A chemically modified graphene oxide wrapped porous hematite nano-architecture as a high rate lithium-ion battery anode material†
Chandrasekar M. Subramaniyam‡
,
Md. Monirul Islam‡,
Taslima Akhter,
Dean Cardillo,
Konstantin Konstantinov*,
Hua Kun Liu* and
Shi Xue Dou
Institute for Superconducting and Electronic Materials (ISEM), Australian Institute for Innovative Materials (AIIM) Facility, Innovation Campus, University of Wollongong, North Wollongong, NSW 2500, Australia. E-mail: konstan@uow.edu.au; hua@uow.edu.au; Fax: +61 2 4221 5731; Tel: +61 2 4221 5765
First published on 25th August 2016
Abstract
Successful fabrication of nanoporous metal oxides with carbonaceous nanomaterials can enhance the conductivity of electrodes as well as advance their electrochemical activity to overcome the stress induced during continuous charge–discharge cycling, and this is an effective way to harness their excellent reversible theoretical capacity. Nanoporous hematite (α-Fe2O3) nanorods have been prepared through an advanced spray precipitation method and nanofabricated with reduced graphene oxide (rGO) sheets by simply mixing solutions. This approach helps to introduce a continuous conductive network in between the nanorods to enhance ion interactions, giving the composite a promising electrochemical response as a negative electrode for the lithium-ion battery (LIB). The nanocomposites delivered an outstanding reversible capacity of 1330 mA h g−1 at 100 mA g−1 for 100 cycles and showed excellent rate retention during cycling at different current densities over long cycle numbers, highlighting the potential of this material with its specially designed nano-architecture as an anode material for high energy LIBs for electric vehicles. Along with the overwhelming electrochemical performance of chemically modified graphene-oxide-wrapped hematite porous nanorods (α-Fe2O3/rGO), the abundance of the hematite source, and the advanced and environmentally friendly synthesis approach show the potential for large-scale preparation of such electrode materials for real world application.
Introduction
There is an overwhelming quest to fabricate the best electrochemical energy storage devices to harness available renewable energy (from solar, hydro, tidal, wind, etc.) as (1) an alternative to fast depleting fossil fuels to build plug-in hybrid electric vehicles1–3 and (2) for long term energy storage with minimal dissipation as a replacement for power grids.4 Ever since commercialized in the 1990s, lithium-ion batteries (LIBs) have found widespread applications in modern portable electronic devices and are still a prime subject of research for materials scientists, as they deliver high power and energy densities, long cycle life, and good safety as compared to supercapacitors and fuel cells.5–9 This has boosted the thirst of researchers to hunt for high performing electrode materials that could store energy efficiently. The discovery of the “conversion reaction” by Tarascon and co-workers10 raised the possibility of using non-layered transition metal oxides, nitrides, fluorides, sulphides, phosphides, and even hydrides11–25 as high performing negative electrodes to replace the commercialized layered graphite. Iron oxide is considered the best conversion reaction electrode due to its high theoretical capacity (1007 mA h g−1), natural abundance, and environmental friendliness.10–15,26–41 In a typical conversion reaction, lithium (Li) reacts with metal oxide to form a polymeric Li2O matrix surrounded by Fe nanoparticles, which, in turn, take part in the reverse reaction when the polarity changes.15,42 This reaction results in fast capacity fade, however, due to the stress induced by accommodating the volume change during cycling and the sluggish reaction kinetics upon charge transfer, while the intrinsic structural changes could damage the electrode when it is cycled at high current densities.42,43 Enormous efforts were made in past decades to circumvent these disadvantages by tuning the iron oxide morphology (such as with one-, two-, and three-dimensional (1D, 2D, and 3D) nanostructures) and particle size, but even so, fabricating a durable iron oxide electrode exhibiting superior reversible energy and power densities remains a great challenge.27–29The use of blended nanostructures, wherein nanostructured active electrode materials are chemically or non-covalently bonded to conductive materials, have proved to be an effective method of achieving high performing electrode materials for LIBs by improving their electrical conductivity and electron transfer. In such hybrid nanocomposites, the contribution due to the strong synergetic effects from integrating the various components could lead to outstanding overall electrochemical performance. Such an approach has shown practical progress in recent decades, such as with α-Fe2O3/reduced graphene oxide (rGO),13,15,37 carbon coated α-Fe2O3/rGO,35,36 α-Fe2O3/carbon nanotube (CNT),15,32,33 carbon coated α-Fe2O3,31,35,36 and α-Fe2O3/graphene.34,36,37,39–41
The uniqueness of these nanostructured composites and their electrochemical performance have made them the object of much research, and they have been reported to be synthesized via electro-spinning, hydrothermal, solvothermal, microwave assisted hydrothermal, chemical vapour deposition, and sol–gel techniques.14,27,28,35–41,44 Moreover, these methods employ surfactants to produce different hierarchical nanostructured morphology, but process scaling poses a greater challenge. The spray precipitation technique, however, possesses several advantages over the above conventional methods, such as (1) requiring less time to produce large amounts of uniform nanoparticles economically without post annealing; (2) avoiding the use of surfactants for precise particle size control by its ability to produce atomized droplet sizes in the range of 20–100 nm, thereby leading to improvement of the magnetic and other physical properties of the nanomaterials.45,46 Nevertheless, an effective method for the preparation of nanoporous α-Fe2O3 nanorods for LIB application by a simple spray precipitation process has been rarely reported.
In our present report, we have explored the nanofabrication of graphitic carbon connected to porous hematite (α-Fe2O3) nanorods. An advanced room temperature spray precipitation method has been utilized to prepare highly porous hematite structures, and ultra-large graphene oxide (GO) nanosheets have been inserted into the composition by low temperature aqueous dispersion to form conductive connections among the nanorods. In situ deoxygenation of the GO content by using ascorbic acid enables the composite to offer a three dimensionally (3D) interconnected conductive network for excellent lithium ion (Li+) penetration throughout the whole surface of the active electrode material. Also, the porosity and surface area created by the nanofabrication, along with the porous structure of the hematite nanorods, decrease the diffusion length to the nanoscale, enhance electrolyte impregnation, and enable this composite to act as a buffer to accommodate stress induced during charge–discharge cycling, which are the major highlights of this nano-architectured electrode material.
Results and discussion
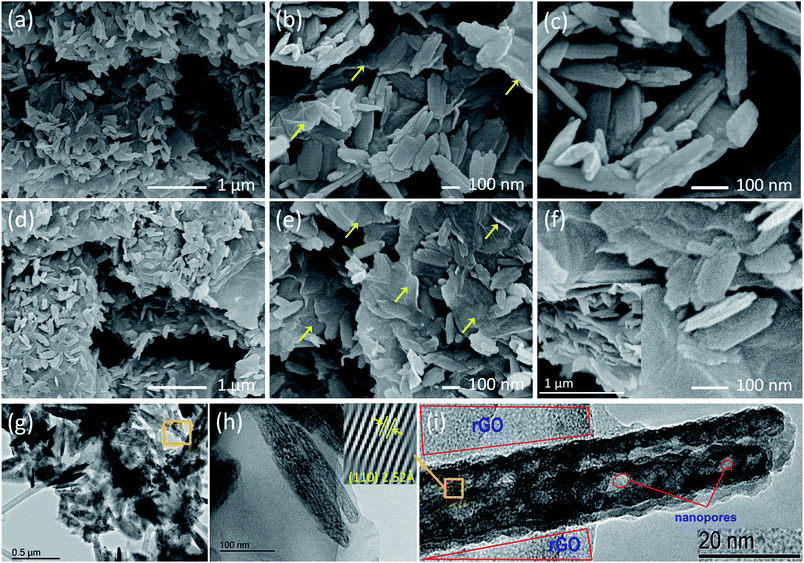
A schematic illustration of the fabrication of nanoporous α-Fe2O3 nanorods is presented in Fig. 1, and the synthesis procedure is explained in the Experimental section. Spraying the Fe3+ precursor into the NaOH solution resulted in formation of an intermediate precursor containing goethite (α-FeO(OH)) nanoparticles. Annealing at 400 °C for 4 h resulted in the formation of nanoporous α-Fe2O3 nanorods. The nanorod morphology may be due to the formation of highly crystalline precursor containing spindle-shaped nanoparticles, which then aggregated to form nanoporous α-Fe2O3 nanorods upon annealing.45–52The individual hematite nanorods contain several nanopores on their structure, which could facilitate uniform and stable dispersion in aqueous medium upon sonication.47 As-prepared graphene oxide (GO), being highly dispersible in water without sonication which helps to maintain its ultra-large sheet size and able to form composites with various nanomaterials easily.48 The addition of this GO to the nanorod dispersion under stirring leads to a homogeneous aqueous dispersion of α-Fe2O3/GO composite.47 Under low temperature stirring (Fig. 1a), the aqueous medium is slowly evaporated, and the van der Waals interaction of the two different materials drives the ultra-large GO sheets to wrap the nanorods as a shell architecture prior to self-agglomeration.51 The addition of ascorbic acid helps to deoxygenate the functional oxygen groups on the GO surface at low temperature to convert the GO content to reduced graphene oxide (rGO), leading to α-Fe2O3/rGO composite.48 In our proposed structure (Fig. 1b), the rGO sheets not only wrap the nanorods to create hollow spaces, but also constitute a continuous network among the nanorods, which helps the composite to benefit from a conductive carbon network in between the porous hematite nanorods (Fig. 1c). Different amounts such as 10 wt% and 30 wt% of GO content along with the respective wt% of hematite nanorods were used in the dispersion to prepare nanostructures with different connectivity of rGO among the hematite nanorods.
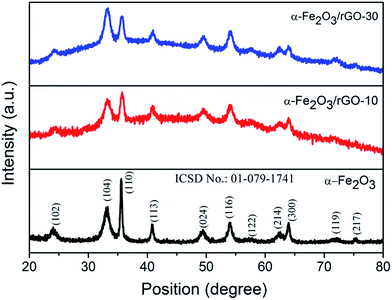
The crystallographic structure and phase purity of the pure α-Fe2O3 nanorods and the α-Fe2O3/rGO composites are presented in Fig. 2. All the diffraction peaks of pure α-Fe2O3 could be assigned to the rhombohedral crystal structure with R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) c space group (ICSD No. 01-079-1741). It could be ascertained that the α-Fe2O3 nanorods were preferentially grown along the [110] axis, which may be due to the controlled velocity of the droplets coming out of the spray nozzle.45 Apparently, no peaks of rGO were identified in either of the α-Fe2O3/rGO nanocomposites, indicating that the α-Fe2O3 nanorods were efficiently distributed over the surface of the rGO and suppressed stacking of layers,13–15 as is evident from the FEGSEM images.
c space group (ICSD No. 01-079-1741). It could be ascertained that the α-Fe2O3 nanorods were preferentially grown along the [110] axis, which may be due to the controlled velocity of the droplets coming out of the spray nozzle.45 Apparently, no peaks of rGO were identified in either of the α-Fe2O3/rGO nanocomposites, indicating that the α-Fe2O3 nanorods were efficiently distributed over the surface of the rGO and suppressed stacking of layers,13–15 as is evident from the FEGSEM images.
The top view FEGSEM images in Fig. 3a and b reveals that the nanocomposites are composed of a uniform distribution of α-Fe2O3 nanorods over rGO layers. The quantity of α-Fe2O3 nanorods varies with the rGO composition. This is well supported by TEM (Fig. 3c), which shows α-Fe2O3 nanorods spread across each layer of rGO without layer stacking. Also, the nanorods feature preferential growth along the [110] axis, as established by XRD data. Nanopores ∼2–5 nm in size are well distributed over the α-Fe2O3 nanorods, as is evident from the high resolution TEM (HRTEM) image. Therefore, this nanostructure represents an integration of several highlighted features which shorten the Li+ diffusion length, since nanorods that are both preferentially orientated along the [110] planes and nanoporous could facilitate easy impregnation with electrolyte and accommodate the stress due to volume changes induced during the charge–discharge process.15–30
Thermogravimetric analysis (TGA) of the composites were carried out and compared with bare hematite nanorods as well as rGO to provide the evidence of reduced graphene oxide and hematite nanorods contents in the final composites. Fig. S1 in ESI† represents the thermal stability of the materials in air. Having the complete degradation of rGO in air at 1000 °C and 3.6 wt% degradation of hematite nanorods at similar condition the α-Fe2O3/rGO-10 shows stability of 88.7 wt%, whereas the α-Fe2O3/rGO-30 composite remain only 67.9 wt%. These results clearly reflect the content of hematite nanorods of 10 wt% and 30 wt% in the α-Fe2O3/rGO-10 and α-Fe2O3/rGO-30 respectively as mentioned in the nanodecoration approach. The Raman spectra in Fig. 4a demonstrate the presence of chemically reduced GO in the α-Fe2O3/rGO nanocomposite. The peaks at 294, 410, and 608 cm−1 indicate the presence of α-Fe2O3 nanorods in the nanocomposites, while two Raman peaks from the hexagonal carbon plane at 1327 cm−1 and 1600 cm−1 could correspond to the D band and the G band of the reduced graphene structure.47 The intensity ratio of the D to the G peaks (ID/IG) was calculated to be 1.13, indicating the change in the carbon structure from GO to rGO as an effect of deoxygenation/reduction on the planar GO surface due to the ascorbic acid.48 Along with Raman analysis, the compositional analysis of the α-Fe2O3/rGO composite is revealed by the XPS analysis, as presented in Fig. 4b–d. The survey spectra (Fig. 4b) show the presence of three key components, C, O, and Fe, in the final composite. The presence of the elements C and Fe confirms the successful composition of hematite nanorods entrapped by the rGO sheets as a continuous network. The high resolution XPS C 1s spectrum of α-Fe2O3/rGO-10 (Fig. 4c) was fitted with three sub-peaks, suggesting the presence of three types of carbon. The peaks at 284.4, 285.5, and 287.5 eV were assigned to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, C–C, and C–O/C
C, C–C, and C–O/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O/O–C
O/O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O.45,50 The high resolution Fe 2p spectrum of α-Fe2O3/rGO-10 (Fig. 4d) nanocomposite contains two distinct peaks at 710.7 and 724.4 eV, conforming the presence of Fe 2p3/2 and Fe 2p1/2, and these can be used to qualitatively determine the ionic state of iron.45,46 Moreover, a satellite peak at 717.5 eV (Fig. 4d) is characteristic of the Fe3+ ions in the nanocomposite.45
O.45,50 The high resolution Fe 2p spectrum of α-Fe2O3/rGO-10 (Fig. 4d) nanocomposite contains two distinct peaks at 710.7 and 724.4 eV, conforming the presence of Fe 2p3/2 and Fe 2p1/2, and these can be used to qualitatively determine the ionic state of iron.45,46 Moreover, a satellite peak at 717.5 eV (Fig. 4d) is characteristic of the Fe3+ ions in the nanocomposite.45
Following, α-Fe2O3/rGO composites were tested as anode materials against Li+/Li0 between 0.02 and 3 V at specific constant current density (mA g−1). Fig. 5a presents discharge–charge curves of rGO, α-Fe2O3, and the α-Fe2O3/rGO-10 and α-Fe2O3/rGO-30 composites at a moderate current density of 100 mA g−1 within a cut-off potential window of 0.02–3.0 V. The initial specific discharge and charge capacities were respectively found to be 285, 139 mA h g−1 (rGO); 1234, 956 mA h g−1 (α-Fe2O3); 1527, 1120 mA h g−1 (α-Fe2O3/rGO-10); and 1198, 974 mA h g−1 (α-Fe2O3/rGO-30). Since the specific capacity of pure rGO is negligible (due to its low theoretical capacity) as compared to the α-Fe2O3/rGO composites, the performance was calculated based on the active mass of α-Fe2O3 in each respective nanocomposite. The first irreversible capacity loss may be attributed to the initial irreversible formation of Li2O and other irreversible processes involving lithium retained in the crystal lattice, the formation of solid electrolyte interphase (SEI), and electrolyte decomposition at low potential, which are the most common for nanostructured anode materials.12–15 From the second cycle onwards, however, the nanocomposites exhibited stable specific capacity with 98–99% coulombic efficiency. The performance of α-Fe2O3 deteriorated with increasing cycle number, while the composites with α-Fe2O3 wrapped in rGO exhibited stable and superior specific discharge capacities. When cycled at 100 mA g−1, α-Fe2O3 delivered only discharge capacity of 562 mA h g−1 up to 100 cycles, while the α-Fe2O3/rGO-10 and α-Fe2O3/rGO-30 nanocomposites exhibited 1320 mA h g−1 and 970 mA h g−1, respectively, as plotted in Fig. 5b. Also, the performance of the latter composite decreases as compared to former composite, which may be caused by stacking of rGO (inset of Fig. 3e) with random distribution of α-Fe2O3, which resulted in this erratic behaviour.
For further optimization, the high rate capability is also of the greatest importance, particularly for high power applications. When subjected to rate capability testing at various current densities of 0.1, 0.2, 0.4, 0.8, 1.0, 1.5, 3.0, 4, and 6 A g−1, the α-Fe2O3/rGO-10 composite exhibited reversible capacity of 1338, 1269, 1215, 1147, 970, 700, 558, 500, and 425 mA h g−1, respectively. Such a remarkable high rate performance is superior to those of most reported α-Fe2O3 based electrode materials, as tabulated in Table 1. Even after cycling at the high current density of 6 A g−1, a reversible capacity of 1380 mA h g−1 could be restored upon cycling at 100 mA g−1 after 100 cycles, as shown in Fig. 5c. Also, to test the long cycle life stability of α-Fe2O3/rGO-10, the composite was subjected to electrochemical testing at high current densities of 1–2 A g−1. The composite delivered a high reversible discharge capacity of 1100 and 844 mA h g−1 for 100 cycles at 1 and 2 A g−1, respectively (Fig. 5d). Also, it exhibited capacity of 445 mA h g−1 at 4 A g−1 over a long run for 200 cycles (Fig. 5e). Such high performance for long cycling at high current densities has been rarely reported. This overwhelming performance benefitted from the unique hierarchical structure and the presence of rGO. α-Fe2O3/rGO-10 composite exhibited an excellent cycling response to continuously varying current densities, even though α-Fe2O3 electrodes suffer from sluggish kinetics. In this paper, we claim that nanoporous α-Fe2O3 nanorods prepared by the practically scalable spray precipitation technique have superior electrochemical performance as negative electrode for lithium ion battery applications.
| Morphology/(wt%) carbonaceous materials | Synthesis method | Potential (V vs. Li+/Li0) | Current rate (mA g−1) | Initial capacity (mA h g−1) | Capacity retention (mA h g−1)/(cycles) | Rate test current rate (mA g−1), (cycle)/capacity (mA h g−1) | Ref. |
|---|---|---|---|---|---|---|---|
| a Note: the table compares the present work with the existing literature: (1) highest first discharge capacity except for ref. 39 and 57; (2) superior long-term cycling stability at 50–100 mA g−1 compared to ref. 14, 27, 35, 38, 40, 54, 56 and 57; (3) excellent rate capability with 10 wt% rGO composite compared to ref. 14, 34, 35, 37–40, 53–57; and (4) the present nanoporous α-Fe2O3 nanorods were synthesized by scalable spray precipitation technique as compared to others mostly synthesized by hydro/solvothermal processes. | |||||||
| Nanoporous α-Fe2O3 nanorods/(10 wt%) rGO | Spray precipitation and solution mixing | 0.02–3 | 100 | 1527 | 1320 (100) | 6000, (10)/425 | Present work |
| α-Fe2O3/(44.2 wt%) rGO | Hydrothermal | 0.01–3 | 500 | ∼1080 | ∼700 (300) | 2000, (5)/∼600 | 14 |
| α-Fe2O3 wrapped by (15 wt%) few layered graphene nanosheets | Dielectric barrier discharge plasma (DBDP) assisted milling | 0.1–3 | 200 | 916 | 758 (300) | 5000, (5)/295 | 34 |
| α-Fe2O3/(20%) rGO | Solvothermal/hydrothermal | 0.01–3 | 100 | 1089.2 | 1787.27 (90) | 1600, (5)/393.75 | 37 |
| α-Fe2O3/(37%) rGO | Hydrothermal | 0.005–3 | 100 | ∼680 | ∼600 (500) | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, (300)/225 000, (300)/225 |
38 |
| α-Fe2O3 nanomesh/(90%) graphene | Chemical vapour deposition | 0.01–3 | 50 | ∼(>6000) | 1692 (50) | 1000, (5)/∼555 | 39 |
| α-Fe2O3 particles enwrapped by 30 wt% graphene | Hydrothermal | 0.001–3 | 50 | 1561 | 1094 (50) | 1000, (10)/572 | 40 |
| α-Fe2O3/(17.1 wt%) graphene | Hydrothermal | 0.05–3 | 200 | 1268 | ∼900 (100) | 2000, (5)/634 | 53 |
| α-Fe2O3/(39.2 wt%) graphene composite | Chemical modified method | 0.005–3 | 100–1000 | 1336 | 806 (60) | 2000, (5)/620 | 54 |
| 289 (60) | |||||||
| α-Fe2O3 nanoparticles over graphene | Hydrothermal | 0.01–3 | 50 | 1369 | 559 (50) | 300, (10)/300 | 55 |
| Hollow α-Fe2O3 spheres constructed 22 wt% graphene | Hydrothermal | 3 | 100 | 1353 | 950 (50) | 1000, (10)/∼700 | 56 |
| Fe2O3@SnO2 nanoparticle decorated (35.5 wt%) graphene flexible films | Hydrothermal | 0.01–3 | 100 | 1620 | 1015 (200) | 2000, (5)/∼535 | 57 |
To provide insight into the electrochemistry of α-Fe2O3 and α-Fe2O3/rGO composite during the discharge–charge process, cyclic voltammetry was performed at 0.1 mV s−1 scan rate between 0.020 and 3.0 V, as shown in Fig. 6a. Upon discharge from open circuit potential (OCP), a peak at 1.63 V may be due to the intercalation of lithium into α-Fe2O3 to form LixFe2O3 without any change in the crystal lattice. On further reduction to lower potential, a distinct peak at 0.57 V represents the formation of the intermediate Li2Fe2O3, which then decomposes to form Fe(0) nanoparticles dispersed around a Li2O matrix at lower potential of 0.02 V. The first 2 steps are irreversible reactions, while the last is reversible for lithium storage. On applying reverse potential, Fe(0) facilitates the charge process, the conversion of Li2O to α-Fe2O3, which is represented by two broad anodic peaks in the range of 1.66–1.92 V and matches well with reports in the literature.13–15,20–25,58,59
1st discharge:
| 4Li+ + α-Fe2O3 → α-LixFe2O3 → α-Li2Fe2O3 → 3Li2O + 2Fe |
1st charge:
| Li2O + Fe → α-Fe2O3 |
The overlapping of subsequent CV cycles shows the reversibility of the 10% rGO composite with a shift in the conversion reaction's cathodic potential to 0.81 V, which may be due to structural changes that occurred during the first cycle. Similar trends were observed in α-Fe2O3/rGO nanocomposites, with slight shifts in the cathodic and anodic peaks during cycling. To show the advantages of rGO backbones, however, our 10% rGO composite exhibited lower resistance to charge transfer compared to α-Fe2O3 and the α-Fe2O3/rGO-30 composite, as is evident from the electrochemical impedance spectroscopy (Fig. 6b). As a result of lower contact resistance and charge-transfer resistance, lithium ion diffusion and electron transfer are facilitated, so as to give the 10 wt% rGO wrapped α-Fe2O3 nanocomposite superior electrochemical performance.59
Conclusions
We have successfully fabricated a nanocomposite consisting of spray precipitation synthesized highly porous hematite nanorods wrapped with chemically modified graphene oxide layers, by following a simple low-temperature soft self-assembly approach. The electrochemical performance of the nanorod architecture in a conductive rGO network shows exceptional energy storage capability as a negative electrode active material for battery application. A comparatively small amount of rGO (10 wt%) interaction creates an outstanding interconnected conductive network among the nanorods to result in a highly (Li+) ion penetrable nanostructure that has revealed superior reversible capacity of 1320 mA h g−1 over 100 cycles at 100 mA g−1 and excellent rate capability at various current densities over prolonged cycling. These environmentally friendly materials in a composite created through a low temperature fabrication approach highlight this material as a promising anode material for high performance lithium ion batteries. The simple fabrication methodology can point the way to the large-scale production of active materials for modern energy devices in future developments.Experimental
All the chemicals employed were of analytical grade and purchased from Sigma Aldrich. They were used as is without any further purifications or treatments.Synthesis of nanoporous α-Fe2O3 nanorods and graphene oxide
The nanoporous hematite nanorods were synthesized via the spray precipitation technique, as reported elsewhere.45–47 In a typical process, the prepared 0.15 M Fe(NO3)3·9H2O precursor was sprayed dropwise using a spray nozzle and peristaltic pump into a 1.5 M NaOH solution, as shown in Fig. 1, resulting in a red-brown precipitate, which was then allowed to rest overnight to allow the partial separation of the solid and liquid phases. The spray precipitation precursor was then subjected to ageing, which changed its colour from brown-red to yellow. After decanting the upper liquid layer, the precipitants were washed several times via centrifugation, and the resultant solid precursor material was vacuum dried for 3 h at 90 °C and then subjected to annealing under argon atmosphere at 400 °C for 4 h in a tubular furnace (Thermotech, Haugesund, Norway). The method for the synthesis of ultra-large graphene oxide (GO) nanosheets in aqueous dispersion has described in our previous reports.48–50Fabrication of reduced graphene oxide wrapped α-Fe2O3 nanorods
Nanoporous α-Fe2O3 nanorods (50 mg) were dispersed in 50 mL deionised (DI) water using probe sonication for 30 min (Sonics VC505 with maximum amplitude of 30%) followed by a 30 min bath sonication to form a stable homogeneous dispersion. As prepared GO (10 wt% or 30 wt% of α-Fe2O3 nanorods) in the liquid crystalline state (5 mg mL−1) was added to the above solution under vigorous stirring. Ascorbic acid in a similar weight ratio to GO was also added as its reducing agent with continued stirring at 80 °C for 6 h. The resultant solid sample was collected, washed with an ethanol–water mixture to remove residual acid, and dried at 45 °C to obtain pure α-Fe2O3/reduced graphene oxide (rGO) nanocomposite. The samples having 10 wt% and 30 wt% rGO are denoted as α-Fe2O3/rGO-10 and α-Fe2O3/rGO-30, respectively. A bare rGO sample was prepared with GO and ascorbic acid for a comparative electrochemical study.Materials characterization
The nanoporous α-Fe2O3 nanorods wrapped with chemically modified rGO sheets were characterized for phase purity, morphology, and electrochemical properties. X-ray diffraction (XRD, GBC MMA) with Cu-Kα irradiation conducted at 1° min−1 scan rate and 0.02° step size was used to identify the phase. Field emission gun scanning electron microscopy (FEGSEM, JOEL JSM-7500, Japan) was used to study the morphology of the nanocomposites. Transmission electron microscopy (TEM, JEOL JEM-ARM200F) operated at 200 kV with resolution of <0.08 nm was used to determine the distribution of rGO and nanopores over the α-Fe2O3 in the nanocomposites. X-ray photoelectron spectroscopy (XPS) analysis was conducted using a PHOIBOS 100 hemispherical analyzer with pass energy of 26.00 eV and 45° take-off angle. Raman spectroscopy was carried out on a HORIBA spectrophotometer (H800) with a microscope objective of 50× and confocal hole size of 1000 m. A 532.81 nm He–Ar laser was used to excite Raman scattering between 200 and 3000 cm−1 using a 200 m grating.Electrochemical analysis
The electrochemical performances of the as-prepared nanocomposites, along with those of their individual components, have been studied with CR2032 half-cell configured coin-cells assembled in an argon filled glove box (MBraun, Germany). The fabricated nanocomposites were blended with carbon black (Super P, TIMCAL Switzerland) as conducting material and sodium alginate (Sigma Aldrich) as a binder in a weight ratio of 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, respectively, using Millipore water as solvent. The slurry was mixed using a planetary mixer (Kurabo Mazerustar, Japan), and the thus-obtained slurry was tape-casted over copper current collector by using the doctor blade technique and vacuum dried at 80 °C overnight. The dried electrodes were cut into circular discs, with each electrode loaded with ∼1 mg cm−2 active materials. Half-cell type coin cells were assembled using the above electrode as working electrode, while Li metal foil was the counter/reference electrode, with Celgard polypropylene film as the separator, which was impregnated with a few drops of commercially available 1 M LiPF6 in 1
1, respectively, using Millipore water as solvent. The slurry was mixed using a planetary mixer (Kurabo Mazerustar, Japan), and the thus-obtained slurry was tape-casted over copper current collector by using the doctor blade technique and vacuum dried at 80 °C overnight. The dried electrodes were cut into circular discs, with each electrode loaded with ∼1 mg cm−2 active materials. Half-cell type coin cells were assembled using the above electrode as working electrode, while Li metal foil was the counter/reference electrode, with Celgard polypropylene film as the separator, which was impregnated with a few drops of commercially available 1 M LiPF6 in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v) ethylene carbonate (EC)
1 (v/v) ethylene carbonate (EC)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) diethyl carbonate (DEC) as electrolyte. All the assembled cells were electrochemically tested in a battery testing analyser (Landt, China CT2001A) at a constant specific current density (mA g−1) between 0.02 and 3.0 V. A Biologic (VMP3) electrochemical workstation was employed to perform cyclic voltammetry (CV) at a 0.1 mV s−1 scan rate and potentiostatic electrochemical impedance spectroscopy (PEIS) in the frequency range of 0.1 MHz to 10 mHz against Li+/Li0.
diethyl carbonate (DEC) as electrolyte. All the assembled cells were electrochemically tested in a battery testing analyser (Landt, China CT2001A) at a constant specific current density (mA g−1) between 0.02 and 3.0 V. A Biologic (VMP3) electrochemical workstation was employed to perform cyclic voltammetry (CV) at a 0.1 mV s−1 scan rate and potentiostatic electrochemical impedance spectroscopy (PEIS) in the frequency range of 0.1 MHz to 10 mHz against Li+/Li0.
Acknowledgements
The authors are grateful to the Commonwealth of Australia, Excellerate Australia and the Automotive Australia 2020 Cooperative Research Centre (CRC) for financial support under project code: AutoCRC 1-111, the Australian Research Council (ARC) for financial support through a Linkage Infrastructure Equipment and Facilities (LIEF) grant no. LE0237478 located at the UOW Electron Microscopy Centre, and the Institute for Semiconducting and Electronic Materials (ISEM) for the use of its infrastructure as part of in-kind support. The authors would also like to thank Dr Tania Silver for critical review of the paper and Mr Zhixin Tai for the technical support.References
- A. S. Arico, P. Bruce, B. Scrosati, J.-M. Tarascon and W. van Schalkwijk, Nat. Mater., 2005, 4, 366–377 CrossRef CAS PubMed.
- M. Armand and J.-M. Tarascon, Nature, 2008, 451, 652–657 CrossRef CAS PubMed.
- J.-M. Tarascon and M. Armand, Nature, 2001, 414, 359–367 CrossRef CAS PubMed.
- B. Dunn, H. Kamath and J.-M. Tarascon, Science, 2011, 334, 928–935 CrossRef CAS PubMed.
- M. Winter and R. J. Brodd, Chem. Rev., 2004, 104, 4245–4270 CrossRef CAS PubMed.
- J. B. Goodenough, Acc. Chem. Res., 2013, 46, 1053–1061 CrossRef CAS PubMed.
- K. Zaghib, A. Mauger, H. Groult, J. B. Goodenough and C. M. Julien, Materials, 2013, 6, 1028–1049 CrossRef CAS.
- J. B. Goodenough, Energy Environ. Sci., 2014, 7, 14–18 CAS.
- J. B. Goodenough and K. Park, J. Am. Chem. Soc., 2013, 135, 1167–1176 CrossRef CAS PubMed.
- P. Poizot, S. Laruelle, S. Grugeon, L. Dupont and J.-M. Tarascon, Nature, 2004, 407, 496–499 Search PubMed.
- P. L. Taberna, S. Mitra, P. Poizot, P. Simon and J.-M. Tarascon, Nat. Mater., 2006, 5, 567–573 CrossRef CAS PubMed.
- P. S. Veluri, A. Shaligram and S. Mitra, J. Power Sources, 2015, 293, 213–220 CrossRef CAS.
- Q. Zhou, Z. Zhao, Z. Wang, Y. Dong, X. Wang, Y. Gogotsiac and J. Qiu, Nanoscale, 2014, 6, 2286–2291 RSC.
- X. Dong, L. Li, C. Zhao, H. K. Liu and Z. Guo, J. Mater. Chem. A, 2014, 2, 9844–9850 CAS.
- Z. Wang, D. Luan, S. Madhavi, Y. Hu and X. W. (David) Lou, Energy Environ. Sci., 2012, 5, 5252–5256 CAS.
- H.-C. Park, S.-J. Kim, M.-C. Kim, D.-M. Kim and K.-W. Park, Ceram. Int., 2016, 42, 1933–1942 CrossRef CAS.
- L. Baggetto, N. A. M. Verhaegh, R. A. H. Niessen, F. Roozeboom, J.-C. Jumas and P. H. L. Nottena, J. Electrochem. Soc., 2010, 157, A340–A347 CrossRef CAS.
- X. Fan, Y. Zhu, C. Luo, T. Gao, L. Suo, S. C. Liou and C. Wang, J. Power Sources, 2016, 307, 435–442 CrossRef CAS.
- P. Liu, J. J. Vajo, J. S. Wang, W. Li and J. Liu, J. Phys. Chem. C, 2012, 116, 6467 CAS.
- J. G. Wang, D. Jin, R. Zhou, C. Shen, K. Xie and B. Wei, J. Power Sources, 2016, 306, 100–106 CrossRef CAS.
- M. Chen, J. Zhang, X. Xia, M. Qi, J. Yin and Q. Chen, Electrochim. Acta, 2016, 195, 184–191 CrossRef CAS.
- S. Liu, H. Zhang, L. Xu, L. Ma and X. Chen, J. Power Sources, 2016, 304, 346–353 CrossRef CAS.
- M. S. Chandrasekar and S. Mitra, Electrochim. Acta, 2013, 92, 47–54 CrossRef CAS.
- Y. Oumellal, A. Rougier, G. A. Nazri, J.-M. Tarascon and L. Aymard, Nat. Mater., 2008, 7, 916–921 CrossRef CAS PubMed.
- L. Aymard, Y. Oumella and J.-P. Bonnet, Beilstein J. Nanotechnol., 2015, 6, 1821–1839 CrossRef CAS PubMed.
- P. S. Veluria and S. Mitra, RSC Adv., 2013, 3, 15132–15138 RSC.
- S. Chaudharia and M. Srinivasan, J. Mater. Chem., 2012, 22, 23049–23056 RSC.
- K. Cao, L. Jiao, H. Liu, Y. Liu, Y. Wang, Z. Guo and H. Yuan, Adv. Energy Mater., 2015, 5, 1401421 CrossRef.
- L. Li, H. Bin Wu, L. Yu, S. Madhavi and X. W. (David) Lou, Adv. Mater. Interfaces, 2014, 1, 1400050 CrossRef.
- P. Wang, M. Gao, H. Pan, J. Zhang, C. Liang, J. Wang, P. Zhou and Y. Liu, J. Power Sources, 2013, 239, 466–474 CrossRef CAS.
- S. L. Chou, J.-Z. Wang, D. Wexler, K. Konstantinov, C. Zhong, H.-K. Liu and S. X. Dou, J. Mater. Chem., 2010, 20, 2092–2098 RSC.
- Z. Cao and B. Wei, J. Power Sources, 2013, 241, 330–340 CrossRef CAS.
- W.-J. Yu, L. Zhang, P.-X. Hou, F. Li, C. Liu and H.-M. Cheng, Adv. Energy Mater., 2016, 6, 1501755 Search PubMed.
- Y. Wang, L. Yang, R. Hu, W. Sun, J. Liu, L. Ouyang, B. Yuan, H. Wang and M. Zhu, J. Power Sources, 2015, 288, 314–319 Search PubMed.
- Y. Wan, Z. Yang, G. Xiong, R. Guo, Z. Liu and H. Luo, J. Power Sources, 2015, 294, 414–419 Search PubMed.
- X. Jiang, X. Yang, Y. Zhu, Y. Yao, P. Zhao and C. Li, J. Mater. Chem. A, 2015, 3, 2361–2369 Search PubMed.
- X. Li, Y. Ma, L. Qin, Z. Zhang and Z. Zhang, J. Mater. Chem. A, 2015, 3, 2158–2165 Search PubMed.
- L. Xiao, M. Schroeder, S. Kluge, A. Balducci, U. Hagemann, C. Schulz and H. Wiggers, J. Mater. Chem. A, 2015, 3, 11566–11574 Search PubMed.
- X. Zhu, X. Song, X. Ma and G. Ning, ACS Appl. Mater. Interfaces, 2014, 6, 7189–7197 Search PubMed.
- W. Xiao, Z. Wang, H. Guo, X. Li, J. Wang, S. Huang and L. Gan, Appl. Surf. Sci., 2013, 266, 148–154 Search PubMed.
- G. Xia, N. Li, D. Li, R. Liu, C. Wang, Q. Li, X. Lü, J. S. Spendelow, J. Zhang and G. Wu, ACS Appl. Mater. Interfaces, 2013, 5, 8607–8614 Search PubMed.
- J. Cabana, L. Monconduit, D. Larcher and M. Rosa Palacín, Adv. Mater., 2010, 22, E170–E192 Search PubMed.
- B. Tian, J. Ś. V. Maurice, C. Pereira-Nabais, A. Seyeux and P. Marcus, J. Phys. Chem. C, 2015, 119, 919–925 Search PubMed.
- J. Qu, Y.-X. Yin, Y.-Q. Wang, Y. Yan, Y.-G. Guo and W.-G. Song, ACS Appl. Mater. Interfaces, 2013, 5, 3932–3936 Search PubMed.
- D. Cardillo, M. Tehei, M. Lerch, S. Corde, A. Rosenfeld and K. Konstantinov, Mater. Lett., 2014, 117, 279–282 Search PubMed.
- D. Cardillo, M. Tehei, S. Hossain, M. Islam, K. Bogusz, D. Shi, D. Mitchell, M. Lerch, A. Rosenfeld, S. Corde and K. Konstantinov, ACS Appl. Mater. Interfaces, 2016, 8, 5867–5876 Search PubMed.
- M. Islam, D. Cardillo, T. Akhter, S. H. Aboutalebi, H. K. Liu, K. Konstantinov and S. X. Dou, Part. Part. Syst. Charact., 2015, 33, 27–37 Search PubMed.
- T. Akhter, M. Islam, S. N. Faisal, E. Haque, A. I. Minett, H. K. Liu, K. Konstantinov and S. X. Dou, ACS Appl. Mater. Interfaces, 2016, 8, 2078–2087 Search PubMed.
- M. Islam, S. H. Aboutalebi, D. Cardillo, H. K. Liu, K. Konstantinov and S. X. Dou, ACS Cent. Sci., 2015, 1, 206–216 Search PubMed.
- M. Islam, A. T. Chidembo, S. H. Aboutalebi, D. Cardillo, H. K. Liu, K. Konstantinov and S. X. Dou, Front. Energy Res., 2014, 2, 1–11 Search PubMed.
- A. Chidembo, S. H. Aboutalebi, K. Konstantinov, M. Salari, B. Winton, S. A. Yamini, I. P. Nevirkovets and H. K. Liu, Energy Environ. Sci., 2012, 5, 5236–5240 Search PubMed.
- S. Velumani and J. A. Ascencio, Appl. Phys. A, 2004, 79, 153–156 Search PubMed.
- H. Zhang, L. Zhou and C. Yu, RSC Adv., 2014, 4, 495–499 Search PubMed.
- Q. Sun, X. Liu, A. B. Djurišić, T. L. Leung, M. Xie, A. M. C. Ng, H. K. Li, Z. Deng and K. Shih, RSC Adv., 2015, 5, 91466–91471 Search PubMed.
- J. Ye, J. Zhang, F. Wang, Q. Su and G. Du, Electrochim. Acta, 2013, 15, 212–217 CrossRef.
- Y. Chen, J. Wang, J. Jiang, M. Zhou, J. Zhu and S. Han, RSC Adv., 2015, 5, 21740–21744 RSC.
- S. Liu, R. Wang, M. Liu, J. Luo, X. Jin, J. Sun and L. Gao, J. Mater. Chem. A, 2014, 2, 4598–4604 CAS.
- Y. Wang, L. Yang, R. Hu, L. Ouyang and M. Zhu, Electrochim. Acta, 2014, 125, 421–426 CrossRef CAS.
- R. Hu, D. Chen, G. Waller, Y. Ouyang, Y. Chen, B. Zhao, B. Rainwater, C. Yang, M. Zhu and M. Liu, Energy Environ. Sci., 2016, 9, 595–603 CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra14626k |
| ‡ These two authors have made equal contributions. |
| This journal is © The Royal Society of Chemistry 2016 |