NaHS inhibits NF-κB signal against inflammation and oxidative stress in post-infectious irritable bowel syndrome†
Shenglan Yang,
Danfang Deng,
Yingying Luo,
Yanran Wu,
Rui Zhu*,
Kaming Xue and
Yanping Zhou
Department of Integrated Traditional Chinese and Western Medicine, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430022, China. E-mail: zhuruism@yeah.net
First published on 30th June 2016
Abstract
In this study, the alleviating role of hydrogen sulfide (H2S) was investigated in a Post-Infectious Irritable Bowel Syndrome (PI-IBS) murine model and Caco-2 cells. Murine PI-IBS model was established using Trichinella spiralis infection and the results showed that mice infected with Trichinella spiralis exhibited marked intestinal inflammatory response and oxidative injury. NaHS, a H2S donor, injection markedly alleviated inflammatory response and oxidative injury and in vitro data further confirmed the anti-inflammatory and antioxidant functions in TNF-α treated Caco-2 cells. Meanwhile, TNF-α exposure reduced cellular H2S level and cystathionine β-synthase (CBS), a H2S synthesis enzyme, further exacerbated TNF-α-induced inflammation in Caco-2 cells. NaHS reversed the effect of TNF-α and CBS siRNA on cellular H2S generation and inflammation. Furthermore, NaHS inhibited Trichinella spiralis induced NF-κB upregulation in Trichinella spiralis infected group. In conclusion, H2S plays a beneficial role in PI-IBS model and TNF-α induced inflammation in vitro, which may involve in NF-κB signaling pathway.
Introduction
Irritable bowel syndrome (IBS) is a common intestinal disease with abdominal pain and bloating.1 IBS patient, who has a history of acute gastrointestinal infection, subsequently developed into post-infectious IBS (PI-IBS).2–4 Visceral hypersensitivity and dysmotility are two major pathophysiological findings. However, the pathological reason is not fully understood, while the current references indicated that gastrointestinal inflammatory response and oxidative stress may contribute to the development of PI-IBS.5–7Hydrogen sulfide (H2S), a biological gasotransmitter, exhibits various physiological functions, including cytoprotection and calcium homeostasis regulation.8 Meanwhile, H2S also has been demonstrated to exhibit an antioxidant and antiinflammatory effects in various pathological situations.9,10 However, little is known about the protective effect of H2S on PI-IBS. Thus, in this study, NaHS, a H2S donor, was used to investigate H2S effects on inflammation and oxidative stress in a murine PI-IBS model and Caco-2 cells.
Methods and materials
Animals
Thirty six Kunming male mice (22.43 ± 1.87) were housed in normal cages with controlled temperature, light/dark cycle, basic diet, and water. Experimental protocol was approved by the Animal Care and Use Committee of the Union Hospital, Tongji Medical College and Huazhong University of Science and Technology.Animal group and Trichinella spiralis infection
Animals were randomly assigned into three groups: a control group (Cont, n = 12), a PI-IBS group (PI-IBS, n = 12), and a NaHS plus PI-IBS group (NaHS, n = 12). Mice in PI-IBS and NaHS group orally administrated 260–280 Trichinella spiralis larvae per mouse (0.2 ml in 0.9% saline) according to previous report.11 The control mice received 0.9% saline instead. Mice in NaHS group intraperitoneally injected with 14 μmol kg−1 NaHS twice a day,12 while mice in the control and PI-IBS group received the same volume of sterile saline alone.Blood samples were harvested from eyes at day 14 after infection. Serum was separated by centrifugation at 3000 × g for 10 min and under 4 °C and then stored at −20 °C for further analysis. Middle section of duodenum, jejunum, ileum, and colon samples were harvested for gene expression and western blotting analysis.
AWR scores
Before slaughter, colorectal distention of each mice was evaluated at day 14 through abdominal withdrawal reflex (AWR).13 Briefly, mice were anesthetized by ether, then a balloon catheter (6-Fr, 2 mm external diameter) was inserted rectally into the descending colon of mildly sedated mice. Each 20 s distention was subsequent with 30 s resting period. 0.25, 0.35, 0.5, and 0.65 ml distention was repeated three times, and the balloon was deflated and withdrawn after assessing AWR. The AWR score was evaluated as follows: 0, no response to distention; 1, brief head movement; 2, contraction of abdominal muscles; 3, lifting of abdomen; 4, body arching and lifting of pelvic structures.Morphological analyses
Ileal samples were fixed using 10% formalin and embedded in paraffin wax. Then the wax were sectioned at 5 μm thick and mounted on slides. The tissues were further dewaxed, hydrated, and stained with hematoxylin eosin (HE). HE stained slides were evaluated in a blinded fashion by two independent investigators according to previous scoring system.14Serum antioxidant indexes
Serum activities of superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), catalase (CAT), total antioxidant capability (T-AOC) and abundances of malondialdehyde (MDA) and total glutathione (GSH) were determined by spectrophotometric kits (Nanjing Jiangcheng Biotechnology Institute, China).Ileal NF-κB activity
Ileal samples were homogenized (1 g tissue in 9 ml saline) and then centrifuged at 3000 × g for 10 min under 4 °C. The supernatants were used for determining NF-κB activity via an ELISA kit (Shanghai Yaji Bio. Tech., China).Cell culture and treatment
The human colon cancer cell line Caco-2 cells were grown in supplemented 10% fetal bovine serum (FBS), 2 mM glutamine and antibiotics (100 units per ml of penicillin and 100 μg ml−1 of streptomycin). Cells were seeded at 1 × 106 cells per well into six-well plates and allowed to attach overnight. Cells were treated with recombinant human TNF-α (2 ng ml−1) (Invitrogen/Life Technologies) to cause inflammation. Cell viability was measured by the CKK-8 assay (Sigma-Aldrich).15 Briefly, 8 × 103 cells were seeded in 96-well plates. The following day, cells were treated with 1, 10, 20, 50, 100, and 200 μM NaHS for 2 days and then assayed.Cellular IL-1β and IL-17 measurement
Cells were harvested after TNF-α and NaHS treatment for IL-1β and IL-17 measurement. Cellular protein concentrations were determined using the BCA protein assay kit (Beyotime, China). IL-1β and TNF-α abundances were assayed using human IL-1β and IL-17 ELISA kits (Boster, China).siRNA transfection
Human cystathionine β-synthase (CBS) siRNA was obtained from Guangzhou RiboBio and transfected into cells using Lipofectamine RNAiMAX reagent according to the manufacturer's instructions (Invitrogen). After transfection for 48 h, the medium over the cells was changed before subsequent treatments.Western blot
Western blotting analysis was conducted to investigate protein expression.16 Briefly, total protein extraction used protein extraction reagents (Thermo Fisher Scientific Inc., Waltham, MA, USA) and 30 μg proteins were separated by a reducing SDS-PAGE electrophoresis. Then the proteins were transferred onto a PVDF membrane (Millipore, MA, USA) and blocked with 5% non-fat milk in Tris-Tween buffered saline buffer for 1.5 hour. The primary antibodies SOD1 (ab195505), SOD2 (ab13533), Gpx1 (ab22604), Gpx4 (ab125066), Akt (ab8805), p-Akt (ab131443), IκBα (ab32518), IκBβ (ab7547), NF-κBp65 (ab86299), p-p65 (ab86299), and CBS (96252) were incubated overnight at 4 °C and the HRP-conjugated secondary antibodies were subsequently incubated for 2 hour at room temperature. Then the membrane developed the blots using Alpha Imager 2200 software (Alpha Innotech Corporation, CA, USA). We digitally quantified the resultant signals and normalized the data to β-actin (ab8226) abundance.Real-time quantitative (RT-PCR)
Total RNA was isolated from liquid nitrogen frozen and ground tissues with TRIZOL regent (Invitrogen, USA) and then treated with DNase I (Invitrogen, USA). The reverse transcription was conducted at 37 °C for 15 min, 95 °C 5 s. Primers used in this study were designed via Primer 5.0 according to mouse gene sequence (ESI Table 1†). β-Actin was chosen as the house-keeping gene to normalize target gene levels. The PCR cycling condition was 36 cycles at 94 °C for 40 s, 60 °C for 30 s and 72 °C for 35 s. The relative expression was expressed as a ratio of the target gene to the control gene using the formula 2−(ΔΔCt), where ΔΔCt = (Cttarget − Ctβ-actin)treatment − (Cttarget − Ctβ-actin)control. Relative expression was normalized and expressed as a ratio to the expression in the control group.Statistical analysis
All data were performed by using the one-way analysis of variance (ANOVA) to test homogeneity of variances via Levene's test and followed with Ducan's multiple comparison test (SPSS 17.0 software). Difference was tested by Ducan's multiple comparison test. Data are expressed as the mean ± SEN. Values in the same row with different superscripts are significant (P < 0.05).Results
Body weight and AWR scores
Body weight was measured and the results showed that Trichinella spiralis infection significantly reduced body weight after day 8 compared with the control group (P < 0.05), while NaHS injection failed to alleviated the growth suppression in the PI-IBS murine model (P > 0.05) (Fig. 1A).AWR score was used to estimate the model of PI-IBS in this study3 (Fig. 1B). Trichinella spiralis infection markedly increased AWR scores at distention volumes of 0.35 and 0.45 ml (P < 0.05), suggesting a good model of PI-IBS with visceral hypersensitivity.
Inflammatory response in mice
Inflammatory response has been widely demonstrated to involve in the progress of PI-IBS. Thus, ileal samples have been stained with H&E and the results showed that a marked infiltration by neutrophils in PI-IBS model was observed and Trichinella spiralis infection significantly increased ileal inflammatory scores (P < 0.05) (Fig. 1). The inflammation in PI-IBS model was significantly alleviated after NaHS injection evidenced by the decreased inflammatory infiltrate and inflammatory score (P < 0.05).Meanwhile, IL-1β, IL-4, IL-6, IL-10, IL-17, and TNF-α expression in the duodenum, jejunum, ileum, and colon have been further determined via RT-PCR (Fig. 2). The results showed that Trichinella spiralis infection markedly upregulated IL-1β, IL-17, and TNF-α in the duodenum, IL-1β, IL-4, IL-6, IL-10, and TNF-α in the jejunum, IL-1β, IL-4, IL-6, IL-10, IL-17, and TNF-α in the ileum, and IL-1β and TNF-α in the colon (P < 0.05). NaHS injection significantly downregulated IL-10 expression in the jejunum and IL-1β, IL-17, and TNF-α expressions in the ileum (P < 0.05), indicating an anti-inflammatory function.
Antioxidant function in mice
Our next focus was to investigate the oxidative stress in IP-IBS model and the antioxidant function after NaHS treatment. As shown at Fig. 3, Trichinella spiralis infection significantly increased serum MDA concentration and inhibited T-AOC and GSH-Px activities (P < 0.05), suggesting an oxidative stress in PI-IBS model. Meanwhile, NaHS alleviated Trichinella spiralis infection induced oxidative stress evidenced by the decreased MDA levels and increased GSH-Px activity (P < 0.05). Western blotting results from the jejunum and ileum showed that NaHS injection markedly alleviated the downregulation of Gpx1 in the jejunum and ileum caused by Trichinella spiralis infection (P < 0.05). Furthermore, CAT and GSH were determined in this study but we failed to notice any significant difference (P > 0.05) (ESI Table 2†).NaHS alleviated inflammation and oxidative stress in TNF-α-challenged Caco-2 cells
TNF-α was used for inducing inflammation in Caco-2 cells according to previous report.17 Firstly, the proper dosage of NaHS was investigated after TNF-α treatment via CKK-8 assay and the results showed that 100 μM markedly increased cell viability compared with other dosages (P < 0.05), thus we used 100 μM for further analysis (Fig. 4). | ||
| Fig. 4 Effects of NaHS on cell viability and inflammation in Caco-2 cells after TNF-α exposure. (A) Cell viability (%), (B) cellular IL-1β level (pg ml−1), and (C) cellular IL-17 level (mg ml−1). | ||
After TNF-α treatment, IL-1β and IL-17 were measured using ELISA kits. As shown at Fig. 3, TNF-α exposure markedly increased cellular IL-1β and IL-17 production (P < 0.05), indicating an inflammatory model in vitro. Although NaHS failed to affect IL-17, IL-1β abundance was significant lower in NaHS group than that in the TNF-α group (P < 0.05).
Antioxidant function of NaHS was further demonstrated in vitro model and the results showed that TNF-α treatment inhibited SOD1 and Gpx1 expression (P < 0.05). In addition, NaHS significantly increased Gpx1 and Gpx4 abundance after TNF-α exposure (P < 0.05) (Fig. 5).
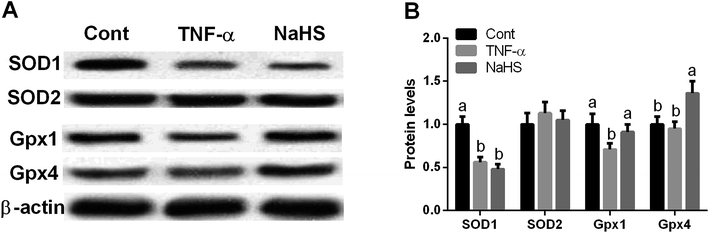 | ||
| Fig. 5 Effects of NaHS on cellular antioxidant function in Caco-2 cells after TNF-α exposure. (A and B) Protein abundances. | ||
Inhibition of H2S synthesis exacerbated TNF-α-induced inflammation in Caco-2 cells
CBS-siRNA was transfected to investigate the effects of H2S system in TNF-α induced inflammation. After TNF-α exposure, cellular H2S was significantly lower and CBS-siRNA further inhibited H2S generation (P < 0.05) (Fig. 6). NaHS markedly enhanced cellular H2S abundance after TNF-α and CBS-siRNA treatment (P < 0.05), suggesting that H2S system involves in TNF-α-induced inflammation in Caco-2 cells.Inhibition of CBS expression markedly exacerbated TNF-α induced inflammation in Caco-2 cells evidenced by the increased IL-1β and IL-17 production (P < 0.05). Meanwhile, blocking CBS-siRNA via NaHS treatment alleviated IL-1β generation after TNF-α and CBS-siRNA treatment (P < 0.05) (Fig. 6).
NF-κB and Akt signals in mice
NF-κB and Akt signals have been determined to investigate the signaling mechanism of Trichinella spiralis infection induced inflammation. As shown at Fig. 7, Trichinella spiralis infection significantly increased ileal NF-κB activity (P < 0.05), while NaHS injection alleviated NF-κB activation (P < 0.05). Western blotting results further demonstrated that NaHS inactivated ileal NF-κB signal evidenced by inhibiting NF-κBp65 phosphorylation in PI-IBS model (P < 0.05). As two upstream proteins of NF-κB signaling pathways, IκBα and IκBβ also have been tested and the results exhibited that Trichinella spiralis infection inhibited IκBα and IκBβ expression (P < 0.05), while IκBα abundance in NaHS group was significant higher than that in PI-IBS group (P < 0.05). Meanwhile, Akt and p-Akt were measured in this study and we failed to notice any significant difference in three groups (P > 0.05).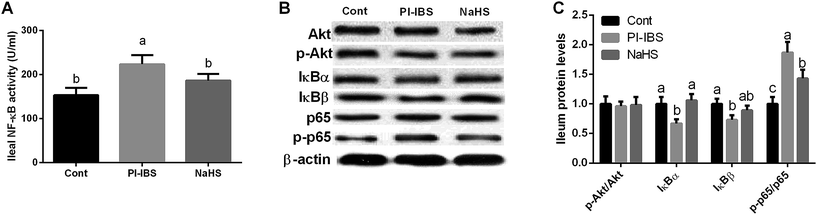 | ||
| Fig. 7 Effects of NaHS on NF-κB and Akt signals in Trichinella spiralis challenged mice. (A) Ileal NF-κB activity (U ml−1) and (B and C) protein abundances. | ||
Discussion
Previous report suggested that orally Trichinella spiralis infection causes serious inflammation and the ileum is the main target in mice.18 Meanwhile, H2S, a biological gasotransmitter, has been revealed to function as an anti-inflammatory agent in various pathological situations.10 However, excess H2S production is detrimental for cells because of its cytotoxic.19 In addition, recent study indicates that sulfide acts as a mucus barrier-breaker in inflammatory bowel diseases.20 Thus, reducing sulfide concentrations to form H2S in the intestine may be a novel therapeutic option for intestinal bowel disease. In this study, we found that H2S played a protective role in Trichinella spiralis infection induced inflammation in mice. Meanwhile, in vitro data indicated that TNF-α exposure disturbed H2S system and NaHS treatment replenished cellular H2S level and exhibited an anti-inflammatory function in Caco-2 cells.In this study, we successfully established a PI-IBS mouse model induced by Trichinella spiralis larvae evidenced by the decreased body weight gain and the disturbed visceral hypersensitivity with AWR scores. Inflammation has been demonstrated to involve in the occurrence and persistence of the symptoms in PI-IBS patient.4,21–26 In this study, we found that Trichinella spiralis infection increased ileal inflammatory score and cytokines expression, including IL-1β, IL-4, IL-6, IL-10, IL-17, and TNF-α, and NaHS injection exhibited an anti-inflammatory function in PI-IBS model. The result have been further demonstrated in vitro model that 100 μM NaHS increased cellular H2S abundance and alleviated TNF-α induced IL-1β generation in Caco-2 cells. Meanwhile, TNF-α exposure disturbed H2S system, which might promote inflammation. In a model of peritoneal fibrosis, exogenous H2S has been reported to ameliorate the pathologic process of peritonitis via attenuating inflammatory events and transforming growth factor-β1 synthesis.27 Rios et al. suggested that H2S attenuates cytokine production, including IL-6 and TNF-α, in an in vitro model of lipopolysaccharide (LPS)-induced inflammation and the mechanism may be through the modulation of chromatin remodeling.28 Thus, we speculated that H2S may serve as an anti-inflammatory agent in PI-IBS murine model and in vitro inflammation. Furthermore, anti-inflammatory effects of H2S also has been observed in other inflammatory diseases, including diabetic cardiomyopathy,29 renal ischemia-reperfusion injury,30 and neuroinflammation.31
Oxidative stress involves in various inflammation related diseases.15,23,32–34 However, the oxidative stress and antioxidant function have not been clearly illustrated in PI-IBS model. In this study, we found that Trichinella spiralis infection induced oxidative stress evidenced by the enhanced serum MDA level. MDA is the key oxidative stress biomarker, which results from a series of reactions during lipid peroxidation caused by free radical species.16,35–37 Meanwhile, we found that serum T-AOC and GSH-Px activities and protein abundance of SOD2, Gpx1, and Gpx4 have been inhibited in PI-IBS model. NaHS treatment markedly alleviated oxidative stress and increased antioxidant enzymes activities and expression in vivo and in vitro. Oxidative stress can induce dysfunction of L-glutamate transporters,38 while H2S can enhance L-glutamate uptake and alleviate oxidative stress-induced brain damage.39
NF-κB is sequestered in the cytoplasm via its inhibitory proteins, IκBs under normal condition,40 the degradation of IκBs contributes to NF-κB activation.41 NF-κB signaling pathway is activated in various inflammations.42 Thus, inhibition of NF-kB signaling pathway has been considered to be a potential target for IBD therapy. In this study, Trichinella spiralis infection significantly decreased IκBs and activated NF-κB signaling pathway in mice. Similarly, Symeonidou et al. found that various genes expression from NF-κB signaling pathway were affected between the specific points of Trichinella spiralis infection via microarray analysis.43 Meanwhile, NaHS injection alleviated the activation of NF-κB signal in PI-IBS model. H2S also has been indicated to inhibit NF-κB signal against inflammatory response.15,44 Thus, we speculated that H2S might plays a protective role in PI-IBS mice via inhibiting NF-κB activation, following with the lower inflammation. This hypothesis has been validated in a rat model of bleomycin-induced pulmonary fibrosis which demonstrated that H2S inhibited NF-κB and exhibited an anti-inflammatory effect.45
In conclusion, Trichinella spiralis infection induced intestinal inflammatory response and oxidative stress. H2S treatment alleviated inflammation and oxidative stress and enhanced antioxidant function in PI-IBS model and Caco-2 cells. The beneficial role of H2S might be associated with NF-κB signaling pathway.
Conflict of interest
There is no conflict of interest.References
- A. Vaiopoulou, G. Karamanolis, T. Psaltopoulou, G. Karatzias and M. Gazouli, World J. Gastroenterol., 2014, 20, 376–383 CrossRef PubMed.
- G. F. Longstreth, W. G. Thompson, W. D. Chey, L. A. Houghton, F. Mearin and R. C. Spiller, Gastroenterology, 2006, 130, 1480–1491 CrossRef PubMed.
- H. Wang, J. Gong, W. Wang, Y. Long, X. Fu, Y. Fu, W. Qian and X. Hou, PLoS One, 2014, 9, e90153 Search PubMed.
- F. Hirai and T. Matsui, Integr. Food, Nutr. Metab., 2015, 2, 148–150 Search PubMed.
- R. Spiller and K. Garsed, Gastroenterology, 2009, 136, 1979–1988 CrossRef PubMed.
- H. A.-R. E. Hendy and A. R. O. A. Gemeai, Integr. Food, Nutr. Metab., 2014, 1, 1–6 Search PubMed.
- S. Mileva, B. Galunska, M. Gospodinova, D. Gerova and D. Svinarov, Integr. Food, Nutr. Metab., 2014, 1, 1–6 Search PubMed.
- Y. Mikami, N. Shibuya, Y. Kimura, N. Nagahara, M. Yamada and H. Kimura, J. Biol. Chem., 2011, 286, 39379–39386 CrossRef CAS PubMed.
- Y. Kimura and H. Kimura, FASEB J., 2004, 18, 1165–1167 CAS.
- M. Bhatia, Handb. Exp. Pharmacol., 2015, 230, 165–180 CAS.
- H. Wang, J. Gong, W. F. Wang, Y. Q. Long, X. C. Fu, Y. Fu, W. Qian and X. H. Hou, PLoS One, 2014, 9(3), e90153 Search PubMed.
- L. R. Benetti, D. Campos, S. A. Gurgueira, A. E. Vercesi, C. E. Guedes, K. L. Santos, J. L. Wallace, S. A. Teixeira, J. Florenzano, S. K. Costa, M. N. Muscara and H. H. Ferreira, Eur. J. Pharmacol., 2013, 698, 463–469 CrossRef CAS PubMed.
- C. Keating, M. Beyak, S. Foley, G. Singh, C. Marsden, R. Spiller and D. Grundy, J. Physiol., 2008, 586, 4517–4530 CrossRef CAS PubMed.
- K. Kawabata, N. H. Tung, Y. Shoyama, S. Sugie, T. Mori and T. Tanaka, J. Evidence-Based Complementary Altern. Med., 2012, 2012, 820415 Search PubMed.
- M. J. McCann, J. E. Dalziel, R. Bibiloni and M. P. G. Barnett, Integr. Food, Nutr. Metab., 2015, 2, 197–204 Search PubMed.
- J. Yin, W. Ren, G. Liu, J. Duan, G. Yang, L. Wu, T. Li and Y. Yin, Free Radical Res., 2013, 47, 1027–1035 CrossRef CAS PubMed.
- H. Zhang, J. Kovacs-Nolan, T. Kodera, Y. Eto and Y. Mine, Biochim. Biophys. Acta, 2015, 1852, 792–804 CrossRef CAS PubMed.
- X. Zhou, L. Dong, B. Yang, Z. He, Y. Chen, T. Deng, B. Huang and C. Lan, Amino Acids, 2015, 47, 2635–2645 CrossRef CAS PubMed.
- X. J. Ooi and K. S. Tan, Appl. Environ. Microbiol., 2016, 82, 2078–2085 CrossRef PubMed.
- N. Ijssennagger, R. van der Meer and S. W. van Mil, Trends Mol. Med., 2016, 22, 190–199 CrossRef CAS PubMed.
- M. Schmulson, M. V. Bielsa, R. Carmona-Sanchez, A. Hernandez, A. Lopez-Colombo, Y. Lopez Vidal, M. Pelaez-Luna, J. M. Remes-Troche, J. L. Tamayo and M. A. Valdovinos, Rev. Gastroenterol. Mex., 2014, 79, 96–134 CAS.
- M. El-Salhy, World J. Gastroenterol., 2012, 18, 5151–5163 Search PubMed.
- Z. Rashti and H. Koohsari, Integr. Food, Nutr. Metab., 2015, 2, 193–196 Search PubMed.
- J. Zhou, B. Liu, C. Liang, Y. Li and Y. H. Song, Trends Endocrinol. Metab., 2016, 27, 335–347 CrossRef CAS PubMed.
- H. Wu, V. Tremaroli and F. Backhed, Trends Endocrinol. Metab., 2015, 26, 758–770 CrossRef CAS PubMed.
- A. Agus, J. Denizot, J. Thevenot, M. Martinez-Medina, S. Massier, P. Sauvanet, A. Bernalier-Donadille, S. Denis, P. Hofman, R. Bonnet, E. Billard and N. Barnich, Sci. Rep., 2016, 6, 19032 CrossRef CAS PubMed.
- Y. Lu, L. Gao, L. Li, Y. Zhu, Z. Wang, H. Shen and K. Song, Nephron, 2015, 131(3), 210–921 CrossRef CAS PubMed.
- E. C. Rios, B. Szczesny, F. G. Soriano, G. Olah and C. Szabo, Int. J. Mol. Med., 2015, 35, 1741–1746 CAS.
- X. Zhou, G. An and X. Lu, Clin. Sci., 2015, 128, 325–335 CrossRef CAS PubMed.
- I. Lobb, J. Zhu, W. Liu, A. Haig, Z. Lan and A. Sener, Can. Urol. Assoc. J., 2014, 8, E413–E418 Search PubMed.
- X. Zhou, Y. Cao, G. Ao, L. Hu, H. Liu, J. Wu, X. Wang, M. Jin, S. Zheng, X. Zhen, N. J. Alkayed, J. Jia and J. Cheng, Antioxid. Redox Signaling, 2014, 21, 1741–1758 CrossRef CAS PubMed.
- J. Yin, W. K. Ren, X. S. Wu, G. Yang, J. Wang, T. J. Li, J. N. Ding, L. C. Cai and D. D. Su, J. Food, Agric. Environ., 2013, 11, 132–139 Search PubMed.
- K. Howell, F. Yan, A. Tokich and K. Ng, Integr. Food, Nutr. Metab., 2015, 2, 184–188 Search PubMed.
- J. Eastep and G. Chen, Integr. Food, Nutr. Metab., 2015, 2, 174–179 Search PubMed.
- T. Watanabe, T. Sato, M. Miyazaki and S. Igawa, Integr. Food, Nutr. Metab., 2015, 2, 156–158 Search PubMed.
- J. V. Virbasius and M. P. Czech, Trends Endocrinol. Metab., 2016, 27, 484–492 CrossRef CAS PubMed.
- Y. Fang, T. Su, X. Qiu, P. Mao, Y. Xu, Z. Hu, Y. Zhang, X. Zheng, P. Xie and Q. Liu, Sci. Rep., 2016, 6, 21018 CrossRef CAS PubMed.
- J. Yin, W. Ren, G. Yang, J. Duan, X. Huang, R. Fang, C. Li, T. Li, Y. Yin, Y. Hou, S. W. Kim and G. Wu, Mol. Nutr. Food Res., 2016, 60, 134–146 CAS.
- M. Lu, L. F. Hu, G. Hu and J. S. Bian, Free Radicals Biol. Med., 2008, 45, 1705–1713 CrossRef CAS PubMed.
- H. S. Shin, S. I. Kang, S. A. Yoon, H. C. Ko and S. J. Kim, Biosci., Biotechnol., Biochem., 2012, 76, 847–849 CrossRef CAS PubMed.
- F. Yan and D. B. Polk, J. Biol. Chem., 1999, 274, 36631–36636 CrossRef CAS PubMed.
- J. Yin, J. Duan, Z. Cui, W. Ren, T. Li and Y. Yin, RSC Adv., 2015, 5, 15479–15486 RSC.
- I. Symeonidou, S. Pappa, A. Kourelis, A. Anogeianaki, I. Frydas, E. Karagouni and M. Hatzistilianou, Int. J. Immunopathol. Pharmacol., 2010, 23, 821–831 CAS.
- X. Zhou, Y. Feng, Z. Zhan and J. Chen, J. Biol. Chem., 2014, 289, 28827–28834 CrossRef CAS PubMed.
- H. Cao, X. Zhou, J. Zhang, X. Huang, Y. Zhai, X. Zhang and L. Chu, Toxicol. Lett., 2014, 224, 387–394 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra13849g |
| This journal is © The Royal Society of Chemistry 2016 |




