Preparation and physical property assessments of liquid-core hydrogel beads loaded with burdock leaf extract
Abstract
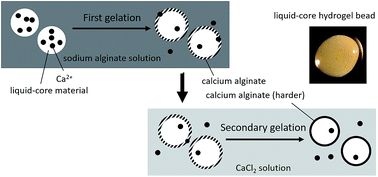
Secondary gelation is an important but overlooked element which has a significant impact on the quality of liquid-core hydrogel beads (LHB). This study firstly searches the optimized extraction conditions of burdock leaf and analyses its functional compounds and antioxidant abilities. Then, we measure loading efficiency, relative hardness, swelling capacity, and outer appearance to evaluate the optimized preparation conditions of LHB loaded with burdock leaf extract (BLE). The results showed that the optimized extraction conditions of burdock leaf were a solid to liquid (95% ethanol) ratio of 1 : 3, and extraction at 80 °C for 90 min. This extraction contained 23.81 mg g−1 dw chlorogenic acids (CGA) and the DPPH scavenging activity was 59.49%. LHB prevented the DPPH scavenging ability of BLE from decreasing during storage. The diameter and swelling capacity of burdock LHB increased, and hardness and loading efficiency decreased with longer gelation times. Relative hardness of the LHB prepared by 1% CaCl2 in the secondary gelation was 5.6-fold higher than that of the control, but there was no significant difference in CGA loading efficiency.

 Please wait while we load your content...
Please wait while we load your content...