Enhanced osteoblast adhesion on amino-functionalized titanium surfaces through combined plasma enhanced chemical vapor deposition (PECVD) method
Mengmeng Luae,
Dan Shaobe,
Ping Wangc,
Danying Chend,
Yidi Zhanga,
Mingqiang Lie,
Jinghui Zhao*a and
Yanmin Zhou*a
aJilin Province Key Laboratory of Tooth Development and Bone Remodeling, Department of Dental Implantology, School and Hospital of Stomatology, Jilin University, Changchun 130021, China. E-mail: zhaojh_1986@126.com; zhouym62@126.com
bCAS Key Laboratory of Bio-Medical Diagnostics, Suzhou Institute of Biomedical Engineering and Technology, Chinese Academy of Sciences, Suzhou 215163, China
cDepartment of Otolaryngology-Head and Neck Surgery, First Hospital of Jilin University, Changchun 130021, China
dShanghai Engineering Research Center of Tooth Restoration and Regeneration, School of Somatology, Tongji University, Shanghai 200072, China
eDepartment of Biomedical Engineering, Columbia University, New York, NY 10027, USA
First published on 26th August 2016
Abstract
Surface modification of titanium and its alloys with positively charged amino-groups has been proved to improve the performance of implants on initial osteoblast function. As a novel chemical approach, plasma enhanced chemical vapor deposition is now applied to introducing bioactive molecules to titanium surfaces with its own advantages. However, there is still little information about the effect of the combined plasma polymerization mode of plasma enhanced chemical vapor deposition on osseointegration of titanium surfaces. In this work, we aim to investigate the effect and mechanism of osteoblast adhesion on amino-functionalized titanium surfaces produced by different plasma polymerization modes of plasma enhanced chemical vapor deposition. The surface morphology and chemistry of these alloys were examined by scanning electron microscopy, scanning probe microscopy and X-ray photoelectron spectroscopy. CCK-8 assay, DAPI staining and flow cytometry showed that all amino-modified surfaces significantly induced osteoblast adhesion compared with pure titanium surfaces without any cytotoxicity. Immunofluorescence staining and western blotting further demonstrated a remarkable elevation of focal adhesion kinase phosphorylation with the increase of integrin α2. More importantly, combined mode (CW + P) mediated surfaces possessed the best improvement of osseointegration. Taken together, our results indicate that amino-functionalizated titanium surfaces promote osteoblast adhesion through upregulation of integrin α2 and p-phosphorylated focal adhesion kinase. These findings will shed some light on the potential of combined plasma enhanced chemical vapor deposition methods for achieving ideal osseointegration on titanium surfaces.
1. Introduction
Titanium (Ti) and its alloys have been widely used for dental/orthopedic implants, and joint replacements for decades due to their excellent mechanical properties, good resistance to corrosion and inertness in physiological environments, as well as superior biocompatibility.1,2 Nevertheless, lack of osseointegration during early healing or infection of the peri-implant tissue can lead to bone resorption and treatment failure.3,4 In order to improve the performance of titanium, like the interaction of implants with the surrounding bone tissue, a variety of mechanical, chemical and physical surface modification technologies has been explored to enhance the direct bone–material interaction.5–7 Conventionally, chemical approach including chemical treatment, electrochemical treatment, sol–gel, and biochemical modification are utilized to immobilize bioactive molecules onto titanium surface.8,9 However, several drawbacks are related to the traditional titanium surface modifications, such as the lack of stability, lower efficiency, and difficulty of modification controlling, etc.10 To surmount these obstacles, high-efficient method, controllable modification effect, and low-temperature treatment is urgently desirable in the context of titanium surface modification for implants.Chemical vapor deposition (CVD) is a chemical process between precursor gas and the surface of metallic implants to facilitate deposition of bioactive coatings with high-purity, super-performance, well-controlled and non-volatile properties.11,12 Plasma enhanced chemical vapour deposition (PECVD) technique, a novel family member of CVDs, has additional benefits such as low deposition temperature, high purity and direct control of reaction parameters.13 This deposition which is operated under low-temperature provides an advantageous possibility to deposit bioactive coatings on titanium surfaces. In PECVD method, one of the hardest challenging problems is some of the plasma polymers, especially those produced by either pulse (P) plasma or lower continuous wave (CW) input energy are unstable in aqueous environments. The instability is due to their swelling properties, loss of low molecular weight molecules, delamination of the deposited films, and interaction between the functional groups and solvent.14 Surface modifications of titanium must be stable through the whole procedure, including sterilization before implantation, as well as the initial reaction between the surface and its biological environment after implantation.15 To meet these challenges, a novel PECVD method with combined plasma polymerization mode (CW + P) is developed to introduce bioactive functional groups to the surfaces of titanium implants. Moreover, there is little information available about the effect of plasma polymerization mode of PECVD on biological characteristics of modified titanium surface currently.
Development of titanium coatings using bioactive molecules is expected to optimize the biological response of implants by enhancing osteoblast adhesion.16,17 Bioactive molecules, such as amine, silanes, siloxanes, phosphoric acid or thiols, can be covalently attached to titanium surfaces with self-assembled monolayers.18,19 It is well known that the positive-charged titanium surface is advantageous concerning osteoblastic focal adhesion.20 Thus in the case of surface modification, amine molecules could provide strong cross-linking and attachment to the titanium surfaces, as well as offer positive charges when protonated in aqueous solutions at physiological pH values. Ammonia plasma treatment can easily equip surfaces with amine molecules. Amino-modification of titanium surfaces requires an interface layer based on metals, and polymers.21
In order to design superior implants for facilitating osseointegration, a better understanding of adhesive function of osteoblast to the bioactive surface of titanium that plays a crucial role in optimizing the implant–bone interface, ultimately improving clinical performance of these medical devices.22 Increasing evidence suggests that new bone growth at the implant–bone interface occurs in two ways, containing distal and contacting new bone growth and formation.23 It is generally believed that new bone growth via contact osteogenesis would be a better explanation for ideal status of the osseointegration. Among multiple types of cells in the bone, osteoblasts are the major functional members for bone formation.24 As the anchor-dependent cells, adhesion and clustering of osteoblasts on the surface of the implant is key for contact osteogenesis to occur.25 Recent findings indicated a key role of integrins in adhesion and spreading of osteoblasts on implant surface.26 Integrins are well known as heterodimeric receptor proteins that link the extra-cellularmatrix (ECM) to the cytoskeleton to regulate osteoblast shape, migration, and survival.27,28 Binding of the integrins to ECM ligands triggers the formation of focal adhesions, multiprotein signaling complexes that link the integrin cytoplasmic tails with the actin cytoskeleton.29 Reversible protein tyrosine phosphorylation, mainly catalyzed by focal adhesion kinase (FAK) and other protein tyrosine kinase or phosphatases, is an important mechanism controlling focal adhesion signaling and turnover to regulate osteoblast movement.30 Based on these observations, we reasoned that amino-functionalization of titanium surfaces prepared by PECVD seems to be advantageous concerning initial osteoblast adhesion compared with pure titanium surfaces.
Herein, we aim to investigate the effect and mechanism of osteoblast adhesion on amino-functionalized titanium surfaces modified by different plasma polymerization modes of PECVD. To address these issues, heptylamine was chosen as the precursor for film deposition on the basis of the good retention of amino groups. Depending on the process conditions, the samples were divided into six groups: CW, P(10%), P(30%), P(60%), CW + P(30%) and Ti (untreated group of pure titanium disc). Besides, advanced surface analytical techniques such as X-ray photoelectron spectroscopy (XPS), scanning electron microscope (SEM), scanning probe microscope (SPM), and contact angle were utilized to analyze the physicochemical properties and stability of these titanium surfaces. More importantly, CCK-8 assay, DAPI staining and flow cytometry were carried out to compare the guidance of cell viability and adhesion between pure titanium and different amino-functionalized titanium. Additionally, immunofluorescence staining and western blotting were analyzed expression of integrin α2 and FAK to further investigate molecular mechanisms of osteoblast adhesion.
2. Experimental section
2.1 Materials
Pure titanium discs of technical purity (cp, grade 2), diameter 14 mm/10 mm and thickness 2.0 mm/1.0 mm, were commercially purchased from Feng Ying Nickel-Titanium Co., Ltd. (Shanxi, China). αMEM, fetal bovine serum, and goat serum were obtained from Gibco Co., Ltd (Carlsbad, CA, USA). 4,6-Diamidine-2-phenylindole (DAPI) was obtained from Invitrogen Co., Ltd. (Carlsbad, CA). TruCount tubes were obtained from Becton Dickinson and Company (BD Biosciences, USA). Cell Counting Kit-8 (CCK-8), dexamethasone (DEXA), β-glycerophosphate (β-GP), and vitamin C were obtained from Sigma-Aldrich (St. Louis, MO, USA). Sodium dodecyl sulfate, glycine, ammonium persulfate, tetramethylethylenediamine, acrylamide, Tris, Tween-20, and proteinase inhibitors were purchased from Beijing Dingguo Biological Technology Co., Ltd (Beijing, China). All reagents were commercially available products with analytical grade purity and used without further purification.2.2 Sample preparation and polymerization reaction
The surface of the titanium disc was progressively polished with carbonate silicon sandpaper no. 600, 1200, 2000, 3000, and 5000, and then a mirror polishing was done using polishing paste on fine fabric cloth to gain a qualified titanium surface. The average roughness (Ra) of final surface was less than 5 nm after polishing. The polished titanium disc was cleaned by ultrasonic cleaning method in acetone, anhydrite ethanol, and nano-pure water for 15 min, respectively. After drying by nitrogen gas, the samples were stored in a dry Petri dish.Plasma deposited films were fabricated on the pure titanium surfaces with an inductively coupled plasma excited by an external copper band electrode with a 13.56 MHz RF. This specially designed external copper band electrode with attached RF matching network is able to ensure more than 95% RF power output into the plasma reaction chamber. The power was outputted in continuous wave (CW) and pulse (P) modes. Pulse generator adjusted duty ratio under the pulse mode. The liquid monomer in a monomer jar was connected to the reaction chamber with a stainless steel tube and the middle of the tube was equipped with a manual flow control valve. A capacitance manometer connected to the chamber measured the density of pressure inside the reaction chamber. The front end of the pump was equipped with a liquid nitrogen cooling apparatus for collecting the excess reactant. The basic intensity of pressure was <1 × 10−3 mbar before discharge of reaction chamber. The titanium surface treatment procedure can be divided into two steps: (1) surface were cleaned and activated by argon (CW 50 W, 10 s, density of pressure controlled at 2.5 × 10−2 mbar); (2) heptylamine exerted glow discharge on the material surfaces so that it modified the plasma characteristics under different plasma parameters and discharge modes. Depending on the process conditions, the samples were divided into 6 groups: CW, P(10%), P(30%), P(60%), CW + P(30%) and Ti (untreated group of pure titanium disc). Different plasma processing parameters are shown in Table 1. As-prepared titanium discs were sterilized through 30 min of UV irradiation for further biology experiments.
| Samples | Continuous wave | Pulse | |||
|---|---|---|---|---|---|
| Radio-frequency power/W | Time/min | Duty cycle/DC | Average power/W | Time/min | |
| CW | 45 | 10 | — | — | — |
| P(10%) | — | — | 1/10 | 4.5 | 30 |
| P(30%) | — | — | 1/3 | 13.5 | 30 |
| P(60%) | — | — | 3/5 | 27 | 30 |
| CW + P(30%) | 45 | 10 | 3/5 | 13.5 | 30 |
| Ti | — | — | — | — | — |
2.3 Characterization
The morphology of amino-functionalized titanium surfaces was observed using scanning electron microscopy (SEM, HITACHI S-3400N, Japan) at 5 kV. Surface morphology and thickness were detected by a Digital Instruments Dimension 3000 Scanning Probe Microscope (SPM). Roughness was obtained from an analysis of the images by Nanoscope III software. Surface chemical composition was analyzed using X-ray photoelectron spectroscopy (XPS). XPS samples' spectra were acquired with an XR50 Mg anode source operating at 150 W and a Phoibos 150 MCD-9 detector (D8 advance, SPECS Surface Nano Analysis GmbH, Germany). High-resolution XPS spectra data were collected using pass energy of 50 eV and energy step size of 0.1 eV. The quantitative analysis was carried out using a CasaXPS fitting program and was based on the collected XPS data. All binding energies were referred to the C 1s neutral carbon peak at 284.6 eV. Three samples were studied for each working condition. The sessile drop method was used to determine the hydrophilicity of the titanium surfaces by a Contact Angle System (OCA15 plus, Dataphysics, Germany). This method was performed by means of an analysis of the geometry of deionized water drop placed on the surface. The standard volume of a drop amounted to 1 μL.2.4 Cell culture
Mouse pre-osteoblastic cell line MC3T3-E1 was purchased from American Type Culture Collection (ATCC, Manassas, VA, USA). MC3T3-E1 cells were cultured in αMEM culture medium with 10% fetal bovine serum (Gibco Co., Ltd) and 100 U mL−1 penicillin/streptomycin. The 4th–5th generation of cultured cells were transferred into bone induction medium (i.e. the previous culture medium with addition of DEXA, vitamin C and β-GP); after day 3, cultured cells were digested with 2.5 g L−1 trypsin, percussed, and single cell suspension was made, adjusted cell density. Cell suspension was planted in 24-well plate (containing larger diameter of titanium discs) or 48-well plate (containing small diameter of titanium discs), in which the titanium disc surfaces have been treated variously after 30 min UV radiation on both sides, respectively. Then cultured in incubator at 37 °C with 5% of CO2 and saturated humidity for various time periods designated according to different experimental methods, respectively.2.5 CCK-8 assay
The treated titanium discs and untreated groups were placed in 24-well plates, the diameter of the discs fit the well. MC3T3-E1 cell density was adjusted to be 5 × 105 mL−1 and planted on the surface of the titanium discs at a volume of 1 mL per well. After cultured for 6, 12, 24, 48, or 72 hours, 100 μL of CCK-8 reagent was added into the wells, respectively, gently shacked and placed in the incubator for 1.5 hours, the cultures from each well were transferred to 96-well plate and the optic density (OD) of each well was measured at 450 nm wavelength. Triplicate wells for each condition were repeatedly measured 3 times per well and a mean value was obtained from each group. The cellular proliferation rate (relative cytotoxicity rate) was calculated by the following equation: RCR = (OD value of experimental group/OD value of pure cell group) × 100%.2.6 Flow cytometry
Adhesion rate of the osteoblast with absolute cell counting was measured through flow cytometry. In brief, suspended MC3T3-E1 cells in serum-free αMEM were seeded onto the treated and untreated surfaces of the titanium discs for 5, 30 and 120 min at a density of 2 × 104 per specimen, and un-adhered osteoblasts in the supernatant were counted and analyzed by flow cytometry. Cell adhesion was then calculated in percent to the cell number of 0 min. Each time point had 3 parallel samples and the samples were counted 3 times in the 6 groups.2.7 DAPI staining
After all culture solution in each well was taken out as described above, the remnants in the wells were rinsed twice, fixed with 4% paraformaldehyde for 20 min, rinsed 3 times with PBS, 5 min each; 10 μg mL−1 of DAPI-staining solution was added and stained for 10 min, then rinsed 3 times with PBS, cover slipped with glycerol, and the coverslip used had the same dimension as the wells; observed and compared under a fluorescent microscope.2.8 Scanning electron microscopy
The titanium discs with treated and untreated groups were placed in 24-well plates, the diameter of the discs fitted the well. MC3T3-E1 cell density was adjusted to be 2 × 104 mL−1 and planted on the surface of the titanium discs at a volume of 1 mL per well. After 12 h, the cells were fixed in 2.5% glutaraldehyde for 2 hours; rinsed 3 times with PBS buffer, 15 min each; fixed with osmium tetroxide at 4 °C for 30 min; rinsed 3 times again with PBS buffer, 10 min each; graded ethanol dehydration at concentrations of 50%, 70%, 80% and 90%, respectively, 5–10 min each, then 3 times dehydration with 100% ethanol, 5–10 min each; ethanol was substituted by t-butanol for 10 min; then samples stayed in t-butanol at −20 °C overnight and freeze-dried, gold-sprayed, and observed under a scanning electron microscope (SEM, HITACHI S-3400N, Japan). Relative cell areas on different titanium discs were calculated through ImageJ (>50 cells per group).2.9 Immunofluorescence staining
The MC3T3-E1 cell density was adjusted to 5 × 104 mL−1 and planted on the surfaces of treated and untreated titanium discs, cultured for 12 hours, then the culture medium was discarded; cells were then rinsed with 0.1 mol L−1 PBS, and fixed in 4% paraformaldehyde solution at room temperature for 20 min. Cells were rinsed again with PBS buffer for 3 times, 10 min each. 0.1% Triton X-100 was added and kept for 10 min, then rinsed with PBS for 3 times. 5% goat serum (Gibco Co., Ltd) was added and kept for 1 h at room temperature. Mouse anti-integrin α2 monoclonal antibody was added, then rabbit anti-p-FAK (Tyr 397)-R: sc-11765-R antibodies and incubated in a humidified chamber at 4 °C overnight and were removed the next day. After 3 PBS washes, cells were incubated for 1 h at RT with fluorescent labeled secondary antibodies, Alexafluor 555 goat anti-rabbit IgG and 488 goat anti-rabbit IgG (Invitrogen, Carlsbad, CA) (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200). Samples were then mounted in glycerol with Hoechest 33342 (Sigma, USA), cover-slipped and evaluated by confocal laser scanning microscopy. The confocal laser scanning microscopy was carried out using an Olympus FV1000 microscope equipped with multi-line argon LASER, 405, 488, 543 nm, and 30 mW laser class 3D laser.
200). Samples were then mounted in glycerol with Hoechest 33342 (Sigma, USA), cover-slipped and evaluated by confocal laser scanning microscopy. The confocal laser scanning microscopy was carried out using an Olympus FV1000 microscope equipped with multi-line argon LASER, 405, 488, 543 nm, and 30 mW laser class 3D laser.
2.10 Western blotting
Immunoblots were performed from total lysates of cells, which adhered to the specimens. Cells were collected and lysed with 0.1 mL of cold RIPA lysis buffer containing 0.02% PMSF. The cell lysate was centrifuged at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g for 30 min at 4 °C. All the samples were measured for total protein concentration using a BCA assay (Pierce, Rockford, IL) to ensure equal loading. Loading buffer was added to 20 μg protein and samples were boiled prior to being resolved on 10% SDS-PAGE gels and then transferred onto PVDF membranes (Bio-Rad). The blots were blocked using 5% nonfat dry milk in PBS at room temperature for 1 h with shaking and then incubated for overnight with primary antibody for integrin α2, phosphorylated-FAK, and FAK. Following three washes with PBS-T (PBS contain 5% Tween-20), the membranes were then incubated with the peroxidase-conjugated secondary antibodies at 1
000 g for 30 min at 4 °C. All the samples were measured for total protein concentration using a BCA assay (Pierce, Rockford, IL) to ensure equal loading. Loading buffer was added to 20 μg protein and samples were boiled prior to being resolved on 10% SDS-PAGE gels and then transferred onto PVDF membranes (Bio-Rad). The blots were blocked using 5% nonfat dry milk in PBS at room temperature for 1 h with shaking and then incubated for overnight with primary antibody for integrin α2, phosphorylated-FAK, and FAK. Following three washes with PBS-T (PBS contain 5% Tween-20), the membranes were then incubated with the peroxidase-conjugated secondary antibodies at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2000 dilution in PBS-T containing 1% non-fat milk for 1 h at room temperature. The membranes were then washed 3 times with PBS-T and the protein bands were visualized with the ECL Western blotting detection system. Immunoblotting for each detected protein was repeated 3 times using lysates from 3 independent experiments.
2000 dilution in PBS-T containing 1% non-fat milk for 1 h at room temperature. The membranes were then washed 3 times with PBS-T and the protein bands were visualized with the ECL Western blotting detection system. Immunoblotting for each detected protein was repeated 3 times using lysates from 3 independent experiments.
2.11 Statistical analysis
The experimental results were analyzed with SPSS19.0 statistical software; the amino-group plasma activation on cell adhesion of the titanium surfaces between treated and untreated groups was compared. Representative data was expressed as![[x with combining macron]](https://www.rsc.org/images/entities/i_char_0078_0304.gif) ± s. The comparison among groups was conducted by using the least significant difference (LSD) method, with single-factor analysis of variance (one-way ANOVA); P < 0.05 was count as significant difference; all experiment results were repeated at least three times.
± s. The comparison among groups was conducted by using the least significant difference (LSD) method, with single-factor analysis of variance (one-way ANOVA); P < 0.05 was count as significant difference; all experiment results were repeated at least three times.
3. Results
3.1 Physico-chemical characterization
The morphology of amino-functionalized titanium surfaces was examined by using SEM and SPM (Fig. 1 and 2), compared to non-treated Ti group, CW, P(10%), P(30%), P(60%), CW + P(30%) group had a smoother surface morphology. However, no apparent differences in surface morphology were seen between all amino-functionalized Ti surfaces. As shown in Table 2, the thickness of the plasma polymer layer on CW, P(10%), P(30%), P(60%), CW + P(30%) was 18.5 ± 2.9, 24.2 ± 3.5, 32.4 ± 5.7, 45.9 ± 6.0, 60.2 ± 7.1 nm, respectively. The roughness of the amino-functionalized titanium surfaces were also shown in Table 2, the Ra of Ti, CW, P(10%), P(30%), P(60%), CW + P(30%) groups is 1.82 ± 0.21, 1.43 ± 0.15, 1.36 ± 0.16, 1.15 ± 0.13, 1.06 ± 0.09, 0.85 ± 0.11 nm, respectively. The layer observed on all amino-functionalized Ti surfaces had a decreased surface roughness compared to control surfaces, while combined CW + P mode group exhibited the lowest surface roughness.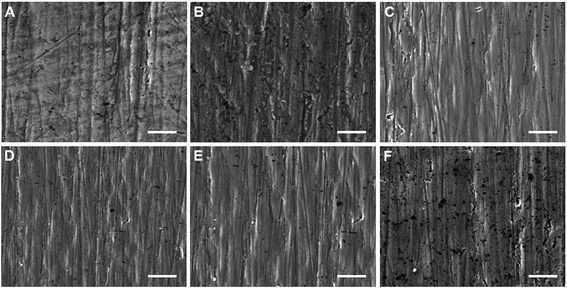 | ||
| Fig. 1 SEM images of the six types of titanium surfaces with different treatment. (A) Ti, (B) CW, (C) P(10%), (D) P(30%), (E) P(60%), and (F) CW + P(30%). Scale bars are 5 μm. | ||
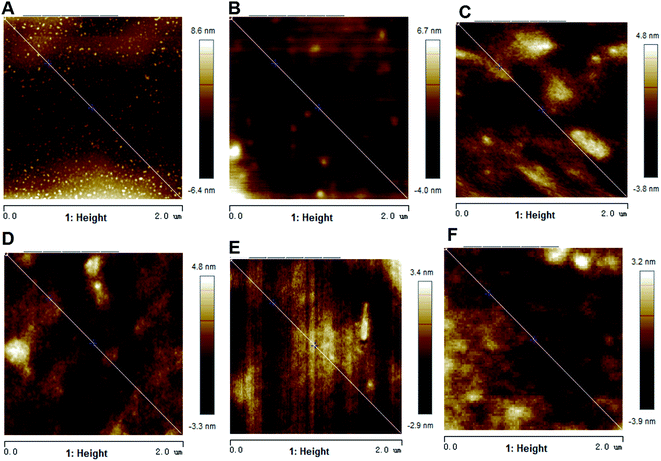 | ||
| Fig. 2 SPM images of the six types of titanium surfaces with different treatment. (A) Ti, (B) CW, (C) P(10%), (D) P(30%), (E) P(60%), and (F) CW + P(30%). | ||
| Sample | Ra (nm) | Thickness (nm) |
|---|---|---|
| Ti (control) | 1.82 ± 0.21 | — |
| CW | 1.43 ± 0.15 | 36.3 ± 3.1 |
| P(10%) | 1.36 ± 0.16 | 24.2 ± 3.5 |
| P(30%) | 1.15 ± 0.13 | 32.4 ± 5.7 |
| P(60%) | 1.06 ± 0.09 | 45.9 ± 6.0 |
| CW + P(30%) | 0.85 ± 0.11 | 60.2 ± 7.1 |
Chemical composition differences of the titanium sample before and after treatment were analyzed by X-ray photoelectron spectroscopy (XPS). As shown in Fig. 3 and Table 3, compared to non-treated Ti group, C, O and N peaks were also observed, but Ti 2p peaks were disappeared on all of amino-functionalized Ti surfaces. More importantly, the intensity of N 1s peak significantly increased on CW + P(30%) group than other treated groups. The quantitative compositional analysis showed that the atomic percentage of nitrogen on Ti, CW, P(10%), P(30%), P(60%), CW + P(30%) is 3.45%, 12.46%, 9.68%, 12.72%, 15.13%, 18.77% respectively. It is clearly demonstrated that the combined CW + P mode possesses the advantages of both CW and P modes providing higher density of primary amines. We further investigated the stable bonding of the functional groups on the different amino-functionalized titanium surfaces by studying the chemical element changes and the retention of –NH2 groups in ultrapure water for 1 h. As shown in Fig. 4, all the mode retained more than 70% of –NH2 groups, while combined mode CW + P exhibited the highest –NH2 groups retention, indicating the higher stability in aqueous solutions. Moreover, the surface hydrophilicity was determined by measuring the contact angle of a water-drop on amino-functionalized titanium surfaces (Table 4). It is clearly observed that all of amino-functionalized titanium surface wettability was significantly increased compared to the non-treated Ti group. While combined mode CW + P enhanced surface wettability to a comparable extent on all of amino-functionalized titanium surfaces.
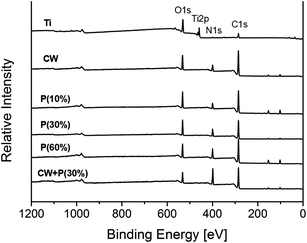 | ||
| Fig. 3 Surface chemistry of six types of titanium surfaces was analyzed by X-ray photoelectron spectroscopy (XPS). | ||
| Ti | C | N | O | |
|---|---|---|---|---|
| Ti (control) | 4.23 | 53.67 | 3.45 | 38.65 |
| CW | 0 | 72.53 | 12.46 | 15.01 |
| P(10%) | 0 | 73.48 | 9.69 | 16.83 |
| P(30%) | 0 | 71.54 | 12.72 | 15.74 |
| P(60%) | 0 | 71.25 | 15.13 | 13.62 |
| CW + P(30%) | 0 | 69.44 | 18.77 | 11.79 |
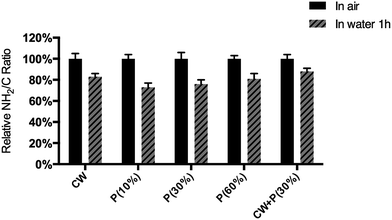 | ||
| Fig. 4 Relative NH2/C ratio on the surface of five types of amino-functionalized titanium surfaces with different treatment, as deposited (in air) and after soaking in water for 1 hour. | ||
| Sample | Water contact angle (°) |
|---|---|
| a All the values are the average of at least five measurements in different points of the samples. | |
| Ti (control) | 83.6 ± 7.8 |
| CW | 42.3 ± 3.5 |
| P(10%) | 47.6 ± 4.6 |
| P(30%) | 35.9 ± 5.3 |
| P(60%) | 33.2 ± 4.1 |
| CW + P(30%) | 28.1 ± 3.7 |
3.2 Osteoblast viability, adhesion and morphology
To determine the effect of amino-functionalization on MC3T3-E1 osteoblast proliferation, cell viability was measured after a 72 h incubation using CCK-8 assay. As shown in Fig. 5, there is no statistical significance in cell viability for each time point between each treated and untreated groups even after 72 h incubation, indicating that all amino-functionalized titanium surfaces are not obviously toxic to cultured osteoblasts.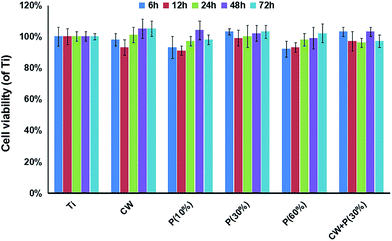 | ||
| Fig. 5 Cell viability of six types of titanium surfaces after 6, 12, 24, 48, 72 h of incubation was measured by CCK-8 assay. The data represent three separate experiments. Mean values ± SD. | ||
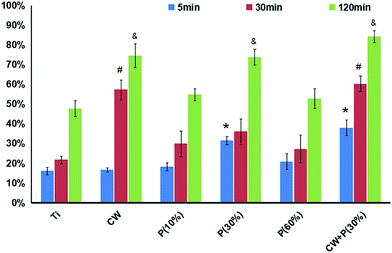
To evaluate whether amino-functionalized titanium can serve as matrix for osteoblast attachment, cell adhesion rate was quantitatively conducted using flow cytometry. As shown in Fig. 6, all the treated titanium surfaces had more cells than non-treated surfaces after 5, 30, and 120 min incubation. Moreover, cell adhesion rate of osteoblasts on the surfaces of titanium discs in groups P(30%) and CW + P(30%) was significantly higher than other untreated samples, while CW + P(30%) showed the highest cell adhesion rate at all time points. We further evaluated osteoblast adhesion using confocal laser scanning microscope (CLSM). DAPI staining results in Fig. 7 showed that the number and density of osteoblasts on the titanium surfaces with combined mode CW + P(30%) for 5 min incubation was increased than those on the untreated titanium surfaces, which are consistent with previous FACS results.
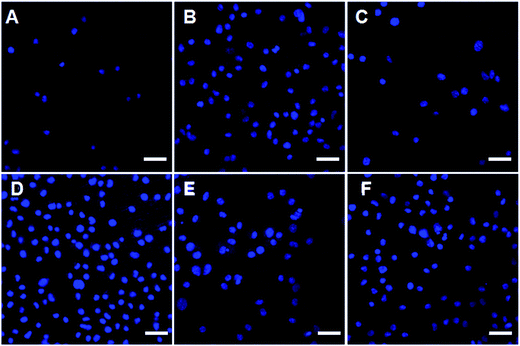 | ||
| Fig. 7 DAPI staining of osteoblasts on six types of titanium surfaces after 5 min incubation. (A) Ti, (B) CW, (C) P(10%), (D) P(30%), (E) P(60%), and (F) CW + P(30%). Scale bars are 40 μm. | ||
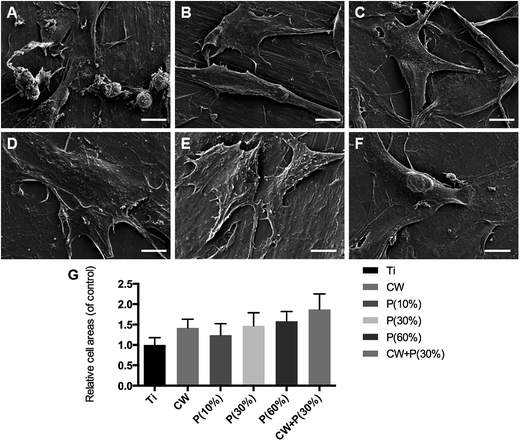
To observe the effect of amino-functionalization on MC3T3-E1 osteoblast morphology, SEM images in Fig. 8A–F showed that osteoblasts in non-treated titanium groups displayed shrinking shaped clusters with smaller adhesion areas and loose adhesion. While osteoblasts in all treated titanium groups displayed stronger adhesion with larger adhesion areas (Fig. 8G). More importantly, osteoblasts in groups P(30%) and CW + P(30%) showed multiple pseudopodia enhancing cell adhesion, especially osteoblasts are fully adhered to the surface of the materials with more extended appearance in CW + P(30%) group. Collectively, the amino-functionalized titanium surface could remarkably accelerate osteoblast adhesion, increase adhesion rate. And the osteoblasts were firmly adhered to the material surface and fully spread.
3.3 Influence of amino-functionalization on integrin α2 and p-FAK expression
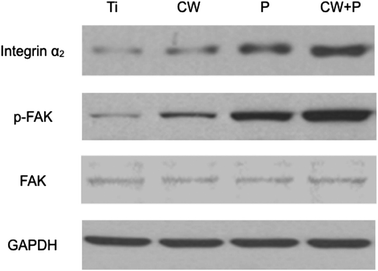
To explore the mechanism of osteoblast adhesion promotion on amino-functionalized titanium surfaces, immunohistochemistry assay were employed to detect the expression of integrin α2 and phosphorylated focal adhesion kinase (p-FAK) of osteoblasts. As shown in Fig. 9, both integrin α2 and p-FAK were expressed in cytoplasm. Expression of two target markers in the treated titanium groups CW, P(30%) and CW + P(30%) was significantly higher than those in the non-treated titanium group, especially in CW + P(30%) group. It is worth noting that the strong overlying yellow fluorescence observed in groups P(30%) and CW + P(30%) relatively indicating the simultaneously overexpression of integrin α2 and p-FAK. Additionally, up-regulated integrin α2 and p-FAK expression were demonstrated in osteoblast on the CW + P(30%) surface using western blot (Fig. 10), which was consistent with the immunohistochemistry results. Taken together, these findings suggested that amino-functionalized titanium surface could promote osteoblasts adhesion through up-regulated expression of adhesion-associated plasma protein integrin α2 and FAK, subsequently activating integrin signaling pathway.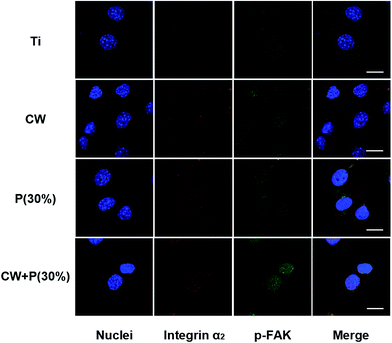 | ||
| Fig. 9 Representative images illustrated the integrin α2 and p-FAK expression of osteoblasts after 12 h incubation with four types of titanium surfaces. Scale bars are 10 μm. | ||
4. Discussion
The clinical goal and most critical factor of dental or orthopaedic implant success are to achieve enhanced and faster osseointegration. Implant osseointegration with surrounding tissue is a direct bone integration with no fibrous tissue between ingrowth.31 In light of this, continuous efforts in the field of biomaterial have focused on design and optimization of bio-interface with stable adhesion to the metal surface, as well as selective control of interactions at the tissue/implant interface.32–34 In this regard, ideal surface modification for implant should be classified into three categories: (1) required density of desired surface chemical functional groups; (2) stable bonding of the bioactive molecules to metallic substrate; (3) employment of nanometer-scale surface features. For implant material fixed in bone, optimal osseointegration needs ideal bone cells' response. As the beginning of the interaction between cells and biomaterial, cell adhesion and spreading quality affects their proliferation and differentiation subsequently. Cell adhesion on the material surface is mainly mediated by integrins. This kind of adhesion is actually the interaction between cell, extracellular matrix proteins and material layer.35 Therefore, this study focused primarily on the effect of activation of amino-functionalized titanium surface on osteoblast adhesion.From experimental results we can know that the amino-functionalized titanium surface showed non-cytotoxicity and suitability for osteoblast growth. Compared with untreated surface, at 5, 30 and 120 min, osteoblast adhesion rates of P(30%) and CW + P(30%) group were significantly higher, which was consistent with previous studies that amino-group functional groups could promote initial osteoblast adhesion and growth on modified surface. SEM images of co-cultured samples for 12 h suggested that osteoblasts in groups CW, P(30%) and CW + P(30%) could adhere more firmly with multiple pseudopodia and larger adhesion areas. Taken together, our findings demonstrated that the combined mode CW + P(30%) possessed better osteoblast adhesion and spreading ability on titanium surface.
The results we obtained by immunohistochemistry and western blotting had shed some light on the involvement of integrin α2 and p-FAK in osteoblast adhesion. Interaction of integrin molecules and ECM protein will affect signal transduction and transcription factors in osteoblasts and osteoblast-specific gene expression. Outside-in signaling is the well-established route for osteoblast–biomaterial interaction in which integrins are the key cell surface proteins.36,37 Integrin adhesion receptors are composed of 18 α subunits and 8 β subunits in mammal.38 Among integrin family, integrin α2 is highly expressed in cells. They promote cell adhesion and proliferation.39 The recruitment of focal adhesion kinase (FAK) to the integrin cytoplasmic tail and phosphorylation at Tyr397 are early events upon integrin engagement by the ECM.40 Recent reports showed that phosphorylation of FAK was critical for bone formation and osteoblast migration and adhesion induced by bone morphogenetic protein (BMP).41 Thus, our study suggested that the enhancement of osteoblast adhesion on the amino-functionalized titanium surfaces was achieved through activation of the integrin signaling pathway.
The ability to modify implant surface chemistry through incorporation of signal recognition ligands and sequences, and to enhance the molecular and cellular response provides an attractive approach for better osseointegration.23 Using amino-groups for covalently binding is an established method of material surfaces bioactivation with immobilized proteins and peptides. Another study by Barbara Nebe et al., also demonstrated that titanium surface functionalization with positive charge amino-groups significantly improved initial steps of cellular contact to the material surface.20 They surmised that these results might be caused by the presence of hyalurona which was negatively charged and pericellular matrix substance. Other study also showed that extracellular matrix fibronectin played a major role in osteoblast adhesion. Fibronectin attached to the main RGD-integrin binding domain on the material surface is extremely sensitive to –NH2.42 Additionally, our study have demonstrated that functionalization of titanium with positively charged amino-groups significantly improved surgical implant osseointegration. Further investigations are necessary to investigate the effect and mechanism of differentiation of the osteoblast on amino-functionalized titanium surfaces based on different plasma polymerization mode of PECVD.
5. Conclusions
In summary, a simple and efficient method named PECVD has been developed to introduce amino-group onto titanium implants. Enhanced wettability of all amino-modified surfaces was correlated with significantly induced osteoblast adhesion compared with pure titanium surface without any cytotoxicity. This amino-functionalization of titanium surface is advantageous concerning integrin α2 and p-FAK expression, leading to enhancement of osteoblast adhesion and proliferation compared with pure titanium surface. More importantly, the combined mode modified titanium surface demonstrated the best improvement of osseointegration. We hope the combined mode of PECVD method would become a promising strategy to achieve ideal osseointegration on titanium surface for better design of surgical implants.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This study was sponsored by the National Natural Science Foundation of China (81070855 and 81200809), Foundation of Science and Technology Development Program of Jilin Province (20130522037JH), Specialized Research Fund for the Doctoral Program of Higher Education of China (20120061110077), and the Graduate Innovation Fund of Jilin University (2015043).Notes and references
- H. J. Rack and J. Qazi, Mater. Sci. Eng., C, 2006, 26, 1269–1277 CrossRef CAS.
- M. Niinomi, Biomed. Mater., 2008, 1, 30–42 Search PubMed.
- P.-I. Bra-nemark, G. A. Zarb, T. Albrektsson and H. M. Rosen, Plast. Reconstr. Surg., 1986, 77, 496–497 Search PubMed.
- Y. T. Sul, D. H. Kwon, B. S. Kang, S. J. Oh and C. Johansson, Clin. Oral Implants Res., 2013, 24, 8–19 CrossRef PubMed.
- X. Liu, P. K. Chu and C. Ding, Mater. Sci. Eng., R, 2004, 47, 49–121 CrossRef.
- B. G. Zhang, D. E. Myers, G. G. Wallace, M. Brandt and P. F. Choong, Int. J. Mol. Sci., 2014, 15, 11878–11921 CrossRef PubMed.
- C. Larsson Wexell, P. Thomsen, B.-O. Aronsson, P. Tengvall, M. Rodahl, J. Lausmaa, B. Kasemo and L. Ericson, Int. J. Biomater., 2013, 2013, 412482 CAS.
- X. Shi, M. Nakagawa, G. Kawachi, L. Xu and K. Ishikawa, J. Mater. Sci.: Mater. Med., 2012, 23, 1281–1290 CrossRef CAS PubMed.
- K.-Y. Hung, S.-C. Lo, C.-S. Shih, Y.-C. Yang, H.-P. Feng and Y.-C. Lin, Surf. Coat. Technol., 2013, 231, 337–345 CrossRef CAS.
- S. Bauer, P. Schmuki, K. von der Mark and J. Park, Prog. Mater. Sci., 2013, 58, 261–326 CrossRef CAS.
- R. Fix, R. G. Gordon and D. M. Hoffman, Chem. Mater., 1991, 3, 1138–1148 CrossRef CAS.
- S. L. Benjamin, C. de Groot, C. Gurnani, A. L. Hector, R. Huang, K. Ignatyev, W. Levason, S. J. Pearce, F. Thomas and G. Reid, Chem. Mater., 2013, 25, 4719–4724 CrossRef CAS PubMed.
- A. Sobczyk-Guzenda, B. Pietrzyk, H. Szymanowski, M. Gazicki-Lipman and W. Jakubowski, Ceram. Int., 2013, 39, 2787–2794 CrossRef CAS.
- M. C. Vasudev, K. D. Anderson, T. J. Bunning, V. V. Tsukruk and R. R. Naik, ACS Appl. Mater. Interfaces, 2013, 5, 3983–3994 CAS.
- J. C. Ruiz, A. St-Georges-Robillard, C. Thérésy, S. Lerouge and M. R. Wertheimer, Plasma Processes Polym., 2010, 7, 737–753 CrossRef CAS.
- M. Kulkarni, A. Mazare, P. Schmuki, A. Iglič and A. Seifalian, Nanomedicine, 2014, 111, 111 Search PubMed.
- C. Chen, S.-M. Zhang and I.-S. Lee, Surf. Coat. Technol., 2013, 228, S312–S317 CrossRef CAS.
- N. Adden, L. J. Gamble, D. G. Castner, A. Hoffmann, G. Gross and H. Menzel, Langmuir, 2006, 22, 8197–8204 CrossRef CAS PubMed.
- M. Godoy-Gallardo, J. Guillem-Marti, P. Sevilla, J. M. Manero, F. J. Gil and D. Rodriguez, Mater. Sci. Eng., C, 2016, 59, 524–532 CrossRef CAS PubMed.
- B. Finke, F. Luethen, K. Schroeder, P. D. Mueller, C. Bergemann, M. Frant, A. Ohl and B. J. Nebe, Biomaterials, 2007, 28, 4521–4534 CrossRef CAS PubMed.
- E. Vanderleyden, S. Mullens, J. Luyten and P. Dubruel, Curr. Pharm. Des., 2012, 18, 2576–2590 CrossRef CAS PubMed.
- J.-K. Lee, D.-S. Choi, I. Jang and W.-Y. Choi, Int. J. Nanomed., 2015, 10, 1145 CAS.
- M. Donati, D. Botticelli, V. La Scala, C. Tomasi and T. Berglundh, Clin. Oral Implants Res., 2013, 24, 738–745 CrossRef PubMed.
- T. Komori, H. Yagi, S. Nomura, A. Yamaguchi, K. Sasaki, K. Deguchi, Y. Shimizu, R. Bronson, Y.-H. Gao and M. Inada, Cell, 1997, 89, 755–764 CrossRef CAS PubMed.
- K. Anselme, Biomaterials, 2000, 21, 667–681 CrossRef CAS PubMed.
- H. Zreiqat, C. Howlett, A. Zannettino, P. Evans, G. Schulze-Tanzil, C. Knabe and M. Shakibaei, J. Biomed. Mater. Res., 2002, 62, 175–184 CrossRef CAS PubMed.
- R. O. Hynes, Cell, 1992, 69, 11–25 CrossRef CAS PubMed.
- K. Minton, Nat. Rev. Mol. Cell Biol., 2013, 14, 401 CrossRef CAS PubMed.
- D. A. Calderwood, S. J. Shattil and M. H. Ginsberg, J. Biol. Chem., 2000, 275, 22607–22610 CrossRef CAS PubMed.
- S. Y. Cheng, G. Sun, D. D. Schlaepfer and C. J. Pallen, Mol. Cell. Biol., 2014, 34, 348–361 CrossRef PubMed.
- M. Vivan Cardoso, K. Vandamme, A. Chaudhari, J. De Rycker, B. Van Meerbeek, I. Naert and J. Duyck, Clin. Implant. Dent. Relat. Res., 2015, 17, 639–645 CrossRef PubMed.
- R. Tejero, E. Anitua and G. Orive, Prog. Polym. Sci., 2014, 39, 1406–1447 CrossRef CAS.
- T. Ma, X.-Y. Ge, S.-N. Jia, X. Jiang, Y. Zhang and Y. Lin, The influence of titanium surfaces treated by alkalis on macrophage and osteoblast-like cell adhesion and gene expression in vitro, RSC Adv., 2015, 5, 81378–81387 RSC.
- X. Cai, K. Ma, Y. Zhou, T. Jiang and Y. Wang, Surface functionalization of titanium with tetracycline loaded chitosan–gelatin nanosphere coatings via EPD: Fabrication, characterization and mechanism, RSC Adv., 2016, 6, 7674–7682 RSC.
- S. Schlie-Wolter, A. Ngezahayo and B. N. Chichkov, Exp. Cell Res., 2013, 319, 1553–1561 CrossRef CAS PubMed.
- P. Brun, M. Scorzeto, S. Vassanelli, I. Castagliuolo, G. Palù, F. Ghezzo, G. M. Messina, G. Iucci, V. Battaglia and S. Sivolella, Acta Biomater., 2013, 9, 6105–6115 CrossRef CAS PubMed.
- T. A. Haas and E. F. Plow, Curr. Opin. Cell Biol., 1994, 6, 656–662 CrossRef CAS PubMed.
- H. Wolfenson, I. Lavelin and B. Geiger, Dev. Cell, 2013, 24, 447–458 CrossRef CAS PubMed.
- R. Bartolome, R. Barderas, S. Torres, M. Fernandez-Acenero, M. Mendes, J. Garcia-Foncillas, M. Lopez-Lucendo and J. Casal, Oncogene, 2014, 33, 1658–1669 CrossRef CAS PubMed.
- C. Ungewiss, Z. H. Rizvi, J. D. Roybal, D. H. Peng, K. A. Gold, D.-H. Shin, C. J. Creighton and D. L. Gibbons, Sci. Rep., 2016, 6, 18652 CrossRef CAS PubMed.
- K. Cai, M. Frant, J. Bossert, G. Hildebrand, K. Liefeith and K. D. Jandt, Colloids Surf., B, 2006, 50, 1–8 CrossRef CAS PubMed.
- C. Kuehn, E. A. Dubiel, G. Sabra and P. Vermette, Acta Biomater., 2012, 8, 619–626 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2016 |