Self-assembly of synthetic liposome-like curcumin nanoparticles†
Nisha Pawar*ab,
Kamla Rawat cd and
H. B. Bohidar*bc
cd and
H. B. Bohidar*bc
aDepartment of Physics, Indian Institute of Technology, Kharagpur, 721302, India. E-mail: pawar.nisha@phy.iitkgp.ernet.in
bSchool of Physical Sciences, Jawaharlal Nehru University, New Delhi-110067, India. E-mail: bohi0700@mail.jnu.ac.in
cSpecial Centre for Nanosciences, Jawaharlal Nehru University, New Delhi-110067, India
dInter University Accelerator Centre (IUAC), New Delhi 110067, India
First published on 19th July 2016
Abstract
The self-assembly of curcumin nanoparticles has gained considerable attention due to their widespread applications in the biological and technological fields. We report the formation of free-energy-driven self-assembled core–shell curcumin nanoparticles. The assembly mimics the morphology of liposome with hydrophobic curcumin particles trapped inside the core and hydrophilic nanoclay platelets forming the corona. Hydrophilic and hydrophobic regions were found to coexist with a soft interface separating them. Herein, a simple theoretical model is used to explain the important role played by the interface in providing stability to the assembly, whereby it provides a repulsive barrier against the attractive hydrophobic forces prevailing between the nanoparticles located in the core. The dimension of the nanoclay platelets beautifully tunes the stability of the self-assembly. It is remarkable that hydrophilic and hydrophobic systems can coexist with soft interfacial forces providing the required stability without the necessity for any surfactant.
1. Introduction
Free-energy-driven self-assembly is an interesting phenomenon observed in soft-matter systems. It plays a key role in the formation of living cells.1 The self-assembly of nanoparticles as the building blocks can organize into hierarchical supramolecular structures by thermodynamic and other constraints.1–5 Such self-assembly is highly sensitive to the balance among various secondary forces, entropy, and the energy in the system. It mainly involves non-covalent interactions, such as hydrogen bonds, the formation of ion pairs, van der Waals forces, hydrophobic interactions, etc.6 Among all the forces, hydrophobic interactions play the key role in modulating the self-assembly of nanoparticles in their dispersion phase. Hydrophobic interactions are attractive forces that emerge when water molecules rearrange in the presence of hydrophobic moieties.7 They are responsible for flocculation in many colloidal systems.8 Recently, Marzan and his group explained quantitatively the role of hydrophobic interactions in modulating the self-assembly of nanoparticles.9 A number of physical phenomena observed in nature are driven by hydrophobic interactions, including the formation of micelles, liposome, protein folding, etc. Liposomes are vesicular structures with an aqueous core.10 Their formation is a result of the self-assembly of phospholipids. They depict the best example of a self-assembly where hydrophobic and hydrophilic forces coexist, but the presence of a surfactant is a necessary condition for their existence. These structures are found to have many applications in the world of biophysics.10–14 They are efficient carriers of hydrophobic and hydrophilic molecules.Tuning different forces experimentally is a great challenge, but it can give rise to the formation of various interesting self-assembled systems with wide applications. The literature shows numerous applications originating from the self-assembly of nanoparticles in different areas.3,15,16,19 Nanoparticles have especially made great contributions to the biological field.17–19 Curcumin is a naturally occurring hydrophobic diketone molecule that can be extracted from the ginger family of plants. It is well known for its antioxidant, anti-inflammatory, antimicrobial, anti-carcinogenic, and immunomodulatory activities.20,21 Numerous reports show its biomedical properties.22–25 With such multi-tasking properties, it suffers from the drawbacks of poor solubility and bioavailability. To overcome these drawbacks, various groups have encapsulated curcumin in liposomes26 and lipid microparticles (such as BSA and chitosan).27,28 The work by Anirban's group29 shows the formation of a polymeric nanoparticle encapsulating the curcumin molecule. The drawbacks can also be efficiently overcome by the formation of an assembly of nano-curcumin structures.19 The assembly of curcumin nanoparticle shows drastic improvements in the properties of curcumin.22–25 It is a great challenge, however, to form stable curcumin nanoparticles, since they evolve or aggregate very quickly.30 There are different methods reported to form nano-sized particles in general, including emulsification-solvent evaporation and emulsification-solvent diffusion and precipitation, but the major problems with these methods is that they produce highly unstable particles and a surfactant is required to avoid their aggregation. Another method reported by Bhawana et al. involved a wet milling method.31 In this method, they used an organic solvent dichloromethane as a solvent for curcumin. The work by Cheng's group showed the formation of curcumin nanoparticles in a binary mixture of water and ethanol, but these nanostructures were found to be highly unstable.30 They showed spontaneous evolution from spherical- to rod-shaped structures. The dispersion instability was due to the large depletion forces provided by the binary solvent. It would be interesting to stabilize these attractive forces prevailing between hydrophobic curcumin nanoparticles and to form a stable assembly of nanoparticles. With such motivation, a repulsive environment was introduced in our system by using anisotropic colloids. The anisotropic colloid LAPONITE® (a nanoclay) has a disk-shaped morphology (diameter of 30 nm, thickness of 1 nm), with dominant repulsive forces.32,33 The motivation behind using anisotropic nanoclay was its interesting properties and rich phase diagram.32
Is it possible to design a tunable self-assembly at the nanoscale where a hydrophobic and hydrophilic environment coexist without surfactants? This work investigates this question and reports the formation of a self-assembly of core–shell curcumin nanoparticles. Nanoclay clusters self-assembled themselves around a curcumin nanoparticle and beautifully stabilized the assembly. The assembly looks like a core–shell arrangement of phospholipids, and the morphology is similar to liposome. Hence, the new assembly, formed by nanoclay platelets encapsulating the hydrophobic nanoparticles (curcumin) confined in an organic phase could be called “claysomes”. In this free-energy-driven arrangement, no surfactant was present, but regardless of this, a liposome-like structure could be formed with remarkably high stability. The existence of such a structure has never been reported so far.
2. Material and methods
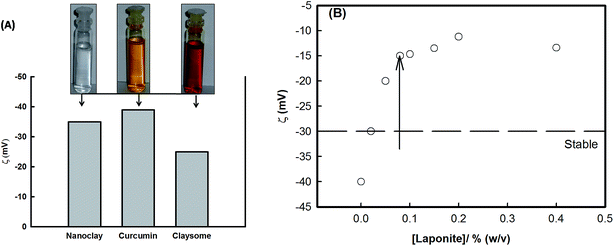
LAPONITE® RD was purchased from Southern Clay Products (USA), and was used as received. Curcumin was purchased from INDSAFF Ltd (India), and was used as received. Dispersion of curcumin (0.08% w/v) and LAPONITE® (0.05–0.5% w/v) were made in ethanol and in deionized water as the solvents, respectively. Curcumin nanoparticles were prepared by dropwise mixing the LAPONITE® dispersion into an ethanolic solution of curcumin under constant stirring at room temperature (25 °C). The change in color of the suspension (inset of Fig. 3(A) in the section below) indicated the presence of a favorable interaction between the two components and the formation of a stable self-assembled structure. This was confirmed from SEM with EDX (Zeiss EV040) and from light scattering experiments (PhotoCor Instruments, USA) data. Also, the surface charge of these structures was investigated by electrophoresis (Zeecom-2000, Microtec Corporation, Japan). The surface tension of the dispersion was monitored by using the Wilhelmy plate method using a tensiometer (Kibron EZ-Piplus, Finland).3. Results and discussion
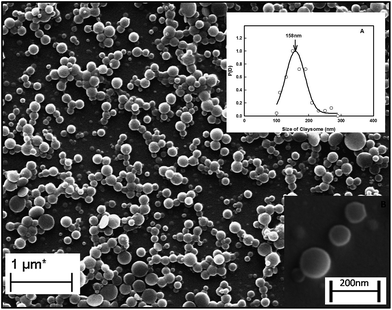
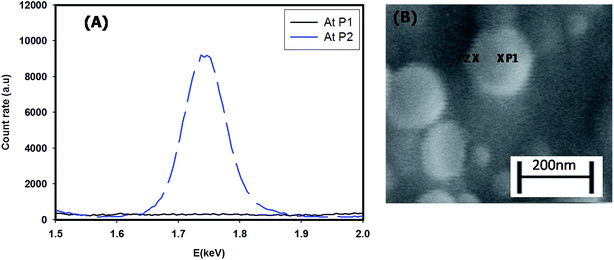
Fig. 1 shows the SEM image of the assembly of the core–shell particles. This clearly shows spherical particles of diameter 158 ± 5 nm (the Gaussian distribution of particle size is given in inset A) with a dark core with a light corona (inset B). The typical core diameter was ∼100 nm and the corona width was of ∼40 nm. The diameter of the assembly was confirmed from light scattering experiments. Analysis of the light scattering data gave a diameter of 100–200 nm, with 161 ± 10 nm as the diameter of maximum number of particles, which was in agreement with size obtained from the SEM data (the Gaussian distribution for the size of particles obtained from DLS is given in Fig. S2 in the ESI†). The location of the clay platelets in the assembly was confirmed from EDAX results. Fig. 2(A) and (B) show the energy dispersion profile of the given structure at two different positions (xP1 and xP2). They clearly show the absence of silicon (one of the major elements in the structure of clay) inside the core (xP1), and its abundance in the corona (xP2).33,34 This confirms that the clay platelets were excluded from the core of these structures. Thus, it was concluded that the core was hydrophobic and predominantly contained curcumin nanoparticles (curcumin is insoluble in water), while the shell (corona) had a propensity of self-assembled platelets (LAPONITE® is insoluble in ethanol). Thus, we have a structure where the core is an organic phase (ethanol) surrounded by an aqueous corona. Due to their solubility preference, curcumin nanoparticles, and LAPONITE® platelets, were preferentially located in the organic, and water phase, respectively.The electrostatic potential on the surface (zeta potential) of core–shell curcumin nanoparticles was measured using electrophoresis (Fig. 3(A)). It is interesting to note that both curcumin and nanoclay were held by the critically balanced repulsive forces, regardless of the fact that at the interface both curcumin and nanoclay had zeta potentials of the same polarity (negative). More details about the different forces responsible for the stability in the complex system are theoretically discussed in a later section. Also, the zeta potential of the dispersion was measured as a function of the concentration of nanoclay at room temperature. This potential increased with the increase in concentration of nanoclay in the system (Fig. 3(B)). Initially, at low concentration of nanoclay, the corona was thinner, which favored the formation of a stable claysome assembly, which was further supported by a higher value of zeta potential. With the increase in concentration of the nanoclay, the corona size increased (>100 nm), and the same occurred with the zeta potential, which in turn produced instability in the complex system.
3.1 Theoretical model
The schematic for the self-assembly of the nanoclay and curcumin nanoparticles is shown in Fig. 4.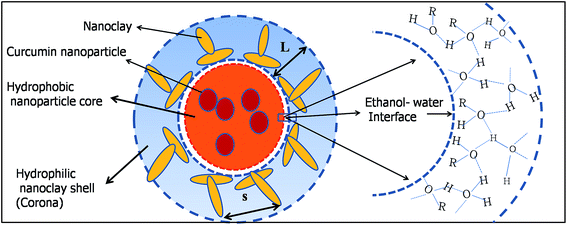 | ||
| Fig. 4 Schematic showing the formation of the claysome structure with nanoclay clusters present in the corona, and hydrophobic curcumin nanoparticles confined inside the hydrophobic core. | ||
The self-assembly formation is an outcome of the balance between the different forces acting in the complex system. The total energy of the system, Etotal, which is the sum of the attractive energy Ea and the repulsive energy Er in the system, is given by:
| Etotal = Ea + Er | (1) |
Two balancing forces play a major role in the formation of the assembly: the attractive hydrophobic interaction dominated by interfacial forces between the two types of particles, and the steric repulsive force among the nanoclay cluster and the hydrophobic environment of the system. Hence, the total energy of the complex core–shell (claysome) assembly is given by the sum of the following three contributions: steric repulsive forces between the neighboring nanoclay clusters (ESR),9 hydrophobic forces (Ehyd),9 and the van der Waals forces (Evand):34
| Etotal = ESR + Ehyd + Evand | (2) |
The three individual contributions can be written as:
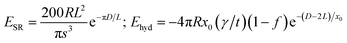 | (3) |
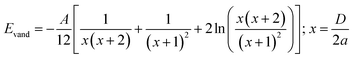 | (4) |
Hence, the total interaction energy is given by:
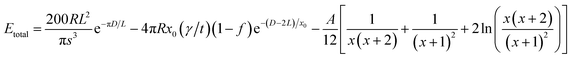 | (5) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixing ratio of ethanol and water, hence f = 0.5, and the corresponding interfacial surface tension was (measured) as γ = 40 mN m−1. The size of curcumin nanoparticles and their cluster size were obtained from SEM measurements as R = 50 nm and a = 150 nm.
1 mixing ratio of ethanol and water, hence f = 0.5, and the corresponding interfacial surface tension was (measured) as γ = 40 mN m−1. The size of curcumin nanoparticles and their cluster size were obtained from SEM measurements as R = 50 nm and a = 150 nm.
3.2 Role of the interface region between the core and shell
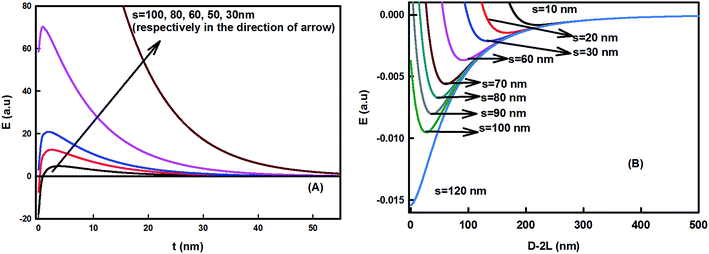
The interface region lying between the hydrophobic core (organic) and the hydrophilic shell (aqueous) played a significant role in the formation of the stable assembly. It sustained the repulsive energy barrier against the attractive hydrophobic energy present inside the core. The barrier inhibits the aggregation of the curcumin nanoparticles and supports the stability of the so-formed claysome assembly (or core–shell nanoparticle). The barrier was found to be highly sensitive toward the dimensions of the nanoclay cluster present in the corona. Fig. 5(A) and 6(A) show the variation of total energy as a function of the thickness of the interface region. The barrier height increased with the increase in length of the nanoclay cluster, and decreased in footprint diameter. It became highly repulsive for L > 80 nm and s < 30 nm.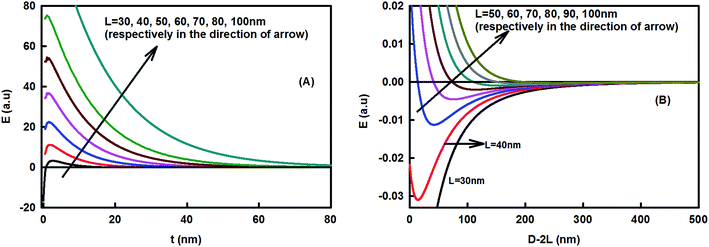 | ||
| Fig. 5 Effect of the length of the nanoclay cluster on the claysome assembly. (A) Variation of energy as a function of the thickness of the interfacial region (s = 60 nm; T = 298 K, f = 0.5; radius of curcumin nanoparticle R = 50 nm, hydrophobic decay length x0 = 1 nm, interfacial tension = 40 mN m−1). (B) Claysome–claysome interaction as a function of interparticle separation D, calculated using eqn (5). The depth of energy minima decreases in the presence of large nanoclay clusters and the energy becomes fully repulsive for L > 80 nm. | ||
 | ||
| Fig. 6 Effect of footprint diameter on the claysome assembly. (A) Variation of energy as a function of the thickness of the interfacial region (L = 60 nm, T = 298 K, f = 0.5, radius of curcumin nanoparticle R = 50 nm, hydrophobic decay length x0 = 1 nm, interfacial tension = 40 mN m−1). (B) Claysome–claysome interaction as a function of interparticle separation D, calculated using eqn (5). The energy minima become deeper for higher footprint diameters and become attractive for s > 100 nm. | ||
The interaction energy between the claysome structures (nanoclay–nanoparticle interactions) was calculated as a function of the interparticle distance. It showed deep minima, as shown in Fig. 5(B) and 6(B). The minima in the energy landscape signified the stability in the assembly of the claysome structures. Claysome structures may form loose flocculates at distances comparable to the long-range minima, but these flocculates may be re-dispersed. Fig. 5(B) and 6(B) show the variation of energy of the complex system as a function of the dimensions of the nanoclay cluster (L and s). These clearly show that the depth of the minima decreased with the increase in length of the nanoclay cluster (L). The energy was highly attractive for L < 40 nm, hence the minimum length of nanoclay cluster required for the formation of a repulsive barrier (the corona) was 40 nm. The energy became highly repulsive for L > 80 nm with no minima. Hence, stable claysome assemblies can only be formed when nanoclay platelets form clusters with a dimension of 40 < L < 80 nm. Also, the interaction between the claysome structure was found to be sensitive to the footprint diameter of the nanoclay, as shown in Fig. 6. With an increase in footprint diameter, the minimum energy becomes more negative, and finally, for s > 100 nm, the interactions are predominately attractive.
4. Conclusions
A stable self-assembly of core–shell nanoparticle (or claysome structures) was formed with curcumin residing in the core and nanoclay in the corona. Particles had a typical size of 150 nm with a negatively charged surface (zeta potential ∼ 25 mV). The stability of particles (formed with 0.05% nanoclay) was confirmed with the zeta potential (as shown in Fig. 3), the energy of the self-assembled system (as shown in Fig. 5(B) and 6(B)), the constant DLS count rate over a period of 20 days (as shown in Fig. S3 in the ESI†), and similar particle size distribution from SEM images taken after a period of 20 days (as shown in Fig. S4 in the ESI†). The assembly demonstrated the coexistence of a hydrophobic (core) and hydrophilic (shell) particle with a soft tunable interfacial region. The main cause for the self-assembly was the intricate balance between the dominant attractive forces among the curcumin nanoparticles and the repulsive forces provided by the nanoclay platelets. The interface region between the hydrophobic and hydrophilic region played a key role in the formation and stabilization. It adequately balanced the repulsive barrier against the attractive hydrophobic forces prevailing among the curcumin nanoparticles (as shown in Fig. 5(A) and 6(A)), which prevented the aggregation of curcumin nanoparticles and resulted in formation of the claysome assembly. The self-assembly of claysome particles was found to be sensitive to the size of the nanoclay cluster, because it tuned the repulsive forces in the system. There exists a critical threshold size of nanoclay cluster (L < 80 nm and s < 100 nm) for the stable formation of these structures. The instability in the self-assembly with the increasing cluster size of nanoclay was supported by the lowering of the zeta potential of the bigger claysome particles. In short, we conclusively demonstrated that liposome-like structures or stable curcumin nanoparticles can be formed in the presence of inorganic clay platelets when the interaction forces are tailored to cause a delicate balance, even in the absence of any surfactants. The claysome structures formed may have different applications in the world of biophysics. The claysome assembly is expected to be sensitive toward the pH of the system, hence it might have application in delivering cargo loaded in the core to a target site.Acknowledgements
The work was supported by Jawaharlal Nehru University visitor's fellowship awarded to NP. NP and KR acknowledge Department of Science and Technology, Government of India-Inspire Faculty Award. We are thankful to Dr Akanksha Sharma for help in SEM measurements at Advanced Research Instrumentation Facility of the University. NP is thankful to Prof. Matthias Weiss for his laboratory facility and useful discussions.References
- Y. Gao, C. Berciu, Y. Kuang, J. Shi, D. Nicastro and B. Xu, ACS Nano, 2013, 7, 9055–9063 CrossRef CAS PubMed.
- G. Helgesen, E. Svasand and A. T. Skjeltorp, J. Phys.: Condens. Matter, 2008, 20, 204127, DOI:10.1088/0953-8984/20/20/204127.
- M. Grzelczak, J. Vermant, E. M. Furst and L. M. Liz-Mirzan, ACS Nano, 2010, 4, 3591–3605 CrossRef CAS PubMed.
- A. K. Boal, F. Ilhan, J. E. DeRouchey, T. Thurn-Albrecht, T. P. Russell and V. M. Rotello, Nature, 2000, 404, 746–748 CrossRef CAS PubMed.
- Y. Xia, T. D. Nguyen, M. Yang, B. Lee, A. Santos, P. Podsiadlo, Z. Tang, S. C. Glotzer and N. A. Kotov, Nat. Nanotechnol., 2011, 6, 580–587 CrossRef CAS PubMed.
- G. M. Whitesides and B. Grzybowski, Science, 2002, 295, 2418 CrossRef CAS PubMed.
- E. E. Meyer, K. J. Rosenberg and J. Israelachvili, PNAS, 2006, 103, 15739–15746 CrossRef CAS PubMed.
- N. I. Lebovka, Adv. Polym. Sci., 2014, 255, 57–96 CrossRef CAS.
- A. S. Iglesias, M. Grzelczak, T. Altantzis, B. Goris, J. Perez-Juste, S. Bals, G. V. Tendeloo, G. V. Stephan, H. Donaldson Jr, B. F. Chmelka, J. N. Israelachvili and L. M. Liz-Marzan, ACS Nano, 2012, 12, 11059–11065 CrossRef PubMed.
- A. Laouini, C. Jaafar-Maalej, I. Limayem-Blouza, S. Sfar, C. Charcosset and H. Fessi, J. Colloid Sci. Biotechnol., 2012, 1, 147–168 CrossRef CAS.
- T. M. Allena and P. R. Cullis, Adv. Drug Delivery Rev., 2013, 65, 36–48 CrossRef PubMed.
- M. J. Ostro and P. R. Cullis, Am. J. Hosp. Pharm., 1989, 46, 1576–1587 CAS.
- A. Samad, Y. Sultana and M. Aqil, Curr. Drug Delivery, 2007, 4, 297–305 CrossRef CAS.
- P. da Silva Malheiros, D. J. Daroit and A. Brandelli, Trends Food Sci. Technol., 2010, 21, 284–292 CrossRef CAS.
- Z. Nie, A. Petukhova and E. Kumacheva, Nat. Nanotechnol., 2010, 5, 15–25 CrossRef CAS PubMed.
- E. Busseron, Y. Ruff, E. Moulin and N. Giuseppone, Nanoscale, 2013, 5, 7098–7140 RSC.
- M. Rad-Malekshahi, L. Lempsink, M. Amidi, W. E. Hennink and E. Mastrobattista, Bioconjugate Chem., 2016, 27, 3–18 CrossRef CAS PubMed.
- R. M. Gorgoll, T. Tsubota, K. Harano and E. Nakamura, J. Am. Chem. Soc., 2015, 137, 7568–7571 CrossRef CAS PubMed.
- W. Lewandowski, M. Fruhnert, J. Mieczkowski, C. Rockstuhl and E. Górecka, Nat. Commun., 2015 DOI:10.1038/ncomms7590.
- M. M. Yallapu, M. Jaggi and S. C. Chauhan, Curr. Pharm. Des., 2013, 19, 1994–2010 CAS.
- Y. Manolova, V. Deneva, L. Antonov, E. Drakalska, D. Momekova and N. Lambov, Spectrochim. Acta, Part A, 2014, 132, 815–820 CrossRef CAS PubMed.
- P. Anand, A. B. Kunnumakkara, R. A. Newman and B. B. Aggarwal, Mol. Pharm., 2007, 4, 807 CrossRef CAS PubMed.
- H. Hatcher, R. Planalp, J. Cho, F. M. Torti and S. V. Torti, Cell. Mol. Life Sci., 2008, 65, 1631 CrossRef CAS PubMed.
- Y. Zhang, C. Yang, W. Wang, J. Liu, Q. Liu, F. Huang, L. Chu, H. Gao, C. Li, D. Kong, Q. Liu and J. Liu, Sci. Rep., 2016, 6, 1–12 Search PubMed.
- X. Yang, Z. Li, N. Wang, L. Li, L. Song, T. He, L. Sun, Z. Wang, Q. Wu, N. Luo, C. Yi and C. Gong, Sci. Rep., 2015, 5, 1–15 Search PubMed.
- D. Wang, S. M. Veena, K. Stevenson, C. Tang, B. Ho, J. D. Suh, V. M. Duarte, K. F. Faull, K. Mehta, E. S. Srivastan and M. B. Wang, Clin. Cancer Res., 2008, 14, 6228–6236 CrossRef CAS PubMed.
- V. Gupta, A. Aseh, C. N. Rios, B. B. Aggarwal and A. B. Mathur, Int. J. Nanomed., 2009, 4, 115–122 CrossRef CAS.
- R. K. Das, N. Kasoju and U. Bora, Nanomedicine, 2010, 6, 153–160 CAS.
- S. Bisht, G. Feldmann, S. Soni, R. Ravi, C. Karikar, A. Maitra and A. Maitra, J. Nanobiotechnol., 2007, 5, 3–21 CrossRef PubMed.
- Y. He, Y. Huang and Y. Cheng, Cryst. Growth Des., 2010, 3, 1021–1024 Search PubMed.
- Bhawana, R. K. Basniwal, H. S. Buttar, V. K. Jain and N. Jain, J. Agric. Food Chem., 2011, 59, 2056–2061 CrossRef CAS PubMed.
- N. Pawar and H. B. Bohidar, Colloids Surf., A, 2009, 333, 120–125 CrossRef CAS.
- B. Ruzicka, E. Zaccarelli, L. Zulian, R. Angelini, M. Sztucki, A. Moussaid, T. Narayanan and F. Sciortino, Nat. Mater., 2011, 10, 56–60 CrossRef CAS PubMed.
- R. K. Pujala, Dispersion Stability, Microstructure and Phase Transition of Anisotropic Nanodiscs, Springer Thesis, 2014, DOI:10.1007/978-3-319-04555-9.
- A. Faghihne jad and H. Zeng, Langmuir, 2013, 29, 12443–12451 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: TEM images depicting the aggregation in the curcumin nanoparticles in a binary mixture of alcohol and water, Gaussian distribution of the size of the core–shell particles from DLS, scattered intensity from particles over a period of 20 days and SEM image of core–shell particles after a period of 20 days. See DOI: 10.1039/c6ra14893j |
| This journal is © The Royal Society of Chemistry 2016 |