Nanoporous ZnO nanostructure synthesis by a facile method for superior sensitivity ethanol sensor applications†
Nguyen Thi Phuong Nhung*b,
Pham Van Tongac,
Chu Manh Hunga,
Nguyen Van Duya,
Nguyen Viet Chiena,
Nguyen Van Vinhb,
Nguyen Thai Tuyenb and
Nguyen Duc Hoa *a
*a
aInternational Training Institute for Materials Science (ITIMS), Hanoi University of Science and Technology (HUST), No. 1, Dai Co Viet Road, Hai Ba Trung, Hanoi, Vietnam. E-mail: ndhoa@itims.edu.vn; Fax: +84 4 38692963; Tel: +84 4 38680787
bPetroVietnam University, 7th Floor, PVMTC Building, Cach Mang Thang Tam Str., Long Toan Ward, Ba Ria-Vung Tau Province, Vietnam
cDepartment of Physics, Faculty of Mechanical Engineering, National University of Civil Engineering (NUCE), No. 55, Giai Phong Str., Hanoi, Vietnam
First published on 30th June 2016
Abstract
Nanoporous ZnO nanostructures were prepared by simple thermal decomposition of plate-like hydrozincite for gas sensor applications. The plate-like hydrozincite obtained through room temperature precipitation had a diameter of 100 nm and a thickness of about 10 nm. After thermal decomposition, nanoporous ZnO nanoparticles on average 30 nm (16.12 nm in crystalline size) in diameter were obtained. Gas-sensing measurements demonstrated that the nanoporous ZnO nanostructures are promising for superior sensitivity ethanol monitoring in lung cancer diagnosis.
1. Introduction
Zinc oxide (ZnO) is one of the most popular metal oxide semiconductors used in gas sensor fabrication; in recent years, it has gained considerable attention because of its ability to sense several gases.1,2 Various methods have been utilized for the fabrication of ZnO nanostructures, including sputtering and thermal oxidation,3 spray pyrolysis,4 precipitation,5 electrochemical deposition,6 template-assisted synthesis,7 and the sol–gel method.8 For instance, Mani et al. reported the facile synthesis of ZnO nanostructures through spray pyrolysis for H2S sensors.9 Jin et al. reported the hydrothermal synthesis of monodisperse ZnO hollow structures for ethanol sensors.10 Mao et al. synthesized porous ZnO nanoparticles by combining precipitation and hydrothermal methods for acetone sensors.11 However, the hydrothermal process requires high temperature treatment, high pressure reactor, and thus consuming high energy, and expensive equipment.3 In addition, such these methods form non-porous materials, which limit their potential gas-sensing applications.5 Recently, researchers have developed a simple pathway to synthesize porous materials by preparing correlative metastable phases and converting them into desired oxides.12,13 The thermal decomposition of metastable phases is highly effective for the fabrication of metal oxides with the ability to control porosity.12 Wahab et al. reported the sol–gel synthesis of hydrozincite and its conversion into ZnO nanoparticles.14 Cheng et al.15 prepared hydrozincite through a solid-state metathesis reaction and studied its thermal decomposition into ZnO. Wang et al.16 prepared hierarchical hydrozincite using a hydrothermal method and converted it to ZnO nanostructures. However, the hydrothermal and sol–gel methods require high temperatures and a long synthesis time. The lack of porosity in the ZnO nanoparticles also limits the sensing performance of materials. Furthermore, the gas-sensing characteristics of the ZnO nanostructures prepared by thermal decomposition of hydrozincite have not been fully investigated.In this communication, we report the simple synthesis of nanoporous ZnO nanostructures through the thermal decomposition of hydrozincite and investigated their gas-sensing characteristics. The plate-like hydrozincite was prepared by a scalable wet chemical precipitation method at room temperature. Thermal decomposition was applied to control the morphology, porosity, and crystalline size of ZnO. Gas-sensing measurements demonstrated that the prepared ZnO nanostructure exhibited exceptional ethanol sensing characteristics.
2. Experimental
The materials used in this study included reagent-grade zinc nitrate, sodium carbonate, and ethanol purchased from Sigma–Aldrich. The nanoporous ZnO nanostructure was synthesized by thermal decomposition of the plate-like hydrozincite. First, hydrozincite was prepared by dropping 50 mL of 1 M zinc nitrate to 10 mL of 1 M sodium carbonate with vigorous stirring at room temperature at a flow rate of 10 mL min−1. Second, the synthesized hydrozincite was collected via centrifugation (4000 rpm) and washed with distilled water several times. The precipitate was then washed with ethanol three times and dried at 60 °C for 4 h. A photo of the precipitated product after drying can be seen in Fig. S1 (ESI†). By weighting the precipitation (2.6 g), and comparing with the theoretical value (2.74 g), we estimated that the yield of product was about 94% of sodium carbonate used. Finally, ZnO was obtained after the thermal decomposition of hydrozincite particles in air at 400 °C for 2.5 h.For sensor fabrication, as-made hydrozincite was dispersed in dimethylformamide solution and spin coated on comb-type interdigitated Pt electrode arrays. The sensor arrays were then heat treated at 400 °C for 2.5 h prior to the gas-sensing measurements. The morphology and composition of the synthesized materials were characterized by scanning electron microscopy (SEM, JEOL 7600F), energy-dispersive X-ray spectroscopy (EDS, Oxford instruments), and X-ray powder diffraction (XRD, D8 Advance Bruker). Thermal decomposition of hydrozincite was studied by dynamic thermogravimetric and differential thermal analyses (TGA and DTA, respectively). The gas-sensing characteristics were measured at different temperatures using a Keithley model 6220 as described elsewhere.
3. Results and discussion
The crystal structure of the as-precipitated product was characterized by powder XRD, and the data are shown in Fig. 1(A). The typical diffraction peaks were indexed to the profile of the standard hydrozincite with monoclinic phase (JCPDS no. 19-1458). The broad XRD peaks revealed the nanostructure of the material, which was consistent with the average crystal size calculated by the Scherrer equation using the strongest peak (401) of 13.76 nm.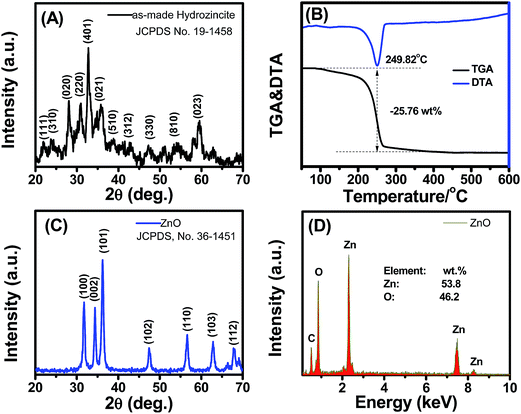 | ||
| Fig. 1 (A) XRD pattern and, (B) TGA & DTA analysis of the as-made hydrozincite; (C) XRD pattern and (D) EDS analysis of the nanoporous ZnO nanostructures. | ||
The TGA and DTA results of hydrozincite synthesized in air are shown in Fig. 1(B). The TGA results showed a total weight loss of 25.76 wt% at temperatures ranging from 100 °C to 400 °C. The weight loss was consistent with the thermal decomposition of hydrozincite into ZnO, in which the theoretical calculation was 25.9%. The DTA results demonstrated an endothermic peak centered at 249.82 °C as a result of the thermal decomposition of hydrozincite, according to the following equation: Zn5(CO3)2(OH)6 → 5ZnO + 2CO2 + 3H2O. This result was consistent with that reported by Nistor et al.,17 who studied the formation and crystallization of disordered nanosized ZnO through the decomposition of commercial hydrozincite.
The XRD pattern of the ZnO nanostructure obtained through the thermal decomposition of hydrozincite is shown in Fig. 1(C). The typical diffraction peaks were well indexed to the profile of the hexagonal crystal structure (JCPDS, no. 36-1451),18 thereby indicating the total decomposition of hydrozincite into ZnO nanostructures. The average crystalline size calculated by the Scherrer equation using the (101) peak was 16.12 nm. We could control the average crystalline size of the ZnO nanoparticles in the range of 16.12 to 35.9 nm by varying the decomposition temperatures from 400 to 700 °C (Fig. S2. ESI†).
The composition of the synthesized ZnO nanostructure was analyzed by EDS, and the data are shown in Fig. 1(D). The presence of O and Zn corresponded to the composition of ZnO. The ratio of [O]/[Zn] = [48.2]/[53.8] was less than one, which confirmed the formation of non-stoichiometric ZnO1−δ. This non-stoichiometric characteristic will result in the n-type nature of ZnO, which is suitable for gas sensor applications.19
The SEM images of the as-made hydrozincite shown in Fig. 2(A) and (B) indicate that the plate-like materials with irregular shapes were obtained through precipitation. The average diameter and thickness of the plate-like particles were less than 100 and 10 nm, respectively. The plate-like particles were distributed randomly but did not agglomerate into a flower-like formation, which caused easy dispersion for the spin-coating synthesis of gas sensors.16,20 The inset in Fig. 2(B) is a photograph of the hydrozincite colloid dispersed in dimethylformamide solution used for sensor fabrication.
The SEM images of the ZnO nanostructures obtained through the thermal decomposition of hydrozincite at 400 °C are shown in Fig. 2(C) and (D). The ZnO nanostructures exhibited irregular shapes different from the plate-like forms of hydrozincite. The average particle size estimated from the SEM images was approximately 30 nm, which was larger than the crystalline size calculated from XRD. This finding revealed the polycrystalline nature of ZnO. Moreover, the surface of the ZnO nanostructures was not smooth but formed from nanograins. The thermal decomposition of hydrozincite resulted in significant weight loss and formed a nanoporous structure in ZnO, as shown in Fig. 2(D). However, the nanoporous structure was damaged with increase of thermal decomposition temperatures (Fig. S3, ESI†). The inset of (D) shows a photograph of the fabricated sensors, which reveals the easy and scalable fabrication of the devices. To confirm the nanoporous structure of the materials, the nitrogen adsorption/desorption isotherm of the sample calcinated at 400 °C was measured using a Gemini VII 2390 Surface Area Analyzer (Fig. S4. ESI†). The specific surface area (SBET), pore volume (Vp), and pore size (Dp) of the nanoporous ZnO sample calcinated at 400 °C are SBET = 37.89 m2 g−1, Vp = 0.049 cm3 g−1, and Dp = 3.01 nm, respectively. The specific surface area of our sample is much higher than that of the aggregated nanoparticles (SBET: 0.49–6.02 m2 g−1),21 and the nanoparticles obtained by the precipitation in the presence of surfactants (SBET: ∼17 m2 g−1).22 In addition, the average pore size of our sample is smaller than that of the aggregated nanoparticles (Dp: 8.96–221.4 nm),23 thus we believed that the nanopores were inside the nanoparticles but not the interspace between nanoparticles.
Ethanol is a volatile organic compound associated with lung cancer; thus, monitoring this gas at low concentrations is important.24 It was reported that metal oxide with smaller grain size, and porous structure showed a higher gas sensitivity,25 thus in this work we selected the sample 400 °C to test with ethanol. The ethanol-sensing characteristics of the fabricated sensor were tested from 250 °C to 400 °C, and the results are shown in Fig. 3. The transient resistances versus time upon exposure to different concentrations are shown in Fig. 3(A). The sensor showed good response and recovery characteristics at all measured temperatures, but the resistance decreased upon exposure to ethanol. The gas-sensing mechanism of the fabricated sensor can be explained through the interactions between ethanol molecules and pre-adsorbed oxygen, as shown by the following equations.
| C2H5OH + 3O2− ↔ 2CO2 + 3H2O + 3e− | (1) |
| C2H5OH + 6O− ↔ 2CO2 + 3H2O + 6e− | (2) |
| C2H5OH + 6O2− ↔ 2CO2 + 3H2O + 12e− | (3) |
The interaction between ethanol molecules and pre-adsorbed oxygen releases electrons back to the crystals and reduces the space–charge layer, resulting in decreased sensor resistance.13 The ethanol-sensing performance improved as the measured temperatures increased from 250 °C to 400 °C. The 90% response and recovery times of the sensor to 50 ppm ethanol measured at 400 °C were approximately 3 and 70 s, respectively. Fig. 3(B) shows the sensor response as a function of ethanol concentrations, which were measured at temperatures ranging from 250 °C to 400 °C. The sensor response increased linearly with ethanol concentrations at all temperatures. At 400 °C, the sensor exhibited the highest response values of 2.2–10.9 to extremely low ethanol concentrations ranging from 2.5 ppm to 50 ppm. Those values are comparable to the responses of the mesoporous ZnO–SnO2 nanofibers (12.8 to 50 ppm at 300 °C),26 and brush-like hierarchical nanostructure (∼10 to 50 ppm at 265 °C).27 The response value of 2.2 to ethanol concentration of 800 ppm under light illumination was reported in ZnO nanoparticles,28 which revealed the superior sensitivity of the nanoporous material.26,29 Superior sensitivity of the synthesized ZnO can be ascribed to the (i) nanoporous nature,12 and the (ii) fine crystalline size of nanoparticles.19 The prepared ZnO nanoparticles have higher external surface area; therefore they provide more sorption sites for the analyte, and thus higher sensitivity. The nanoporous structure of ZnO allowed the ethanol molecules to penetrate easily into the sensing material and change its conductivity, whereas the small crystalline size (∼16.12 nm) is close to two times of the Debye length of ZnO, thus enabled the total depletion and maximized the sensitivity.30
4. Conclusion
In summary, the ZnO nanostructures were successfully synthesized via simple, saleable, and inexpensive thermal decomposition of hydrozincite prepared by precipitation. The particle and crystalline sizes of the ZnO nanostructures were easily controlled by varying the temperature of thermal decomposition. The gas-sensing measurements indicated that the nanoporous ZnO nanostructures showed high sensitivity, fast response/recovery times, and good selectivity to ethanol. These findings demonstrate the highly promising applications of the nanoporous ZnO nanostructures.Acknowledgements
This work was supported by the Vietnam National Foundation for Science and Technology Development (NAFOSTED) under grant number 103.02-2013.62. SEM images were characterized at Laboratory of Electron Microscopy and Microanalysis (BKEMMA).References
- S. Wang, X. Gao, J. Yang, Z. Zhu, H. Zhang and Y. Wang, RSC Adv., 2014, 4, 57967–57974 RSC.
- K. Diao, Y. Huang, M. Zhou, J. Zhang, Y. Tang, S. Wang, T. Liu and X. Cui, RSC Adv., 2016, 6, 28419–28427 RSC.
- V. Galstyan, E. Comini, C. Baratto, G. Faglia and G. Sberveglieri, Ceram. Int., 2015, 41, 14239–14244 CrossRef CAS.
- T. Tharsika, A. S. M. A. Haseeb, S. A. Akbar and M. Thanihaichelvan, Ceram. Int., 2015, 41, 5205–5211 CrossRef CAS.
- V. Sanna, N. Pala, V. Alzari, D. Nuvoli and M. Carcelli, Mater. Lett., 2016, 162, 257–260 CrossRef CAS.
- R. Dalvand, S. Mahmud and J. Rouhi, Mater. Lett., 2015, 160, 444–447 CrossRef CAS.
- Y. Chen, X. Li, X. Li, J. Wang and Z. Tang, Sens. Actuators, B, 2016, 232, 158–164 CrossRef CAS.
- S. Ameen, D.-R. Park, M. Shaheer Akhtar and H. S. Shin, Mater. Lett., 2016, 164, 562–566 CrossRef CAS.
- G. K. Mani and J. B. B. Rayappan, Mater. Lett., 2015, 158, 373–376 CrossRef CAS.
- W. X. Jin, S. Y. Ma, Z. Z. Tie, X. L. Xu, X. H. Jiang, W. Q. Li, T. T. Wang, Y. Lu and S. H. Yan, Mater. Lett., 2015, 159, 102–105 CrossRef CAS.
- Y. Z. Mao, S. Y. Ma, W. Q. Li, J. Luo, L. Cheng, D. J. Gengzang and X. L. Xu, Mater. Lett., 2015, 157, 151–154 CrossRef CAS.
- N. D. Hoa and S. A. El-Safty, Chem.–A Eur. J., 2011, 17, 12896–12901 CrossRef CAS PubMed.
- J. Zhang, S. Wang, M. Xu, Y. Wang, B. Zhu, S. Zhang, W. Huang and S. Wu, Cryst. Growth Des., 2009, 9, 3532–3537 CAS.
- R. Wahab, S. G. Ansari, Y. S. Kim, M. A. Dar and H.-S. Shin, J. Alloys Compd., 2008, 461, 66–71 CrossRef CAS.
- J. Cheng and K. Poduska, Nanomaterials, 2013, 3, 317–324 CrossRef CAS.
- Z. Wang, D. Chu, L. Wang, L. Wang, Y. Zhang and X. Chen, Mater. Lett., 2016, 169, 99–102 CrossRef CAS.
- S. V. Nistor, L. C. Nistor, M. Stefan, D. Ghica, G. Aldica and J. N. Barascu, Cryst. Growth Des., 2011, 11, 5030–5038 CAS.
- M. Jiao, N. V. Chien, N. Van Duy, N. D. Hoa, N. Van Hieu, K. Hjort and H. Nguyen, Mater. Lett., 2016, 169, 231–235 CrossRef CAS.
- H. Van Han, N. D. Hoa, P. Van Tong, H. Nguyen and N. Van Hieu, Mater. Lett., 2013, 94, 41–43 CrossRef.
- C. Yan and D. Xue, J. Phys. Chem. B, 2006, 110, 11076–11080 CrossRef CAS PubMed.
- S. Musić, Đ. Dragčević, S. Popović and M. Ivanda, Mater. Lett., 2005, 59, 2388–2393 CrossRef.
- Y. Wang, C. Ma, X. Sun and H. Li, Inorg. Chem. Commun., 2002, 5, 751–755 CrossRef CAS.
- M. Thirumavalavan, K.-L. Huang and J.-F. Lee, Materials, 2013, 6, 4198–4212 CrossRef CAS.
- G. Peng, U. Tisch, O. Adams, M. Hakim, N. Shehada, Y. Y. Broza, S. Billan, R. Abdah-Bortnyak, A. Kuten and H. Haick, Nat. Nanotechnol., 2009, 4, 669–673 CrossRef CAS PubMed.
- T. Kida, S. Fujiyama, K. Suematsu, M. Yuasa and K. Shimanoe, J. Phys. Chem. C, 2013, 117, 17574–17582 CAS.
- X. Song, Z. Wang, Y. Liu, C. Wang and L. Li, Nanotechnology, 2009, 20, 075501 CrossRef PubMed.
- Y. Zhang, J. Xu, Q. Xiang, H. Li, Q. Pan and P. Xu, J. Phys. Chem. C, 2009, 113, 3430–3435 CAS.
- Z. Q. Zheng, J. D. Yao, B. Wang and G. W. Yang, Sci. Rep., 2015, 5, 11070 CrossRef CAS PubMed.
- P.-P. Wang, Q. Qi, R.-F. Xuan, J. Zhao, L.-J. Zhou and G.-D. Li, RSC Adv., 2013, 3, 19853 RSC.
- C.-C. Lin, H.-P. Chen, H.-C. Liao and S.-Y. Chen, Appl. Phys. Lett., 2005, 86, 183103 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra11531d |
| This journal is © The Royal Society of Chemistry 2016 |


