while at 210 K five methyl groups were eventually detected. This can be understood on the basis of the correlation times deduced from NMR. At 160 K, the correlation times of all three methyl groups are very similar, but τc for two another motions is almost one order of magnitude higher, making this rotation too slow to be observed with the available instrumental resolution. However, with increasing temperature the difference between the correlation times is reduced at 210 K, and eventually correlation times of all five methyl groups span into the instrument time window, and therefore all of them contribute to the observed EISF.
At 210 K, the ratio between the NMR deduced correlation times of two and three methyl groups is about 3/4, implying that in principle the quasielastic broadening should be fitted with two Lorentzians of different width. Nevertheless, the statistic quality of the QENS experiment does not allow to discriminate between these different groups.
In order to answer the pending question about the assignment of particular rotors, the relaxed scans of related coordinates were performed in the framework of periodic DFT calculations. These calculations were performed after full optimization of the conventional unit-cell (Z = 4) at the atmospheric pressure conditions. Starting from the Cc initial model, the full-geometry optimization with each exchange–correlation functional was performed without any symmetry constraints (C1 symmetry). Furthermore, the calculations were also performed for a single molecule in a cubic (25 Å) box to elucidate the crystal-packing effect.
It is important to note that full crystal optimization without symmetry constraints eventually results in ill-defined structures with pure GGA functionals, because of the well-known deficiency of semi-local DFT to describe dispersion interactions. Particularly, while the OCH2CH3⋯π contacts have been already identified as the structure stabilizing,3 the chain conformation was found to be easily disturbed when the van der Walls forces treatment is neglected. Such structure disturbance have been particularly observed for more repulsive functionals (i.e. hard, in the sense of the exchange enhancement, Fx, factor), that is rPBE and PBE, which have been eventually excluded from further discussion. Wrong estimation of cohesive energy is reflected in a large overestimation of the related a and c cell-constants, exceeding 10% w.r.t. experimental data from 140 K.3 Much smaller deviations in full crystal optimization were found for soft-GGA (PBEsol), with much less repulsive nature and with vdW-corrected DFT (PBE-D2 and PBE-TS) functionals. These results were eventually used for assignment of the relaxation events.
The estimated reorientation barriers are collected in Fig. 10. For simplicity, the values were taken as the average for each pair of rotors, which can be approximately described as symmetry equivalent. Among the tested models, PBE-TS is the most theoretically justified approach. PBEsol, presented for comparison, is only able to deal with short- and middle-range electron correlation. The impact of long-range electron correlation can only be mimicked with semi-local DFT by employing ad hoc, dispersion corrections to the total energy. While PBE-D2 is the most standard approach employed in most of the solid-state DFT codes, it is actually recognized as an obsolete approach, where the portion of the dispersion corrections only depends on fixed parametrization for each element. On the contrary, Tkatchenko–Scheffler corrections are able to take into account variations in vdW contributions of atoms due to their local chemical environment, since the dispersion coefficients and damping function are charge-density dependent.
By comparing the calculations performed for the crystal and molecular models one can note that the influence of the crystallographic field is rather small. This supports the previous remarks derived from CP/MAS NMR study about a considerable LCDP mobility in solid-state. Nevertheless, there is a dramatic difference in the activation barrier estimated for the tert-butyl motion, particularly with PBE-TS, which describe the system as the most crowded. It is hence important to analyze t-butyl dynamics in more detail. One should mention that rigid-scan calculations predict tert-butyl reorientation to be absolutely improbable, with the reorientation barriers of the order of 81 (PBEsol), 108 (PBE-D2) and 105.7 kJ mol−1 (PBE-TS). By inspection of the crystal environment it is clear that there is a steric hindrance present. The crystal packing results in the clash between the phenyl ring and potentially rotating tert-butyl fragment, which becomes blocked. Nevertheless, the relaxed coordinates scan reveals the path for the tert-butyl motion. Actually, tert-butyl dynamics was found to be clearly promoted by the earlier discussed flexibility of the (tert-butoxycarbonyl)vinyl chain. While the steric clash is still present, the chain dynamics can promote tert-butyl motion, interpreted as very large-amplitude libration up to the barrier, which, however, cannot be directly crossed. Such reorientation mechanism is supported by theoretical calculations, which provide reasonable values of about 25–30 kJ mol−1 with each functional, that is close to the activation barrier of ca. 26 kJ mol−1 estimated with NMR relaxometry.
Regarding the methyl groups dynamics, all DFT calculations unequivocally assign the lowest-energy barriers with the 2,6-DHP substituted methyl groups (CH3 no. I in Fig. 1). Such assignment is not surprising due to the well-known tunneling tendency of pyridine derivatives. Such an effect is promoted chemically, as DHP ring is not aromatic and the presence of a lone electron pair on the nitrogen atom leads to its quantum inversion. The absence of dramatic differences between the molecular and periodic models suggests that there is no considerable influence of the hydrogen-bonding, which has been already identified as of medium strength.3
All the calculations show clearly that the remaining methyl groups are characterized by higher energies. While the difference in the activation energies is smaller than 3 kJ mol−1, their assignment is not obvious. Nevertheless, by combining experimental and theoretical data one can provide an unambiguous interpretation, as referring to the quantitative QENS analysis of methyl groups contributions. Among the five remaining methyl groups, the activation barriers for the propionate methyls (CH3 no. II) are the lowest, independently on the applied level of theory. These groups contribute to the high-temperature T1 relaxation minimum, described by the activation barrier of 10.0 kJ mol−1. The QENS analysis clearly shows that one more methyl group contributes here, so it has to be assigned to the local-symmetry inequivalent methyl group no. IV (see Fig. 1). Two remaining groups, CH3 no. III in tert-butyl are characterized by higher energy, with the 1H NMR activation barrier of 12.9 kJ mol−1. Such assignment is fully supported by PBE-TS calculations, where CH3 no. II and IV give an average activation barrier of 10.9 kJ mol−1, and CH3 no. III are described by the activation energy of 13.03 kJ mol−1. It seems that PBE-D2 overestimates the dispersion contributions for the CH3 no. IV, interacting with an adjacent hydrogen atom in the phenyl ring. In that way it contradicts with the conclusions clearly delivered by QENS. Such interaction depends directly on the CH3⋯H distance, so dynamics of the (tert-butoxycarbonyl)vinyl chain becomes important again. Any stochastic deformation of this chain as well as any phenyl ring flip motion will weaken this contact, lowering its barrier, since H⋯H is the only force affecting methyl no. IV. On the contrary, methyl groups no. III are more crowded, being in contact with adjacent tert-butyl groups. In that way, librations of the tert-butyl and the (butoxycarbonyl)vinyl moieties are expected to lift the related barriers. Nevertheless, further analysis of their mutual relation would only be possible with the use of high-quality molecular dynamics, which at the moment is impossible due to the long time-scale of the analyzed motions.
4. Conclusions
Molecular dynamics in bulk LCDP has been explored by employing different experimental techniques such as 13C NMR, 1H NMR, INS and QENS. The experimental study was supported with theoretical calculations for solid-state, providing clear assignments of the 13C NMR shifts; vibrational features as well as reorientation motions observed therein. NMR spin–lattice relaxation and QENS measurements, performed on spectrometers with different resolution (IN5 and IN16) and across wide temperature range (2–400 K), have shown that both quantum tunneling and classical reorientation of all methyl groups are found. The temperature dependence of the correlation times τc established from both, NMR and QENS experiments stay in good mutual agreement. Fast motion of two methyl groups (no. I), followed by the motion of the three methyl groups of no. II and IV (with similar energy barriers) and two methyl groups of no. III (with highest energy barriers) were observed. It was also found that reorientation of the tert-butyl moiety is much slower than methyl groups motions. tert-Butyl dynamics has been interpreted as a very large-amplitude libration rather than classical reorientation, due to steric clash present. Such motion was found to be promoted by earlier revealed flexibility of the (tert-butoxycarbonyl)vinyl chain. The activation parameters for all the different reorientations determined experimentally were compared to theoretical results. Among the tested models, PBE augmented with Tkatchenko–Scheffler dispersion corrections delivers reasonable description of the reorientation barriers, in line with experimental findings.Acknowledgements
This work has been partially financed by the National Science Centre of Poland, Grant No. 2015/17/B/ST5/00104 and by the operating program POKL 4.1.1. Financial support from the National Centre for Research and Development under research grant “Nanomaterials and their application to biomedicine”, contract number PBS1/A9/13/2012, is also gratefully acknowledged. This research was supported in part by PL-Grid Infrastructure (Grant ID: solid state 2015; latticedynamics). K. Drużbicki acknowledges the funds from Polish Government Plenipotentiary for JINR in Dubna (Grant No. 44/27-01-2015/7/1121/5) and the OMUS scholarship for outstanding young scientists at FLNP; JINR. Prof. Mark Johnson (ILL) is acknowledged for the helpful discussions on the interpretation and calculations of tunneling spectra.References
- P. L. McCormack and A. J. Wagstaff, Lacidipine: a review of its use in the management of hypertension, Drugs, 2003, 63, 2327–2356 CrossRef CAS PubMed.
- I. Simonic, S. Zupancic, A. Golobic, L. Golic and B. Stanovnik, The crystal structure of lacidipine phototransformation product, Acta Chim. Slov., 2008, 55, 458–461 CAS.
- K. Drużbicki, J. Mielcarek, A. Kiwilsza, L. Toupet, E. Collet, A. Pajzderska and J. Wąsicki, Computationally assisted (Solid-State DFT) structural (X-ray) and vibrational spectroscopy (FT-IR, FT-RS, TDs-THz) characterization of the cardiovascular drug-lacidipine, Cryst. Growth Des., 2015, 15, 2817–2830 Search PubMed.
- W. T. Dixon, J. Schaefer, M. D. Sefcik, E. O. Stejskal and R. A. McKay, Total suppression of sidebands in CPMAS C-13 NMR, J. Magn. Reson., 1982, 49, 341–345 CAS.
- P. A. Egelstaff and P. Schofield, On the evaluation of the thermal neutron scattering law, Nucl. Sci. Eng., 1962, 12, 260–270 Search PubMed.
- S. J. Clark, M. D. Segall, C. J. Pickard, P. J. Hasnip, M. J. Probert, K. Refson and M. C. Payne, First principles methods using CASTEP, Z. Kristallogr., 2005, 220, 567–570 CAS.
- K. Refson, S. J. Clark and P. R. Tulip, Variational density-functional perturbation theory for dielectrics and lattice dynamics, Phys. Rev. B: Condens. Matter Mater. Phys., 2006, 73, 155114–155136 CrossRef.
- J. Perdew, K. Burke and M. Ernzerhof, Generalized gradient approximation made simple, Phys. Rev. Lett., 1996, 77, 3865–3868 CrossRef CAS PubMed.
- B. Hammer, L. B. Hansen and J. K. Nørskov, Improved adsorption energetics within density-functional theory using revised Perdew–Burke–Ernzerhof functionals, Phys. Rev. B: Condens. Matter Mater. Phys., 1999, 59, 7413–7421 CrossRef.
- J. P. Perdew, A. Ruzsinszky, G. I. Csonka, O. A. Vydrov, G. E. Scuseria, L. A. Constantin, X. Zhou and K. Burke, Restoring the density-gradient expansion for exchange in solids and surfaces, Phys. Rev. Lett., 2008, 100, 136406–136410 CrossRef PubMed.
- A. Tkatchenko and M. Scheffler, Accurate molecular van der Waals interactions from ground-state electron density and free-atom reference data, Phys. Rev. Lett., 2009, 102, 073005–073009 CrossRef PubMed.
- S. Grimme, Semiempirical GGA-type density functional constructed with a long-range dispersion correction, J. Comput. Chem., 2006, 27, 1787–1799 CrossRef CAS PubMed.
- S. Baroni, P. Giannozzi and A. Testa, Green's-function approach to linear response in solids, Phys. Rev. Lett., 1987, 58, 1861–1864 CrossRef CAS PubMed.
- S. Baroni, A. Dal Corso, S. de Gironcoli and P. Giannozzi, Phonons and related crystal properties from density-functional perturbation theory, Rev. Mod. Phys., 2001, 73, 515–561 CrossRef CAS.
- A. Ramirez-Cuesta, aCLIMAX 4.0.1, the new version of the software for analyzing and interpreting INS spectra, Comput. Phys. Commun., 2004, 157, 226–238 CrossRef CAS.
- C. Pickard and F. Mauri, All-electron magnetic response with pseudopotentials: NMR chemical shifts, Phys. Rev. B: Condens. Matter Mater. Phys., 2001, 63, 245101–245115 CrossRef.
- J. R. Yates, C. Pickard and F. Mauri, Calculation of NMR chemical shifts for extended systems using ultrasoft pseudopotentials, Phys. Rev. B: Condens. Matter Mater. Phys., 2007, 76, 024401–024412 CrossRef.
- A. Pajzderska, K. Drużbicki, M. A. Gonzalez, J. Jenczyk, B. Peplińska, M. Jarek, J. Mielcarek and J. Wąsicki, Experimental and solid-state computational study of structural and dynamic properties in the equilibrium form of temazepam, J. Phys. Chem. B, 2014, 118, 6670–6679 CrossRef CAS PubMed.
- R. Goc, Computer calculation of the Van Vleck second moment for materials with internal rotation of spin groups, Comput. Phys. Commun., 2004, 162, 102–112 CrossRef CAS.
- A. Grunenberg, B. Keila and J.-O. Henckb, Polymorphism in binary mixtures, as exemplified by nimodipine, Int. J. Pharm., 1995, 118, 11–21 CrossRef CAS.
- M. S. Morales-Rios, A. Martınez-Richa, Z. Hernandez-Gallegos, A. Hernandez-Barragan, R. Vera-Graziano and P. Joseph-Nathan, Studies of dihydropyridines by X-ray diffraction and solid state 13C NMR, Z. Naturforsch., B: J. Chem. Sci., 2007, 62, 549–555 CAS.
- K. Drużbicki, A. Pajzderska, A. Kiwilsza, J. Jenczyk, D. Chudoba, M. Jarek, J. Mielcarek and J. Wąsicki, In search of the mutual relationship between the structure, solid-state spectroscopy and molecular dynamics in selected calcium channel blockers, Eur. J. Pharm. Sci., 2016, 85, 68–83 CrossRef PubMed.
- V. V. N. K. V. Prasada Raju, Studies towards process research and development of few biologically active compounds containing dihydropyridine and purine nitrogen heterocycles, PhD Thesis, Jawaharlal Nehru Technological University, India, 2010.
- J. H. Van Vleck, The dipolar broadening of magnetic resonance lines in crystals, Phys. Rev., 1948, 74, 1168–1183 CrossRef.
- E. R. Andrew, Nuclear magnetic resonance, Cambridge University Press, 2009 Search PubMed.
- J. Haupt, Einfluß von Quanteneffekten der Methylgruppenrotation auf die Kernrelaxation in Festkörpern, Z. Naturforsch., A: Astrophys., Phys. Phys. Chem., 1971, 26, 1578–1589 CAS.
- W. Muller-Warmuth, R. Schuler, M. Prager and A. Kollmar, A Nuclear relaxation and neutron scattering study of the rotational states of methyl groups in solids with low hindering barriers, J. Magn. Reson., 1979, 34, 83–95 Search PubMed.
- W. Muller-Warmuth, R. Schuler, M. Prager and A. Kollmar, Rotational tunnelling in methylpyridines as studied by NMR relaxation and inelastic neutron scattering, J. Chem. Phys., 1978, 69, 2382–2392 CrossRef.
- N. Bloembergen, E. M. Purcell and R. V. Pound, Relaxation effects in nuclear magnetic resonance absorption, Phys. Rev., 1948, 73, 679–712 CrossRef CAS.
- E. R. Andrew, W. S. Hinshaw, M. G. Hutchins and R. Sjoblom, Proton magnetic relaxation and molecular motion in polycrystalline amino acids, Mol. Phys., 1977, 34, 1695–1706 CrossRef CAS.
- E. N. M. R. Szcześniak, Study of molecular dynamics and phase transitions in solid 2-methyl-2-propanol, Mol. Phys., 1986, 59, 679–688 CrossRef.
- E. Szcześniak, Molecular dynamics and phase transitions in solid 2-methyl-2-propanethiol studied by 1H NMR relaxation, Mol. Phys., 1986, 58, 551–560 CrossRef.
- M. Prager and W. Langel, Intermolecular interaction in Sn(CH3)4 an inelastic neutron scattering study of matrix isolated molecules, J. Chem. Phys., 1986, 85, 5279–5285 CrossRef CAS.
- M. Prager, K. H. Dupree and W. Muller-Warmuth, Rotational tunnelling and methyl group reorientation in tetramethyltin [Sn(CH3)4] by inelastic neutron scattering and nuclear magnetic resonance, Z. Phys. B: Condens. Matter, 1983, 51, 3090–3098 CrossRef.
- J. Colmenero, A. J. Moreno and A. Alegria, Neutron scattering investigations on methyl group dynamics in polymers, Prog. Polym. Sci., 2005, 30, 1147–1184 CrossRef CAS.
- R. T. Azuah, L. R. Kneller, Y. Qiu, P. L. W. Tregenna-Piggott, C. M. Brown, J. R. D. Copley and R. M. Dimeo, DAVE: A comprehensive software suite for the reduction, visualization, and analysis of low energy neutron spectroscopic data, J. Res. Natl. Inst. Stand. Technol., 2009, 114, 341–358 CrossRef CAS.
- M. Bee, Quasielastic Neutron Scattering: Principles and Applications in Solid State Chemistry, Biology and Materials Science, Hilger, Bristol, 1988 Search PubMed.
- M. Schlaak, J. C. Lassegues, A. Heidemann and R. E. Lechner, Reorientations in crystalline (CH3)3NHCl studied by quasielastic neutron scattering, Mol. Phys., 1977, 33, 111–123 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |