One-pot syntheses of N-(α-fluorovinyl)azole derivatives from N-(diphenylmethylene)-2,2,2-trifluoroethanamine†
Donghua Zhang,
Haihua Liu,
Pengcheng Zhu,
Weidong Meng and
Yangen Huang*
College of Chemistry, Chemical Engineering and Biotechnology, Donghua University, 2999 North Renmin Road, Shanghai 201620, China. E-mail: hyg@dhu.edu.cn
First published on 27th July 2016
Abstract
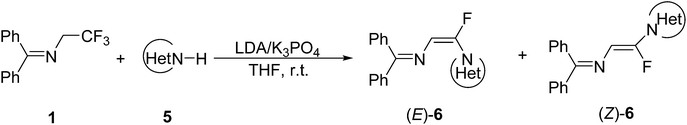
N-(Diphenylmethylene)-2,2,2-trifluoroethanamine acts as a versatile fluorine-containing building block, and from which N-(α-fluorovinyl)azole derivatives were readily prepared in good yields with relatively high stereoselectivity by using various N–H containing heterocycles as nucleophiles via vinylic substitution reactions (SNV) in the presence of LDA and K3PO4 in a one-pot process.
Introduction
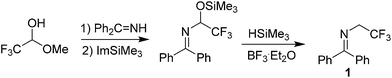
The incorporation of a fluorine-containing group into an organic molecule alters its physical, chemical, and biological properties in such a way that it often enhances the molecule's desirable properties, including higher levels of metabolic stability, solubility, lipophilicity, bioavailability and biopotency.1 Hence, the development of efficient and mild methods for the preparation of organofluorine compounds have attracted considerable attention in a broad area of organic chemistry.2 Among them, special interest has focused on fluoroalkenes which as versatile building blocks can be transformed to many valuable functionalities.3 Nowadays, numerous approaches4 such as carbonyl olefination methods for synthesizing vinyl fluoride have been developed, and many applications of vinyl fluoride in pharmaceuticals and fluoropolymers have been found.5 The major transformation of vinyl fluoride in synthesis includes nucleophilic vinylic substitution reaction (SNV)6 and metal mediated C–F bond activated cross coupling reaction.7N-(diphenylmethylene)-2,2,2-trifluoroethanamine 1 was firstly reported by Langlois in 2002 (Scheme 1).8 Comparing to ethyl N-(diphenylmethylene)glycinate which served as a versatile synthon,9 the application of 1 in synthetic chemistry is rare though the protons near trifluoromethyl group are acidic. Only one example involving the transformation of 1 was realized by anodic α-acetoxylation.10 The reason is that the anion intermediate from deprotonation of 1 with strong base is not stable, which easily undergoes β-fluorine elimination to form fluoroenamine. The fluoroenamine usually is not stable which readily reacted with water to give the corresponding amide and hydrogen fluoride in an addition-elimination process.11 Our investigations for designing new fluorine-containing building blocks, recently led us to find that the gem-difluoroenamine 4 is stable and can be isolated. Considering that 4 is a typical vinyl fluoride, the transformation with 4 is worth to try because it may provide an efficient strategy for preparation of fluoroenamines.
In 2014, Cao and co-workers reported the SNV reaction of 2,2-difluorovinylarene with various N–H-containing heterocycles in the presence of K3PO4 affording the (E)-N-α-fluorovinyl derivatives of azoles in excellent yields with relatively high stereoselectivity.6i Considering the typical vinyl fluoride structure of 4, N–H-containing heterocycles would also be suitable nucleophiles. In this paper, we report an efficient method for synthesizing N-(α-fluorovinyl)azole derivatives from 1 in a one-pot process, in which the isolation of gem-difluoroenamine 4 is not necessary. Especially, the resulting fluoroenamine are stable solid and did not undergo hydrolysis on contacting with water.
Results and discussion
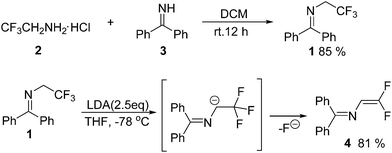
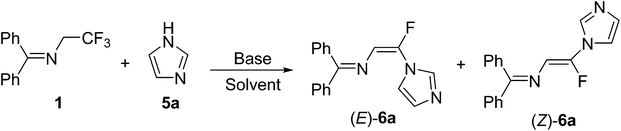
The literature method for preparation of 1 used methyl hemiketal of fluoral as a starting material in a three-step process (Scheme 1).8,12 However, we found that trifluoroethylamine hydrochloride 2 as a cheap and commercial available fluorinated compound can react with diphenylmethanimine 3 smoothly with simple stirring at room temperature in dichloromethane to afford 1 in 85% yield (Scheme 2). And the reaction can scale up to more than 20 gram. The gem-difluoroenamine 4 was then prepared in 81% yield through dehydrofluorination of 1 with LDA as a base. It was found that 4 is stable during the aqueous workup process.With N-(diphenylmethylene)-2,2,2-trifluoroethanamine 1 in hand, we focused on the one-pot synthesis of N-(α-fluorovinyl)azoles. Keeping LDA as the dehydrofluorination base, the reaction of 1 with 1H-imidazole 5a was carried out in the presence of K3PO4 using THF as a solvent (Table 1). It was observed that the nucleophilic substitution of vinylfluoride took place smoothly to give the corresponding product 6a in 80% yield with good stereoselectivity (E/Z = 88![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
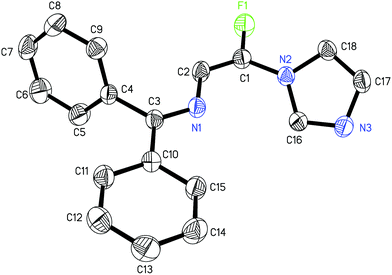
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12) (entry 1). The effect of the second base for deproton of 5a was studied. Na2CO3, K2CO3, Cs2CO3 or EtONa gave nearly the same yields and E/Z selectivities as that of K3PO4 (entries 1–5), but only gem-difluoroenamine 4 was obtained in the absence of the second base (entry 6). Furthermore, 2 equiv. of K3PO4 is necessary to promise a reasonable yield, the decrease in the amount of K3PO4 (e.g., 1 equiv.; entry 8) led to an obvious decrease in the yield, while the increase in the amount of K3PO4 from 2 equiv. to 3 equiv. resulted in no significant improvement of yield (entry 7). The reaction proceeded efficiently at room temperature and the increase of reaction temperature is not necessary (entries 9–10). An investigation of the solvent effect showed that the ethereal solvents were better than other solvents, and tetrahydrofuran (THF) gave the highest yield (Table 1, entries 5, 11–14). Thus, the optimized reaction conditions are as follows: 1H-imidazole 5a (1.0 equiv.), imine 1 (1.1 equiv.), LDA (2.5 equiv.), K3PO4 (2.0 equiv.), and THF as solvent, stirring for 1 h at −78 °C then 16 h at room temperature. It is need to be pointed out that only (E)-6a was obtained after purification by flash chromatography, and the configuration of (E)-6a was confirmed by X-ray single crystal analysis (Fig. 1).13 Usually, the trans 3JH–F coupling constant in Z isomer is higher than the cis 3JH–F coupling constant in E isomer in the 1H NMR spectrum of vinylfluoride,14 so the configuration of other major products were tentatively assigned according to the 3JH–F coupling constant.
12) (entry 1). The effect of the second base for deproton of 5a was studied. Na2CO3, K2CO3, Cs2CO3 or EtONa gave nearly the same yields and E/Z selectivities as that of K3PO4 (entries 1–5), but only gem-difluoroenamine 4 was obtained in the absence of the second base (entry 6). Furthermore, 2 equiv. of K3PO4 is necessary to promise a reasonable yield, the decrease in the amount of K3PO4 (e.g., 1 equiv.; entry 8) led to an obvious decrease in the yield, while the increase in the amount of K3PO4 from 2 equiv. to 3 equiv. resulted in no significant improvement of yield (entry 7). The reaction proceeded efficiently at room temperature and the increase of reaction temperature is not necessary (entries 9–10). An investigation of the solvent effect showed that the ethereal solvents were better than other solvents, and tetrahydrofuran (THF) gave the highest yield (Table 1, entries 5, 11–14). Thus, the optimized reaction conditions are as follows: 1H-imidazole 5a (1.0 equiv.), imine 1 (1.1 equiv.), LDA (2.5 equiv.), K3PO4 (2.0 equiv.), and THF as solvent, stirring for 1 h at −78 °C then 16 h at room temperature. It is need to be pointed out that only (E)-6a was obtained after purification by flash chromatography, and the configuration of (E)-6a was confirmed by X-ray single crystal analysis (Fig. 1).13 Usually, the trans 3JH–F coupling constant in Z isomer is higher than the cis 3JH–F coupling constant in E isomer in the 1H NMR spectrum of vinylfluoride,14 so the configuration of other major products were tentatively assigned according to the 3JH–F coupling constant.
| Entry | Temp (°C) | Base (equiv.) | Solvent | Yieldb (%) | E/Zc |
|---|---|---|---|---|---|
| a Reaction conditions: 1 (0.44 mmol), imidazole 5a (0.4 mmol), solvent (5 mL), LDA (2.5 eq.) at −78 °C for 1 h, then at rt for 16 h.b Yields determined by 19F NMR which using benzotrifluoride as the internal standard.c The ratios of E/Z isomers were determined by 19F NMR.d No product was detected. | |||||
| 1 | 25 | Na2CO3 (2) | THF | 74 | 88![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 12 |
| 2 | 25 | K2CO3 (2) | THF | 74 | 86![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14 14 |
| 3 | 25 | Cs2CO3 (2) | THF | 72 | 86![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14 14 |
| 4 | 25 | EtONa (2) | THF | 76 | 87![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13 13 |
| 5 | 25 | K3PO4 (2) | THF | 80 | 88![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 12 |
| 6 | 25 | None | THF | NR | |
| 7 | 25 | K3PO4 (3) | THF | 76 | 88![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 12 |
| 8 | 25 | K3PO4 (1) | THF | 60 | 84![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16 16 |
| 9 | 40 | K3PO4 (2) | THF | 75 | 88![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 12 |
| 10 | 60 | K3PO4 (2) | THF | 76 | 89![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 11 |
| 11 | 25 | K3PO4 (2) | Et3N | 22 | 89![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 11 |
| 12 | 25 | K3PO4 (2) | n-Hexane | 12 | 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 15 |
| 13 | 25 | K3PO4 (2) | Et2O | 58 | 88![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 12 |
| 14 | 25 | K3PO4 (2) | CH2Cl2 | N.D.d | |
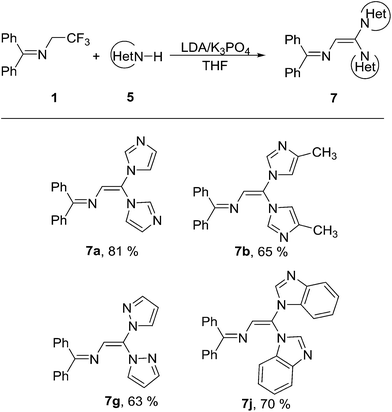
With the optimized condition in hand, the scope of nitrogen-containing heterocyclic compounds as nucleophiles was investigated, and the results were shown in Table 2. Similarly to 5a, substituted imidazoles reacted smoothly to give the N-(α-fluorovinyl)azoles in moderate to good yields, and only pure E-isomers (6a, 6c–f) as major product were obtained after column chromatography. However, both isomers (E)-6b and (Z)-6b could be isolated for 4-methylimidazole (5b) due to their relatively lower stereoselectivity. The reactions of imine 1 with pyrazole (5g), 1H-1,2,3-triazole (5h), 1H-1,2,4-triazole (5i), 1H-benzo[d]imidazole (5j–l), 5-nitro-1H-indazole (5m) and 1H-benzo[d][1,2,3]triazole (5n) were also proceeded well to afford the expected N-(α-fluorovinyl)azoles in moderate to good yields. The best yield (81%, 6k) was achieved by using 2-methyl-1H-benzo[d]imidazole (5k) as the nucleophile. It is noteworthy that the azole bearing a strong electron-withdrawing group such as NO2 (5-nitro-1H-indazole, 5m) also reacted smoothly but with Z-isomer as the major product. The stereoselective outcome of the reaction is consistent with the reported methods in which terminal vinylic fluorine was substituted by heterocycle6i or cyanide anion15 via vinylic nucleophilic substitution reaction (SNV).
| Entry | Azole 5 | Product 6 | Yieldb (%) | Isomer ratioc (E/Z) |
|---|---|---|---|---|
| a Reaction conditions: azoles 5a–n (0.4 mmol), 1 (0.44 mmol), K3PO4 (0.8 mmol), THF (5 mL), LDA (2.5 eq.) at −78 °C for 1 h, then at rt for 16 h.b Isolated yields.c Determined by19F NMR of the crude product.d Not obtained after column chromatography.e Total yield of (E/Z)-6g which can't be isolated from each other. | ||||
| 1 |  |
(E)-6a | 70 | 89![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 11 |
| (Z)-6a | —d | |||
| 2 |  |
(E)-6b | 45 | 74![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 26 26 |
| (Z)-6b | 20 | |||
| 3 |  |
(E)-6c | 51 | 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 15 |
| (Z)-6c | —d | |||
| 4 |  |
(E)-6d | 60 | 91![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 9 |
| (Z)-6d | —d | |||
| 5 |  |
(E)-6e | 61 | 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
| (Z)-6e | —d | |||
| 6 | 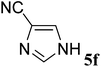 |
(E)-6f | 75 | 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7 |
| (Z)-6f | —d | |||
| 7 | 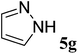 |
(E)-6g | 73e | 37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 63 63 |
| (Z)-6g | ||||
| 8 | 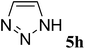 |
(E)-6h | 46 | 62![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 38 38 |
| (Z)-6h | 9 | |||
| 9 | 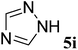 |
(E)-6i | 43 | 63![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 37 37 |
| (Z)-6i | 12 | |||
| 10 |  |
(E)-6j | 68 | 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 15 |
| (Z)-6j | 5 | |||
| 11 |  |
(E)-6k | 81 | 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
| (Z)-6k | —d | |||
| 12 |  |
(E)-6l | 45 | 82![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18 18 |
| (Z)-6l | —d | |||
| 13 |  |
(E)-6m | 9 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 87 87 |
| (Z)-6m | 46 | |||
| 14 |  |
(E)-6n | 46 | 79![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 21 21 |
| (Z)-6n | 7 | |||
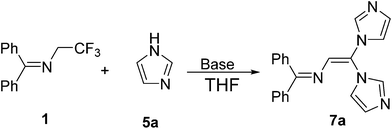
In order to expand the application of 1 as a building block, we further examined the reactivity of the C–F bond in the N-(α-fluorovinyl)azoles 6 towards azoles. As shown in Table 3, the one-pot reaction of 1 equiv. of 1 with 2 equiv. of 1H-imidazole 5a under the aforementioned optimized reaction condition gave diazole substituted enamine 7a in 40% yield accompanied with trace 6a (Table 3, entry 1). The yield of 7a increased slightly (from 40% to 55%) with the increase of reaction temperature from 25 °C to 70 °C (Table 3, entries 2–4). The best result (yield 81%) was achieved when the amount of K3PO4 was increased from 2 equiv. to 4 equiv. (Table 3, entry 6). Under the optimized reaction condition (Table 3, entry 6), 4-methylimidazole (5b), pyrazole (5g), 1H-benzo[d]imidazole (5j) were readily reacted with 1 to afford the trisubstituted alkene 7b, 7g and 7j in 65%, 63% and 70% yield, respectively (Table 4). However, attempts to get the trisubstituted alkenes containing two different type of azoles were failed to achieve acceptable yields no matter the two different azoles were added in one portion or stepwise.
| Entry | Amt of 5a (equiv.) | Temp (°C) | Base (equiv.) | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: 1 (0.4 mmol), THF (5 mL), LDA (2.5 eq.) at −78 °C for 1 h, then at different temperatures for 16 h.b Isolated yield. | ||||
| 1 | 2.0 | 25 | K3PO4 (2) | 40 |
| 2 | 2.1 | 25 | K3PO4 (2) | 43 |
| 3 | 2.0 | 50 | K3PO4 (2) | 52 |
| 4 | 2.0 | 70 | K3PO4 (2) | 55 |
| 5 | 2.0 | 70 | K3PO4 (3) | 62 |
| 6 | 2.0 | 70 | K3PO4 (4) | 81 |
| 7 | 2.0 | 70 | K3PO4 (5) | 62 |
Conclusion
In summary, we have described a versatile fluorine-containing building block N-(diphenylmethylene)-2,2,2-trifluoroethanamine 1 from which a facile and straightforward method for the synthesis of N-(α-fluorovinyl)azoles was developed. The gem-difluoroenamine can be prepared by utilizing the good leaving tendency of fluoride ion. N-(α-fluorovinyl)azoles were then prepared in moderate to good yield by the reaction of 1 with various N–H-containing heterocycles in the absence of metal catalyst under mild reaction conditions via vinylic nucleophilic substitution reaction (SNV). Notably, the C–F bond cleavage of –CF3 in 1 could occur one by one in a one-pot process to afford the corresponding fluorinated or non-fluorinated products. This method can be considered as a valuable strategy for direct access to various N-functionalized fluoroenamines and polysubstituted enamines.Experimental section
General information
All reagents were of analytical grade, obtained from commercial suppliers, and used without further purification. All solvents were dried by standard methods prior to use. 1H NMR and 13C NMR spectra were recorded on a 400 spectrometer (400 MHz for 1H and 100 MHz for 13C) using TMS as internal standard. The 19F NMR spectra were obtained using a 400 spectrometer (376 MHz). DMSO-d6 or CDCl3 was used as the NMR solvent in cases and benzotrifluoride as the internal standard. High-resolution mass spectra (HRMS) were acquired in the electron impact mode (EI) using a TOF mass analyzer.Synthesis of N-(diphenylmethylene)-2,2,2-trifluoroethanamine (1)
Under nitrogen atmosphere, trifluoroethylamine hydrochloride 2 (4.88 g, 36.0 mmol), diphenylmethanimine 3 (5.44 g, 30.0 mmol) and CH2Cl2 (50 mL) were added to a 100 mL round bottom flask. The reaction mixture was stirred at room temperature for 12 h, and then concentrated in vacuum. The crude product was purified by flash chromatography on silica gel using petroleum ether as eluent to afford pure 1 as white crystal (6.71 g, yield 85%). 1H NMR (400 MHz, CDCl3) δ 7.80–7.30 (m, 8H), 7.15 (d, J = 7.3 Hz, 2H). 3.85 (q, J = 9.2 Hz, 2H). 19F NMR (376 MHz, CDCl3) δ −75.8 (t, J = 9.2 Hz).Synthesis of N-(2,2-difluorovinyl)-1,1-diphenylmethanimine (4)
To a solution of 1 (0.263 g, 1.0 mmol) in THF (5 mL) was added dropwise LDA (1.7 mL, 2.5 mmol, 1.5 M in THF) at −78 °C. The mixture was stirred at −78 °C for further 2 h. After warming to room temperature, the reaction mixture was quenched by brine (8 mL), extracted with ethyl acetate (3 × 10 mL). After drying over anhydrous MgSO4 and concentration of the combined organic phases, the crude product was purified by flash chromatography on silica gel using petroleum ether as eluent to afford pure 4 as yellow liquid (0.197 g, yield 81%). 1H NMR (400 MHz, DMSO-d6) δ 7.65 (s, 1H), 7.63 (d, J = 1.6 Hz, 1H), 7.62–7.56 (m, 3H), 7.50 (t, J = 7.2 Hz, 1H), 7.44 (t, J = 7.3 Hz, 2H), 7.30–7.24 (m, 2H), 6.01 (dd, J = 18.2, 0.7 Hz, 1H). 13C NMR (101 MHz, DMSO-d6) δ 168.0 (dd, J = 12.2, 3.4 Hz), 162.2, 159.3, 159.2, 156.3 (d, J = 15.5 Hz), 140.0 (t, J = 21.4 Hz), 136.4, 132.6, 131.1, 130.8, 130.2, 130.1 (d, J = 5.0 Hz), 130.0 (d, J = 16.5 Hz), 98.4 (dd, J = 43.6, 9.7 Hz). 19F NMR (376 MHz, DMSO-d6) δ −86.4 (dd, J = 22.4, 18.1 Hz), −96.0 (d, J = 22.2 Hz). IR (neat) νmax 3067, 1710, 1237, 693 cm−1; HRMS (EI) calcd for C15H12F2N. [M + H]+ 244.0932, found 244.0932.General procedure for preparation of N-(α-fluorovinyl)azoles
Under nitrogen atmosphere, a solution of 1 (0.116 g, 0.44 mmol), imidazole 5a (0.027 g, 0.4 mmol) and K3PO4 (0.17 g, 0.8 mmol) in THF (5 mL) was stirred for 30 min at −78 °C. Then LDA (0.67 mL, 1.0 mmol, 1.5 M in THF) was added dropwise, the reaction mixture was stirred at −78 °C for 1 h and at room temperature for further 16 h. The reaction mixture was quenched by brine (10 mL), extracted with ethyl acetate (3 × 10 mL). After drying over anhydrous MgSO4 and concentration of the combined organic phases, the crude product was purified by flash chromatography (silica gel, petroleum ether/ethyl acetate 5/1) to afford (E)-6a as yellow solid (83 mg, yield 70%), mp 102–105 °C; 1H NMR (400 MHz, CDCl3) δ 8.40 (s, 1H), 7.66 (d, J = 7.0 Hz, 3H), 7.52 (d, J = 6.4 Hz, 3H), 7.45–7.40 (m, 1H), 7.37 (t, J = 7.3 Hz, 2H), 7.25–7.17 (m, 3H), 6.62 (d, J = 7.3 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 167.2 (d, 2JCF = 11.6 Hz), 149, 146.5, 138.7, 137, 135.1, 130.7, 129.5, 129.2, 128.9 (d, 1JCF = 17.5 Hz), 128.4 (d, 3JCF = 10.1 Hz), 117.4, 109.5, 109.1, 19F NMR (376 MHz, CDCl3) δ −114.2 (d, J = 6.4 Hz); IR (neat) νmax 3042, 1673, 1219 cm−1; HRMS (EI) calcd for C18H14FN3, [M]+ 291.1169, found 291.1172.(E)-6b yield 45% (55 mg), yellow solid; mp 108–109 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.04 (s, 1H), 7.64 (d, J = 6.9 Hz, 3H), 7.51 (t, J = 5.9 Hz, 3H), 7.46–7.41 (m, 2H), 7.38–7.33 (m, 2H), 6.90 (s, 1H), 6.81 (d, J = 3.4 Hz, 1H), 2.28 (s, 3H). 13C NMR (100 MHz, DMSO-d6) δ 169.9 (d, J = 11.5 Hz), 149.7, 147.1, 140.4, 139.8, 136.2, 132.7, 131.1, 130.6, 130.1, 130.0, 129.5, 128.4, 117.5, 117.0, 10.8 (d, J = 2.7 Hz). 19F NMR (376 MHz, DMSO-d6) δ −101.6 (d, J = 2.9 Hz). IR (neat) νmax 3059, 2927, 1670, 1424, 1202, 690 cm−1; HRMS (EI) calcd for C19H16FN3. [M + H]+ 306.1399, found 306.1401.
(Z)-6b yield 20% (25 mg), yellow solid. mp 103–105 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.26 (s, 1H), 7.62 (dd, J = 11.9, 6.9 Hz, 5H), 7.55–7.46 (m, 4H), 7.38–7.31 (m, 2H), 6.54 (d, J = 6.5 Hz, 1H), 2.24 (s, 3H). 13C NMR (100 MHz, DMSO-d6) δ 168.1 (d, J = 11.2 Hz), 139.5, 139.2, 137.9, 135.7, 132.0, 130.5, 130.1, 129.6 (d, J = 12.1 Hz), 129.4, 115.1, 110.7, 110.3, 14.5. 19F NMR (376 MHz, DMSO-d6) δ −110.8 (d, J = 7.0 Hz). IR (neat) νmax 3061, 2923, 1671, 1411, 1224, 693 cm−1; HRMS (EI) calcd for C19H16FN3. [M + H]+ 306.1399, found 306.1401.
(E)-6c yield 51% (75 mg), white solid; mp 108–111 °C; 1H NMR (400 MHz, DMSO-d6) δ 7.75 (s, 1H), 7.73–7.69 (m, 2H), 7.62–7.57 (m, 3H), 7.50–7.43 (m, 4H), 7.42–7.36 (m, 4H), 7.30 (s, 1H), 7.11 (dd, J = 6.3, 2.7 Hz, 2H), 6.70 (d, J = 2.0 Hz, 1H). 13C NMR (100 MHz, DMSO-d6) δ 170.2 (d, J = 11.2 Hz), 150.2, 149.0, 147.6, 139.5, 136.1, 132.7, 131.5, 131.1, 130.9, 130.6 (d, J = 16.5 Hz), 130.2, 130.1, 129.9, 129.7, 128.9, 125.1 (d, J = 3.6 Hz), 118.4, 118.0. 19F NMR (376 MHz, DMSO-d6) δ −98.8 (d, J = 1.1 Hz). IR (neat) νmax 3061, 1674, 1292, 1120, 694 cm−1; HRMS (EI) calcd for C24H18FN3. [M]+ 367.1487, found 367.1485.
(E)-6d yield 60% (81 mg), yellow solid; mp 124–128 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.99 (s, 1H), 8.57 (s, 1H), 7.65 (d, J = 5.0 Hz, 3H), 7.61 (d, J = 7.4 Hz, 2H), 7.55 (d, J = 7.0 Hz, 1H), 7.49 (t, J = 7.4 Hz, 2H), 7.43–7.34 (m, 2H), 6.82 (d, J = 5.2 Hz, 1H); 13C NMR (100 MHz, DMSO-d6) δ 171.3 (d, J = 11.3 Hz), 149.2, 148.3, 145.8, 139.7, 139.0, 136.0, 133.1, 131.4, 130.7, 130.2, 130.0, 121.7, 115.0 (d, J = 43.0 Hz). 19F NMR (376 MHz, DMSO-d6) δ −112.12 (d, J = 5.6 Hz). IR (neat) νmax 3061, 1675, 1547, 1268, 693 cm−1; HRMS (EI) calcd for C18H13FN4O2. [M + H]+ 337.1093, found 337.1095.
(E)-6e yield 61% (85 mg), yellow solid; mp 150–152 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.89 (s, 1H), 7.65 (dd, J = 5.0, 1.6 Hz, 3H), 7.56–7.49 (m, 3H), 7.48–7.42 (m, 2H), 7.38 (dd, J = 6.5, 2.9 Hz, 2H), 6.93 (d, J = 2.7 Hz, 1H), 2.50 (s, 3H). 13C NMR (100 MHz, DMSO-d6) δ 171.4 (d, J = 11.6 Hz), 147.7, 147.4, 147.3, 144.7, 139.0, 135.5, 132.7, 130.9, 130.1, 129.8 (d, J = 6.0 Hz), 129.4, 123.8 (d, J = 2.5 Hz), 119.1, 118.6, 14.5. 19F NMR (376 MHz, DMSO-d6) δ −104.0 (d, J = 3.0 Hz); IR (neat) νmax 3061, 2923, 1678, 1550, 1375, 1269, 695 cm−1; HRMS (EI) calcd for C19H15FN4O2. [M + H]+ 351.1249, found 351.1252.
(E)-6f yield 75% (95 mg), white solid; mp 100–101 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.81 (s, 1H), 8.63 (s, 1H), 7.65 (d, J = 5.3 Hz, 3H), 7.60 (d, J = 7.5 Hz, 2H), 7.58–7.53 (m, 1H), 7.49 (t, J = 7.4 Hz, 2H), 7.41–7.33 (m, 2H), 6.76 (d, J = 5.5 Hz, 1H). 13C NMR (100 MHz, DMSO-d6) δ 170.3, 170.2, 147.7, 144.9, 140.6, 139.0, 135.3, 132.3, 130.6, 129.8 (d, J = 17.2 Hz), 129.3 (t, J = 16.5 Hz), 115.5, 114.3, 113.6 (d, J = 44.0 Hz). 19F NMR (376 MHz, DMSO-d6) δ −111.5 (d, J = 5.3 Hz). IR (neat) νmax 3053, 2238, 1673, 1550, 1545, 1205, 694 cm−1; HRMS (EI) calcd for C19H13FN4 [M]+ 316.1127, found 316.1124.
(E/Z)-6g yield 73% (84 mg), yellow solid; mp 97–105 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.51 (s, 0.37H), 8.22 (s, 0.64H), 7.93 (s, 0.36H), 7.78 (s, 0.66H), 7.71–7.31 (m, 10H), 6.76 (d, J = 21.8 Hz, 0.63H), 6.68 (s, 0.37H), 6.64 (d, J = 4.7 Hz, 0.37H), 6.57 (s, 0.62H). 13C NMR (100 MHz, DMSO-d6) δ 168.1 (d, J = 11.5 Hz), 150.7, 148.2, 143.0, 139.0, 135.3, 134.6, 131.6, 130.1, 129.7, 129.2, 129.0, 111.7, 111.3, 108.4 (d, J = 1.8 Hz). 19F NMR (376 MHz, DMSO-d6) δ −109.9 (d, J = 3.6 Hz), δ −111.8 (d, J = 22.9 Hz); IR (neat) νmax 3052, 1653, 1438, 1384, 762, 687 cm−1; HRMS (EI) calcd for C18H14FN3. [M + H]+ 292.1241, found: 292.1245.
(E)-6h yield 46% (54 mg), yellow solid; mp 94–96 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.88 (s, 1H), 8.10 (s, 1H), 7.65 (d, J = 5.7 Hz, 3H), 7.54 (dd, J = 11.7, 7.4 Hz, 3H), 7.46 (t, J = 7.5 Hz, 2H), 7.41–7.34 (m, 2H), 6.87 (d, J = 4.4 Hz, 1H). 13C NMR (100 MHz, DMSO-d6) δ 170.2 (d, J = 11.5 Hz), 147.8, 145.2, 138.5, 134.9, 133.8, 131.9, 130.2, 129.4 (d, J = 17.5 Hz), 129.0, 128.8, 128.4, 114.8 (d, J = 43.8 Hz). 19F NMR (376 MHz, DMSO-d6) δ −110.7 (d, J = 4.9 Hz). IR (neat) νmax 3055, 1673, 1238, 1188 cm−1; HRMS (EI) calcd for C17H13FN4. [M]+ 292.1119, found 292.1124.
(Z)-6h yield 9% (10 mg), yellow solid; mp 148–152 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.65 (s, 1H), 7.96 (s, 1H), 7.69 (d, J = 7.5 Hz, 2H), 7.57 (dd, J = 20.3, 6.0 Hz, 4H), 7.48 (t, J = 7.4 Hz, 2H), 7.38–7.30 (m, 2H), 6.85 (d, J = 21.1 Hz, 1H). 13C NMR (100 MHz, DMSO-d6) δ 170.1 (d, J = 3.6 Hz), 146.44, 143.7, 138.7, 135.0, 134.5, 131.9, 130.2, 129.4 (d, J = 9.3 Hz), 128.9 (d, J = 19.2 Hz), 124.9, 112.2 (d, J = 7.7 Hz). 19F NMR (376 MHz, DMSO-d6) δ −107.9 (d, J = 21.4 Hz). IR (neat) νmax 3097, 1683, 1238 cm−1; HRMS (EI) calcd for C17H13FN4. [M]+ 292.1127, found 292.1136.
(E)-6i yield 43% (50 mg), yellow solid; mp 83–85 °C; 1H NMR (400 MHz, DMSO-d6) δ 9.25 (s, 1H), 8.43 (s, 1H), 7.65 (d, J = 6.1 Hz, 3H), 7.54 (t, J = 9.3 Hz, 3H), 7.46 (t, J = 7.4 Hz, 2H), 7.38 (d, J = 7.6 Hz, 2H), 6.85 (d, J = 3.8 Hz, 1H). 13C NMR (100 MHz, DMSO-d6) δ 170.5 (d, J = 11.5 Hz), 153.9, 149.0 (d, J = 2.0 Hz), 148.6, 146.0, 139.2, 135.6, 132.4, 130.8, 130.1, 129.8, 129.6, 129.4, 115.8, 115.4. 19F NMR (376 MHz, DMSO-d6) δ −112.2 (d, J = 3.6 Hz). IR (neat) νmax 3061, 1673, 1436, 1216 cm−1; HRMS (EI) calcd for C17H13FN4. [M]+ 292.1130, found 292.1124.
(Z)-6i yield 12% (14 mg), yellow solid; mp 116–119 °C; 1H NMR (400 MHz, DMSO-d6) δ 9.10 (s, 1H), 8.27 (s, 1H), 7.71 (d, J = 7.8 Hz, 2H), 7.62 (d, J = 5.4 Hz, 3H), 7.59–7.53 (m, 1H), 7.50 (t, J = 7.3 Hz, 2H), 7.35 (d, J = 7.3 Hz, 2H), 6.79 (d, J = 21.2 Hz, 1H). 13C NMR (100 MHz, DMSO-d6) δ 169.6 (d, J = 3.5 Hz), 154.2, 147.3, 144.9, 144.6, 139.2, 135.5, 132.1, 130.5, 129.9, 129.6, 129.3 (d, J = 19.1 Hz), 110.8 (d, J = 7.0 Hz). 19F NMR (376 MHz, DMSO-d6) δ −111.2 (d, J = 21.2 Hz). IR (neat) νmax 3061, 1682, 1280 cm−1; HRMS (EI) calcd for C17H13FN4. [M]+ 292.1121, found 292.1124.
(E)-6j yield 68% (97 mg), yellow solid; mp 108–111 °C; 1H NMR (400 MHz, CDCl3) δ 8.65 (s, 1H), 7.86 (d, J = 4.6 Hz, 1H), 7.61 (d, J = 7.7 Hz, 3H), 7.53 (d, J = 6.9 Hz, 3H), 7.43–7.29 (m, 5H), 7.25 (d, J = 5.9 Hz, 2H), 6.84 (d, J = 4.9 Hz, 1H). 13C NMR (100 MHz, CDCl3) δ 167.6 (d, 3JCF = 12.0 Hz), 149.7, 147.2, 144.4, 143.3, 138.5, 135.2, 132.5, 130.9, 130.1, 129.3, 128.9 (d, 3JCF = 6.7 Hz), 128.4, 124.4, 123.7, 120.6, 112.6 (d, 2JCF = 14.8 Hz), 112.3 (d, 1JCF = 26.7 Hz); 19F NMR (376 MHz, CDCl3) δ −111.1 (d, J = 4.0 Hz); IR (neat) νmax 3059, 1669, 1223 cm−1; HRMS (EI) calcd for C22H16FN3. [M]+ 341.1324, found 341.1328.
(Z)-6j yield 5% (7 mg), yellow solid; mp 118–120 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.54 (s, 1H), 7.78 (dd, J = 7.0, 1.6 Hz, 1H), 7.75–7.71 (m, 2H), 7.63–7.54 (m, 5H), 7.53–7.48 (m, 2H), 7.42–7.33 (m, 4H), 6.62 (d, J = 20.6 Hz, 1H); 13C NMR (100 MHz, DMSO-d6) δ 168.5 (d, J = 3.5 Hz), 143.5, 143.2, 138.9, 135.1, 131.6, 130.0, 129.29 (d, J = 15.4 Hz), 129.0 (d, J = 8.4 Hz), 124.9, 124.1, 120.7, 113.5 (d, J = 12.6 Hz), 111.6 (d, J = 2.2 Hz); 19F NMR (376 MHz, DMSO-d6) δ −99.0 (d, J = 21.4 Hz); IR (neat) νmax 3052, 1671, 1222 cm−1; HRMS (EI) calcd for C22H16FN3. [M]+ 341.1402, found 341.1401.
(E)-6k yield 81% (118 mg), white solid. mp 144–146 °C; 1H NMR (400 MHz, DMSO-d6) δ 7.74–7.63 (m, 4H), 7.51 (dd, J = 5.9, 3.2 Hz, 1H), 7.46 (t, J = 6.8 Hz, 1H), 7.42–7.33 (m, 8H), 7.00 (d, J = 1.5 Hz, 1H), 2.63 (s, 3H). 13C NMR (100 MHz, DMSO-d6) δ 170.6 (d, J = 11.7 Hz), 153.5 (d, J = 3.4 Hz), 148.9, 146.3, 143.9, 139.6, 136.3, 136.0, 132.9, 131.3, 130.6, 130.2, 129.7, 125.0, 124.8, 120.5, 119.6, 119.1, 112.4, 15.6. 19F NMR (376 MHz, DMSO-d6) δ −102.0 (d, J = 0.4 Hz). IR (neat) νmax 3058, 2963, 1674, 1269, 693 cm−1; HRMS (EI) calcd for C23H18FN3. [M + H]+ 356.1558, found 356.1558.
(E)-6l yield 45% (64 mg), yellow solid; mp 158–160 °C; 1H NMR (400 MHz, DMSO-d6) δ 7.65 (d, J = 7.1 Hz, 3H), 7.46 (t, J = 7.1 Hz, 3H), 7.42 (d, J = 7.4 Hz, 2H), 7.37 (t, J = 7.7 Hz, 2H), 7.28 (d, J = 7.7 Hz, 1H), 7.17 (d, J = 7.7 Hz, 1H), 7.12 (t, J = 7.6 Hz, 1H), 6.99 (t, J = 7.6 Hz, 1H), 6.90 (s, 1H), 13C NMR (100 MHz, DMSO-d6) δ 169.5 (d, J = 12.1 Hz), 155.2 (d, J = 3.4 Hz), 148.7, 146.1, 144.9, 139.8, 136.1, 135.0 (d, J = 4.5 Hz), 132.6, 131.0, 130.4, 129.9 (d, J = 10.2 Hz), 124.0, 120.9, 119.7, 119.2, 116.9, 110.5. 19F NMR (376 MHz, DMSO-d6) δ −103.3. IR (neat) νmax 3433, 3064, 1671, 1550, 1414, 1261, 691 cm−1; HRMS (EI) calcd for C22H17FN4. [M + H]+ 357.1506, found 357.1510.
(E)-6m yield 9% (14 mg), yellow solid; mp 170–172 °C; 1H NMR (400 MHz, DMSO-d6) δ 9.40 (s, 1H), 8.80 (s, 1H), 8.20 (d, J = 9.3 Hz, 1H), 7.94 (d, J = 9.2 Hz, 1H), 7.67 (d, J = 5.4 Hz, 3H), 7.61 (d, J = 7.5 Hz, 2H), 7.55 (t, J = 7.2 Hz, 1H), 7.50–7.41 (m, 4H), 6.98 (d, J = 5.4 Hz, 1H). 13C NMR (100 MHz, DMSO-d6) δ 170.9 (d, J = 11.1 Hz), 149.7, 148.0, 147.4, 147.2, 138.5, 135.0, 131.9 (s), 131.9, 130.4 (d, J = 11.5 Hz), 129.5 (s), 129.0 (d, J = 17.2 Hz), 124.3, 123.7 (d, J = 2.6 Hz), 116.7, 115.0, 114.9 (d, J = 43.5 Hz). 19F NMR (376 MHz, DMSO-d6) δ −112.3 (d, J = 5.5 Hz). IR (neat) νmax 3061, 1660, 1337, 1216 cm−1, 685 cm−1; HRMS (EI) calcd for C22H15FN4O2 [M]+ 386.1172, found 386.1179.
(Z)-6m yield 43% (66 mg), yellow solid; mp 156–158 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.68 (s, 1H), 8.55 (s, 1H), 8.17 (s, 2H), 7.75 (d, J = 7.2 Hz, 2H), 7.58 (ddd, J = 21.5, 12.9, 7.5 Hz, 6H), 7.42 (d, J = 6.6 Hz, 2H), 6.78 (d, J = 21.2 Hz, 1H). 13C NMR (100 MHz, DMSO-d6) δ 169.15 (d, J = 3.5 Hz, 1H), 149.4, 148.8, 146.7, 140.5, 139.9, 139.2, 136.4, 132.6, 131.1, 130.5, 130.1 (d, J = 12.4 Hz), 129.9, 129.4, 124.9, 119.0, 112.8 (d, J = 10.2 Hz), 109.1 (d, J = 6.1 Hz). 19F NMR (376 MHz, DMSO-d6) δ −106.6 (d, J = 22.0 Hz). IR (neat) νmax 3061, 1664, 1525, 1191, 691 cm−1; HRMS (EI) calcd for C22H15FN4O2 [M]+ 386.1174, found 386.1179.
(E)-6n yield 46% (59 mg), yellow solid; mp 108–110 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.28 (d, J = 8.3 Hz, 1H), 7.87 (d, J = 8.3 Hz, 1H), 7.75 (t, J = 7.6 Hz, 1H), 7.63 (dd, J = 14.0, 7.3 Hz, 4H), 7.45–7.38 (m, 3H), 7.28 (d, J = 4.0 Hz, 4H), 7.07 (d, J = 1.9 Hz, 1H). 13C NMR (100 MHz, DMSO-d6) δ 170.7 (d, J = 11.9 Hz), 147.4, 145.2, 144.8, 138.8, 135.3, 134.1, 132.3, 130.5 (d, J = 19.8 Hz), 129.9, 129.3 (d, J = 9.1 Hz), 126.3, 120.7, 118.3, 117.8, 112.6; 19F NMR (376 MHz, DMSO-d6) δ −105.87 (d, J = 1.6 Hz); IR (neat) νmax 3023, 1674, 1292, 1200 cm−1, 1024, 698 cm−1; HRMS (EI) calcd for C21H15FN4. [M + H]+ 343.1351, found 343.1353.
(Z)-6n yield 7% (10 mg), yellow solid; mp 114–117 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.23 (d, J = 8.4 Hz, 1H), 7.87 (d, J = 8.3 Hz, 1H), 7.76 (t, J = 9.8 Hz, 3H), 7.65–7.51 (m, 7H), 7.43 (d, J = 7.2 Hz, 2H), 6.88 (d, J = 20.9 Hz, 1H). 13C NMR (100 MHz, DMSO-d6) δ 170.9 (d, J = 3.7 Hz), 145.9, 139.3, 135.7, 132.9, 132.6, 130.9 (d, J = 20.0 Hz), 130.1 (d, J = 3.0 Hz), 129.7, 129.5, 126.8, 121.2, 115.3 (d, J = 10.0 Hz), 112.0 (d, J = 3.0 Hz). 19F NMR (376 MHz, DMSO-d6) δ −105.7 (d, J = 21.6 Hz). IR (neat) νmax 3055, 1677, 1291, 1200 cm−1; HRMS (EI) calcd for C17H13FN4. [M + H]+ 343.1352, found 343.1354.
Typical procedure for preparation of 7
Under nitrogen atmosphere, a solution of 1 (0.4 mmol), 5 (0.8 mmol) and K3PO4 (1.6 mmol) in THF (5 mL) was stirred at −78 °C for 30 min, then LDA (1.0 mmol, 0.67 mL, 1.5 mol L−1 in THF) was added dropwise. After stirring at −78 °C for 1 h, the reaction mixture was heated to 70 °C and stirred for further 16 h. After cooling to room temperature, the reaction mixture was quenched with H2O (10 mL), extracted with ethyl acetate, filtered and washed by ethyl acetate (15 mL × 3). After drying over anhydrous MgSO4 and concentration of the combined organic phases, the crude product was purified by flash chromatography (silica gel, CH2Cl2/MeOH 20/1).7a yield 81% (110 mg), yellow solid; mp 108–110 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.11 (s, 1H), 7.92 (s, 1H), 7.65–7.58 (m, 5H), 7.57–7.53 (m, 1H), 7.48 (t, J = 7.4 Hz, 2H), 7.45–7.41 (m, 2H), 7.34 (d, J = 13.1 Hz, 2H), 7.16 (s, 1H), 7.09 (s, 1H), 6.86 (s, 1H). 13C NMR (100 MHz, DMSO-d6) δ 170.3, 140.1, 139.2, 138.3, 135.6, 132.2, 130.9, 130.5, 130.1, 129.7, 129.5, 127.7, 124.0, 120.4 (d, J = 17.7 Hz). IR (neat) νmax 3061, 1644, 1478, 1301, 696 cm−1; HRMS (EI) calcd for C21H17N5 [M]+ 339.1480, found 339.1484.
7b yield 65% (74 mg), yellow solid; mp 132–134 °C; 1H NMR (400 MHz, DMSO-d6) δ 7.92 (s, 1H), 7.70–7.60 (m, 5H), 7.51 (d, J = 7.0 Hz, 3H), 7.43 (dd, J = 16.4, 7.2 Hz, 4H), 7.06 (s, 1H), 6.93 (s, 1H), 6.88 (s, 1H), 2.10 (s, 3H), 1.92 (s, 3H). 13C NMR (100 MHz, DMSO-d6) δ 170.0, 140.3, 139.8, 139.2, 136.6, 135.6, 132.1, 130.5, 129.5 (dd, J = 26.8, 14.8 Hz), 129.1, 127.7, 127.2, 125.8, 115.2, 14.3, 9.6. IR (neat) νmax 3096, 2920, 1643, 1483, 1431, 1286, 697 cm−1; HRMS (EI) calcd for C23H21N5 [M]+ 367.1792, found 367.1797.
7g yield 63% (83 mg), yellow sold; mp 130–132 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.38 (d, J = 1.8 Hz, 1H), 7.88 (s, 1H), 7.70 (s, 1H), 7.62 (t, J = 7.6 Hz, 3H), 7.58–7.49 (m, 4H), 7.43 (dd, J = 20.4, 7.3 Hz, 4H), 7.23 (s, 1H), 6.65 (s, 1H), 6.46 (s, 1H). 13C NMR (100 MHz, DMSO-d6) δ 169.2, 142.6 (d, J = 4.4 Hz), 139.3, 136.4, 136.0, 134.1, 131.9, 131.2, 130.3, 129.8, 129.7, 129.1, 122.5, 108.3, 107.4. IR (neat) νmax 3051, 1642, 1448, 1395, 751, 694 cm−1; HRMS (EI) calcd for C21H17N5 [M]+ 339.1488, found 339.1484.
7j yield 70% (122 mg), yellow solid; mp 186–188 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.86 (s, 1H), 8.64 (s, 1H), 7.83 (d, J = 8.0 Hz, 1H), 7.77 (d, J = 8.0 Hz, 1H), 7.67–7.51 (m, 8H), 7.45 (t, J = 7.5 Hz, 2H), 7.29 (td, J = 7.4, 4.0 Hz, 2H), 7.24 (s, 1H), 7.17 (dt, J = 18.5, 7.6 Hz, 2H), 6.91 (d, J = 8.1 Hz, 1H), 6.63 (d, J = 8.1 Hz, 1H). 13C NMR (101 MHz, DMSO-d6) δ 170.6, 146.5, 144.4, 144.0, 139.1, 135.7, 133.6 (d, J = 3.0 Hz), 132.2, 130.5, 129.6 (dd, J = 26.9, 8.3 Hz), 127.1, 126.0, 125.0, 124.9 (d, J = 26.4 Hz), 124.2, 123.9, 121.1 (d, J = 17.3 Hz), 111.5 (d, J = 18.3 Hz). IR (neat) νmax 3064, 1641, 1491, 1450, 740, 696 cm−1; HRMS (EI) calcd for C29H21N5 [M]+ 439.1801, found 439.1797.
Acknowledgements
We thank the Innovation Program of Shanghai Municipal Education Commission (No. 13ZZ047) and the Fundamental Research Funds for the Central Universities (2232015D3-13) for financial support.References
- (a) T. Hiyama, Organofluorine Compounds; Chemistry and Applications, Springer, New York, 2000; and references therein Search PubMed; (b) B. E. Smart, J. Fluorine Chem., 2001, 109, 3–11 CrossRef CAS; (c) A. M. Thayer, Chem. Eng. News, 2006, 84, 15–24 CrossRef; (d) P. Kirsch, Modern Fluoroorganic Chemistry, Wiley-VCH, Weinheim, 2004 Search PubMed; (e) J.-P. Bégué and D. Bonnet-Delpon, J. Fluorine Chem., 2006, 127, 992–1012 CrossRef; (f) K. Müller, C. Faeh and F. Diederich, Science, 2007, 317, 1881–1886 CrossRef PubMed; (g) S. Purser, P. R. Moore, S. Swallow and V. Gouverneur, Chem. Soc. Rev., 2008, 37, 320–330 RSC; (h) Y. Zhou, J. Wang, Z. Gu, S. Wang, W. Zhu, J. L. Aceña, V. A. Soloshonok, K. Izawa and H. Liu, Chem. Rev., 2016, 116, 422–518 CrossRef CAS PubMed.
- For recent reviews in this area, see: (a) M. F. Kuehnel, D. Lentz and T. Braun, Angew. Chem., Int. Ed., 2013, 52, 3328–3348 CrossRef PubMed; (b) G. K. Surya Prakash and J. Hu, Acc. Chem. Res., 2007, 40, 921–930 CrossRef PubMed; (c) M. C. Pacheco, S. Purser and V. Gouverneur, Chem. Rev., 2008, 108, 1943–1981 CrossRef CAS PubMed; (d) V. Gouverneur and K. Seppelt, Chem. Rev., 2015, 115, 563–565 CrossRef CAS PubMed; (e) P. A. Champagne, J. Desroches, J.-D. Hamel, M. Vandamme and J.-F. Paquin, Chem. Rev., 2015, 115, 9073–9174 CrossRef CAS PubMed.
- (a) M. F. Kuehnel, P. Holstein, M. Kliche, J. Krueger, S. Matthies, D. Nitsch, J. Schutt, M. Sparenberg and D. Lentz, Chem.–Eur. J., 2012, 18, 10701–10714 CrossRef CAS PubMed; (b) G. Chelucci, Chem. Rev., 2012, 112, 1344–1462 CrossRef CAS PubMed; (c) Y. Liu, M. Deng, Z. Zhang, X. Ding, Z. Dai and J. Guan, Chin. J. Inorg. Chem., 2012, 32, 661–666 CrossRef CAS; (d) Y. Takahira and Y. Morizawa, J. Am. Chem. Soc., 2015, 137, 7031–7034 CrossRef CAS PubMed.
- (a) C. Lim, C. A. Wesolowski and D. J. Burton, J. Fluorine Chem., 2014, 159, 21–28 CrossRef CAS; (b) M. Hu, C. Ni, L. Li, Y. Han and J. Hu, J. Am. Chem. Soc., 2015, 137, 14496–14501 CrossRef CAS PubMed; (c) S. Chen, C. Xu, L. Lu and Q. Shen, Chin. J. Chem., 2013, 31, 901–907 CrossRef CAS; (d) C. S. Thomoson, H. Martinez and W. R. Dolbier, J. Fluorine Chem., 2013, 150, 53–59 CrossRef CAS; (e) G. K. S. Prakash, A. Shakhmin, M. Zibinsky, I. Ledneczki, S. Chacko and G. A. Olah, J. Fluorine Chem., 2010, 131, 1192–1197 CrossRef CAS; (f) K. Sano, T. Fukuhara and S. Hara, J. Fluorine Chem., 2009, 130, 708–713 CrossRef CAS; (g) Q. Shao and Y. Huang, Chem. Commun., 2015, 51, 6584–6586 RSC.
- (a) P. A. Bartlett and A. Otake, J. Org. Chem., 1995, 60, 3107–3111 CrossRef CAS; (b) T. Allmendinger, E. Felder and E. Hungerbuhler, Tetrahedron Lett., 1990, 31, 7301–7304 CrossRef CAS; (c) T. Allmendinger, P. Furet and E. Hungerbuhler, Tetrahedron Lett., 1990, 31, 7297–7300 CrossRef CAS; (d) J. T. Welch and J. Lin, Tetrahedron, 1996, 52, 291–304 CrossRef CAS; (e) E. Villiers, S. Couve-Bonnaire, D. Cahard and X. Pannecoucke, Tetrahedron, 2015, 71, 7054–7062 CrossRef CAS; (f) S. Borkar and A. Sen, Macromolecules, 2005, 38, 3029–3032 CrossRef CAS; (g) K. L. Berry and J. H. Peterson, J. Am. Chem. Soc., 1951, 73, 5195–5197 CrossRef CAS.
- (a) J. D. Moody, D. Van Derveer, D. W. Smith Jr and S. T. Iacono, Org. Biomol. Chem., 2011, 9, 4842–4849 RSC; (b) G. Jin, J. Zhang, W. Wu and S. Cao, J. Fluorine Chem., 2014, 168, 240–246 CrossRef CAS; (c) J. Yang, A. Mao, Z. Yue, W. Zhu, X. Luo, C. Zhu, Y. Xiao and J. Zhang, Chem. Commun., 2015, 51, 8326–8329 RSC; (d) N. Suzuki, T. Fujita and J. Ichikawa, Org. Lett., 2015, 17, 4984–4987 CrossRef CAS PubMed; (e) X. Zhang, Y. Lin, J. Zhang and S. Cao, RSC Adv., 2015, 5, 7905–7908 RSC; (f) X. Zhang, W. Dai, W. Wu and S. Cao, Org. Lett., 2015, 17, 2708–2711 CrossRef CAS PubMed; (g) V. V. Rudyuk, D. V. Fedyuk and L. M. Yagupolskii, J. Fluorine Chem., 2004, 125, 1465–1471 CrossRef CAS; (h) C. Liu, E. Shi, F. Xu, Q. Luo, H. Wang, J. Chen and X. Wan, Chem. Commun., 2015, 51, 1214–1217 RSC; (i) Y. Xiong, X. Zhang, T. Huang and S. Cao, J. Org. Chem., 2014, 79, 6395–6402 CrossRef CAS PubMed; (j) J. Ichikawa, M. Fujiwara, Y. Wada, T. Okauchi and T. Minami, Chem. Commun., 2000, 1887–1888 RSC; (k) H. Koroniak, J. Walkowiak, K. Grys, A. Rajchel, A. Alty and R. Du Boisson, J. Fluorine Chem., 2006, 127, 1245–1251 CrossRef CAS; (l) K. Fuchibe, H. Jyono, M. Fujiwara, T. Kudo, M. Yokota and J. Ichikawa, Chem.–Eur. J., 2011, 17, 12175–12185 CrossRef CAS PubMed; (m) J. Ichikawa, M. Yokota, T. Kudo and S. Umezaki, Angew. Chem., Int. Ed., 2008, 47, 4870–4873 CrossRef CAS PubMed; (n) X.-J. Yang and J.-T. Liu, Chin. J. Chem., 2006, 24, 1418–1420 CrossRef CAS; (o) C. M. Timperley, M. J. Waters and J. A. Greenall, J. Fluorine Chem., 2006, 127, 249–256 CrossRef CAS.
- (a) W. Dai, J. Xiao, G. Jin, J. Wu and S. Cao, J. Org. Chem., 2014, 79, 10537–10546 CrossRef CAS PubMed; (b) H. Saijo, H. Sakaguchi, M. Ohashi and S. Ogoshi, Organometallics, 2014, 33, 3669–3672 CrossRef CAS; (c) W. Xu, H. Sun, Z. Xiong and X. Li, Organometallics, 2013, 32, 7122–7132 CrossRef CAS; (d) M. Ohashi, H. Saijo, M. Shibata and S. Ogoshi, Eur. J. Org. Chem., 2013, 2013, 443–447 CrossRef CAS; (e) M. E. Slaney, M. J. Ferguson, R. McDonald and M. Cowie, Organometallics, 2012, 31, 1384–1396 CrossRef CAS; (f) B. M. Kraft, E. Clot, O. Eisenstein, W. W. Brennessel and W. D. Jones, J. Fluorine Chem., 2010, 131, 1122–1132 CrossRef CAS; (g) G. Landelle, P. A. Champagne, X. Barbeau and J.-F. Paquin, Org. Lett., 2009, 11, 681–684 CrossRef CAS PubMed; (h) S. Yamada, T. Takahashi, T. Konno and T. Ishihara, Chem. Commun., 2007, 3679–3681 RSC; (i) J. Ichikawa, K. Sakoda, J. Mihara and N. Ito, J. Fluorine Chem., 2006, 127, 489–504 CrossRef CAS.
- T. Billard and B. R. Langlois, J. Org. Chem., 2002, 67, 997–1000 CrossRef CAS PubMed.
- (a) F.-S. He, J.-H. Jin, Z.-T. Yang, X. Yu, J. S. Fossey and W.-P. Deng, ACS Catal., 2016, 6, 652–656 CrossRef CAS; (b) S. Lou, G. M. McKenna, S. A. Tymonko, A. Ramirez, T. Benkovics, D. A. Conlon and F. Gonzalez-Bobes, Org. Lett., 2015, 17, 5000–5003 CrossRef CAS PubMed; (c) F. Xu, M. J. Zacuto, Y. Kohmura, J. Rosen, A. Gibb, M. Alam, J. Scott and D. Tschaen, Org. Lett., 2014, 16, 5422–5425 CrossRef CAS PubMed; (d) S. Alazet, L. Zimmer and T. Billard, Chem.–Eur. J., 2014, 20, 8589–8593 CrossRef CAS PubMed; (e) Y.-H. Shi, Z. Wang, B. Hu, M. Wang, J. S. Fossey and W.-P. Deng, Org. Lett., 2011, 13, 6010–6013 CrossRef CAS PubMed; (f) Q. Li, C.-H. Ding, X.-L. Hou and L.-X. Dai, Org. Lett., 2010, 12, 1080–1083 CrossRef CAS PubMed; (g) L. Kiss, S. Mangelinckx, R. Sillanpaeae, F. Fueloep and N. De Kimpe, J. Org. Chem., 2007, 72, 7199–7206 CrossRef CAS PubMed; (h) F. A. Davis, Y. Zhang and H. Qiu, Org. Lett., 2007, 9, 833–836 CrossRef CAS PubMed.
- D. Baba and T. Fuchigami, Tetrahedron Lett., 2003, 44, 3133–3136 CrossRef CAS.
- (a) H. Wójtowicz-Rajchel, H. Koroniak and A. Katrusiak, Eur. J. Org. Chem., 2008, 368–376 CrossRef; (b) W. R. Dolbier Jr and X. X. Rong, J. Org. Chem., 1997, 62, 1576–1577 CrossRef; (c) X. Li, H. Pan and X. Jiang, Tetrahedron Lett., 1987, 28, 3699–3702 CrossRef CAS.
- T. Billard, B. R. Langlois and G. Blond, Tetrahedron Lett., 2000, 41, 8777–8780 CrossRef CAS.
- ESI.†.
- W. R. Dolbier, Guide to Fluorine NMR for Organic Chemists, John Wiley & Sons, Inc., Hoboken, New Jersy, USA, 2009, p. 20 Search PubMed.
- J. Zhang, C. Xu, W. Wu and S. Cao, Chem.–Eur. J., 2016, 22, 9902–9908 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1455899. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6ra12187j |
| This journal is © The Royal Society of Chemistry 2016 |