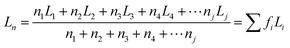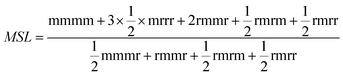Microstructure of polypropylene and active center in Ziegler–Natta catalyst: effect of novel salicylate internal donor
Qian Zhoua,
Ailian Wanga,
Huayi Li*b,
Zhi Luoa,
Tao Zhenga,
Liaoyun Zhang*a and
Youliang Hub
aCollege of Chemistry and Chemical Engineering, University of Chinese Academy of Sciences, Beijing 100049, China. E-mail: zhangly@ucas.ac.cn
bBeijing National Laboratory for Molecular Sciences, CAS Key Laboratory of Engineering Plastics, Institute of Chemistry, Chinese Academy of Sciences, Beijing 100190, China. E-mail: lihuayi@ucas.ac.cn
First published on 25th July 2016
Abstract
Five salicylates with different sizes of hydrocarbon substituents were firstly synthesized and employed as ecofriendly internal donors of the Ziegler–Natta catalyst for propylene polymerization. The influences of these salicylates and traditional, industrial, internal donor diisobutyl phthalates on the microstructure of polypropylene and active center in a Ziegler–Natta catalyst were studied. It was found that the catalyst activities of the catalysts containing salicylate internal donors with a proper volume were higher than the catalysts containing diisobutyl phthalate internal donors. GPC results showed that the molecular weights of polypropylene prepared by salicylate internal donors were lower than those prepared by diisobutyl phthalate, which indicated that the polypropylene chains produced by salicylate internal donors were easier to transfer than those prepared by diisobutyl phthalate internal donors. Deconvolution of the GPC curves exhibited that as the volume of the salicylate internal donor increased some of the active centers for low molecular weight transferred into the active centers for high molecular weight. The results of 13C-NMR and SSA both suggested that a salicylate internal donor with an appropriate catalyst size volume was beneficial for increasing the isotactic sequence length, isotacticity index and regular triads “mm” of polypropylene. However, further increasing the volume of the salicylate internal donor in a catalyst would lead to the polypropylene chain containing more stereo-defects. Moreover, the active centers with different stereospecificity parameters, piso, in the catalyst could explain the trend of stereo-defects in polypropylene chains when different internal donors were used. In addition, it was found that the isotactic sequence length and isotacticity index of polypropylene prepared by isobutyl 2-benzyloxy-3,5-isopropyl benzoate were close to that produced by a diisobutyl phthalate internal donor. Moreover, the lamella thickness distribution of the polypropylene produced by a salicylate internal donor was broad, which might have potential application for expanded polypropylene materials.
Introduction
Driven by continuous developments in catalyst technology and polymerization processes, polypropylene has many excellent properties and has become one of the most widely used thermoplastic resins.1–3 At present, the forth Ziegler–Natta catalyst is one of the most widely used catalyst systems for propylene polymerization,4 which always contains MgCl2-supported TiCl4, an internal donor, triethyl aluminium (TEA) and an external donor.5 Since internal donors are applied to the Ziegler–Natta catalyst system, it has become a key component for improving comprehensive properties of polypropylene.6–8 Many kinds of compounds9–14 are used as Ziegler–Natta catalyst internal donors for research. Now, phthalate3 is the most widely used internal donor for the industrial production of polypropylene. However, phthalates are harmful to the human body, and the European Union and the United States have both restricted the use of phthalates in certain plastics. Our group uses aliphatic diesters as ecofriendly internal donors15 and obtains catalysts with high catalytic activity and polypropylene with high isotacticity. However, the synthesis of aliphatic diesters is difficult on an industrial scale. Salicylic acid is known for its ability to ease aches and pains and is a component of aspirin.16 Moreover, some salicylates are often used in cosmetics as a fragrance additive and UV light absorber. However, there are no reports on salicylate as an internal donor for polypropylene polymerization. In addition, it was found that the internal donor could strongly impact the active center of the Ziegler–Natta catalyst and the microstructure of polypropylene,15,17,18 which determines the properties and industrial applications of polypropylene. Successive self-nucleating and annealing (SSA) thermal fractionation19,20 can give lamella thickness and lamella thickness distribution of polypropylene, and both properties are related to the isotactic sequence length and isotactic sequence distribution of polypropylene. In addition, 13C-NMR21,22 can be used not only to measure the stereo-defect distribution of polypropylene, but also to analyze the stereoselectivity of the active center in the Ziegler–Natta catalyst. Based on the results of 13C-NMR and DSC, a DSC curve model23 could be used to analyze the active center with a different stereoselectivity in the Ziegler–Natta catalyst. In our study, five salicylates with different size hydrocarbon substituents were synthesized and employed as ecofriendly internal donors of the Ziegler–Natta catalyst for propylene polymerization. It was found that the catalyst activity of catalysts containing SID internal donors with a proper volume was higher than that of catalysts containing DIBP internal donors. Deconvolution of the GPC curves indicated that as the volume of the SID internal donor increased, some of the active centers for low molecular weight transformed into active centers for high molecular weight. The results of 13C-NMR and SSA both suggested that the SID internal donor with an appropriate catalyst size volume was beneficial for increasing the isotactic sequence length, isotacticity index and regular triads “mm” of polypropylene, while further increasing the volume of SID internal donors in the catalyst would lead to the polypropylene chain containing more stereo-defects. The DSC curve model was used to study the stereospecificity parameter, piso, of active centers in the catalyst, which could be used to explain the trend of isotactic sequence length of polypropylene prepared by different internal donors. In addition, it was found that the isotactic sequence length and isotacticity index of polypropylene prepared by SID-4 were very close to that produced by DIBP internal donors.Experimental
Materials
Salicylic acid, 3-methyl salicylic acid, 3,5-isopropyl salicylic acid, 3,5-tert-butylation salicylic acid, benzoyl chloride, trimethylacetyl chloride, sodium bicarbonate, chloroform, propylene, hydrogen, triethylaluminium (TEA), diisopropyldimethoxysilane (donor-P) and diisobutyl phthalate (DIBP) were purchased from Aladdin in reagent grade and were used without further purification.Synthesis of the salicylate internal donor
The synthesis method for the five salicylate internal donors was based on the literature.24 The purity of salicylate used for propylene bulk polymerization was above 98%. The synthetic route for salicylate and the structures of the five salicylates are shown in Schemes 1 and 2, respectively. As a typical example, the synthesis process of isobutyl 2-benzyloxybenzoate (SID-1) was carried out as follows:A 100 ml flask contained 40 ml isobutanol, 0.3 mol salicylic acid and 0.5 ml concentrated sulfuric acid. The mixture was stirred at 120 °C for 5 h. When water no longer appeared in the water separator, the heater was turned off. After the formation of isobutyl salicylate was confirmed by gas chromatography, isobutyl salicylate was purified by decompressing distillation. Isobutyl salicylate (0.05 mol), 0.08 mol benzoyl chloride and 0.005 mol bismuth(III) oxychloride were stirred at 40 °C for 6 h. After isobutyl 2-benzyloxybenzoate was confirmed by gas chromatography, the mixture was stirred with aqueous sodium hydrogen carbonate. The organic phase was extracted from the water phase, and then a transparent and oily liquid 2-benzyloxybenzoate was obtained by decompressing distillation.
The synthesis processes for the other salicylates, including isobutyl 2-benzyloxy-3-methyl benzoate (SID-2), isobutyl 2-trimethyloxy-3-methyl benzoate (SID-3), isobutyl 2-benzyloxy-3,5-isopropyl benzoate (SID-4) and isobutyl 2-benzyloxy-3,5-tert-butyl benzoate (SID-5), were similar to that of SID-1. 1H NMR (DMSO) δ (ppm): SID-1: 0.81 (d, 6H), 1.73 (m, 1H), 3.92 (d, 2H), 7.25 (t, 2H), 7.60 (m, 3H), 7.72 (d, 1H), 8.07 (d, 1H), 8.19 (d, 2H); SID-2: 0.82 (d, 6H), 1.21 (s, 9H), 1.92 (m, 1H), 2.13 (s, 3H), 4.01 (d, 2H), 7.08 (t, 1H), 7.38 (d, 1H), 7.80 (d, 1H); SID-3: 0.85 (d, 6H), 1.80 (m, 1H), 3.95 (d, 2H), 7.42 (d, 2H), 7.60 (t, 3H), 7.75 (d, 1H), 8.25 (d, 2H); SID-4: 0.85 (d, 6H), 1.14 (d, 12H), 1.91 (m, 1H), 2.81 (m, 1H), 2.99 (m, 1H), 3.96 (d, 2H), 7.47 (s, 1H), 7.53 (t, 2H), 7.66 (m, 2H), 8.16 (d, 2H); SID-5: 0.88 (d, 6H), 1.39 (s, 18H), 1.78 (m, 1H), 3.90 (d, 2H), 7.54 (t, 2H), 7.63 (t, 1H), 7.93 (s, 2H), 8.25 (d, 2H).
Preparation of catalyst
The Ziegler–Natta catalysts with salicylate as the internal donor were prepared in a reactor under dry nitrogen conditions. Magnesium ethoxide (5 g) and 150 ml dry toluene were added to a glass reactor under nitrogen at room temperature. Then, 50 ml TiCl4 was added to reactor by a funnel over half an hour at 5 °C. Two millilitres of salicylate internal donor was injected into the reactor after dripping the TiCl4. The mixture was stirred at 110 °C for 2 h. After reaction, the supernatant was removed, and the residue was washed by toluene. The precipitated solid was treated again with a mixture of 150 ml toluene and 50 ml titanium tetrachloride at 110 °C for 2 h. After discarding the supernatant, the residue was washed with toluene and hexane, respectively. A solid catalyst was obtained.Propylene bulk polymerization
Firstly, 800 g of propylene gas and 0.3 MPa of hydrogen gas were charged with a reactor. The C(H2)/C(propylene) ratio was 2.59 mmol mol−1. Then, 20 mg of the Ziegler–Natta catalyst, 12 mmol TEA in n-hexane solution and 0.39 mmol donor-P were added to the system. The reactor was heated to 70 °C and stirred at 300 rpm for an hour. Then, the propylene was exhausted, and polypropylene was obtained as a white powder.UV-Vis spectra
The content of Ti in the catalyst was measured by UV-Vis spectra (UV-1800). Firstly, the catalyst was dissolved in a dilute sulfuric acid solution. Then, titanium was converted to [TiO(H2O2)]SO4 by the addition of H2O2. A spectrophotometer was used to record UV-Vis spectra of the resultant solutions of catalyst complexes.Gas chromatography
The ester content of the catalysts was determined on a gas chromatograph (clarus 580) manufactured by PE in the US.Differential scanning calorimetry
A Mettler 822e differential scanning calorimeter was used to measure the thermal properties of polypropylene. Polypropylene (2–4 mg) was put in an aluminium pan. To erase the thermal history, the pan with polypropylene was heated from 50 °C to 200 °C at a heating rate of 50 °C min−1. It was held at 200 °C for 5 min in a nitrogen atmosphere. Then, polypropylene in the pan was cooled to 50 °C and held at 50 °C for 5 min. Finally, the polypropylene was heated to 200 °C at 10 °C min−1.Gel permeation chromatography
PL-GPC 220 high-temperature gel permeation chromatography (Polymer Laboratories Ltd.) was used to measure the molecular weights (Mn and Mw) and the polydispersity index (PI) of polypropylene. The measuring temperature was 413.15 K. 1,2,4-Trichlorobenzene was the solvent for polypropylene. The injection volume was 100 ml, and the flow rate was 1.0 ml min−1. The calibration was made by used linear polystyrene as the standard sample.Successive self-nucleation and annealing
Successive self-nucleation and annealing (SSA) technology has been widely used to analyze the lamellar thickness and isotactic sequence length of polymers.25 The method includes several heating and cooling steps: (1) DSC heating of polypropylene to 200 °C at 50 °C min−1 and then holding for 5 min; this step is for the erasure of the crystalline thermal history of polypropylene. (2) Cooling polypropylene to 50 °C at 20 °C min−1 and holding for 5 min. (3) Heating polypropylene from 50 °C to a partial melting temperature denoted Ts at 20 °C min−1. (4) Keeping polypropylene at Ts for 10 min. (5) Cooling polypropylene from Ts to 50 °C at 20 °C min−1. (6) Repeat steps ‘c’ to ‘e’ at increasingly a new lower Ts, which were varied from 164 to 144 °C, at 5 °C intervals for a total five self-nucleation/annealing steps. (7) Finally, heating polypropylene from 50 °C to 200 °C at 10 °C min−1. Then, multiple melting curves (SSA curve) were obtained. Pijpers gave the method for looking at the optimum Ts temperatures of polypropylene in the literature.26Nuclear magnetic resonance spectroscopy
DMX 300M (Bruker) was used to test the 13C-NMR spectra of polypropylene. Polypropylene (80–100 mg) was dissolved in 0.5 ml of deuterated o-dichlorobenzene at 383 K. The o-dichlorobenzene solvent was used to provide the internal lock signal with its highest peak at 132.700 ppm. The number of pulses was more than 5000; pulse angle was 30; spectrum width was 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Hz; and relaxation delay was 7 s. All spectra were completely proton decoupled.
000 Hz; and relaxation delay was 7 s. All spectra were completely proton decoupled.
Results and discussion
Components and catalytic activity of the Ziegler–Natta catalyst
Five salicylates with different hydrocarbyl substituents on the phenyl group were firstly prepared, as Scheme 2 shows. Then, the five salicylates (SID) and di-butyl phthalate (DIBP) were used as internal donors to prepare six Ziegler–Natta catalysts, as Table 1 lists. Table 1 also displays the Ti content and ester content in the Ziegler–Natta catalyst. As shown in Table 1, the Ti contents and ester contents in SID catalysts were both lower than that of the DIBP catalyst, indicating that the Ti and ester contents in the catalysts could be affected by the types of internal donors. Besides, comparing Cat-1, Cat-3 and Cat-4, the ester content of the catalysts increased from Cat-1 to Cat-4, while their Ti content decreased from Cat-1 to Cat-4. It was reported that the ester can coordinate with Mg atoms on the (110) facets of MgCl2 crystallites,27 which could block surface sites on the MgCl2 support and lead to Ti hardly coordinating with the support. Therefore, the trend of relative Ti and ester content was contrary. Previous research found that an increase in the ester content in a catalyst was beneficial for improving the catalyst activity of the Ziegler–Natta catalyst.28 Table 1 shows that the catalyst activity increased from 6.6 × 105 g PP per g Ti to 24.1 × 105 g PP per g Ti as the ester content of catalyst increased from 1.1% to 2.2%. The trend of catalyst activity and ester content in the catalyst also matched previous conclusions. In addition, it was found that catalyst activities of the SID catalyst (except for SID-1) were much higher than that of the DIBP catalyst, which indicated that SID internal donors with a proper volume were more beneficial for the catalyst activity than DIBP internal donors in catalysts.| Samples | Donor | Catalytic activity (×105 g PP per g Ti) | Tia (wt%) | Esterb (wt%) |
|---|---|---|---|---|
| a The weight percent of Ti in the catalyst.b The weight percent of ester in the catalyst. | ||||
| Cat-1 | SID-1 | 6.6 | 2.5 | 1.1 |
| Cat-2 | SID-2 | 13.7 | 1.7 | 1.9 |
| Cat-3 | SID-3 | 10.3 | 1.8 | 1.7 |
| Cat-4 | SID-4 | 24.1 | 0.9 | 2.2 |
| Cat-5 | SID-5 | 23.7 | 0.7 | 1.9 |
| Cat-D | DIBP | 8.3 | 2.7 | 6.8 |
Molecular weight and polydispersity index of polypropylene
Table 2 lists the molecular weight and polydispersity index of polypropylenes prepared by different internal donors. It was shown that the polydispersity index of the polypropylene prepared by the SID internal donors ranged from 5.15 to 7.19, which was similar to that prepared by the DIBP internal donors. Table 2 also shows that the molecular weights of the polypropylene prepared by SID internal donors were all lower than that prepared by DIBP, which indicated that the polypropylene chain produced by the SID internal donors was easier to transfer than that prepared by the DIBP internal donors. Furthermore, it was also found that the average molecular weight, Mw, of polypropylene successively increased from SID-1 to SID-5. In order to deeply analyze the trend of the molecular weight of polypropylene prepared by different internal donors, GPC curves were deconvoluted by Schulz–Flory distribution functions.| Donor | Mn × 10−4 (g mol−1) | Mw × 10−4 (g mol−1) | Mw/Mn |
|---|---|---|---|
| SID-1 | 4.53 | 26.39 | 5.82 |
| SID-2 | 5.13 | 26.45 | 5.15 |
| SID-3 | 4.25 | 27.24 | 6.40 |
| SID-4 | 4.99 | 27.38 | 5.48 |
| SID-5 | 3.83 | 27.55 | 7.19 |
| DIBP | 7.54 | 43.26 | 5.74 |
Active centers based on the molecular weight of polypropylene
The Mw/Mn ratio of polypropylene prepared by a single active center catalyst was narrow (2–3), and the wider distribution of molecular weight indicated that the catalyst contained multiple active centers. Judging by the polydispersity index of polypropylene, catalysts with SID and DIBP internal donors both contained several types of active centers. Fig. 1 showed that GPC curves of polypropylene prepared by SID and DIBP internal donors were both deconvoluted into five kinds of Flory compounds with small polydispersity indices, which could correspond to different active centers in the catalyst as it did in the literature.29 Comparing the active centers in the catalyst with different SID internal donors, it was found that each type of active center in different SID catalysts produced polypropylene with a similar molecular weight; however, the relative content of each kind of active center in the five SID catalysts changed regularly. The relative contents of and
and  increased from Cat-1 to Cat-5, while the relative contents of
increased from Cat-1 to Cat-5, while the relative contents of  ,
, 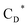 and
and  all decreased from Cat-1 to Cat-5 (Table 3). It was indicated that as the volume of the SID internal donor in the catalyst increased some of
all decreased from Cat-1 to Cat-5 (Table 3). It was indicated that as the volume of the SID internal donor in the catalyst increased some of  ,
,  and
and  transferred into
transferred into  and
and  . In addition, it was found that the molecular weight of the polypropylene prepared by each kind of active center in the DIBP catalyst was obviously different with that in the SID catalyst, which indicated that the active centers in the SID catalyst were different with that in the DIBP catalyst. The molecular weight of polypropylene mainly depended on the hydrogen response, as the chain transfer to hydrogen was more sensitive than the chain transfer to a monomer or TEA.27 The sensitivity of the chain transfer to hydrogen was primarily dependent on the regioselectivity of the active center, which had a great relationship with the proportion of secondary (2,1-) monomer insertion.30 Taking into account the same addition amount of hydrogen for SID and DIBP systems, the different molecular weights of polypropylene prepared by active centers in SID and DIBP catalysts were ascribed to the different internal donors in the catalyst. Although it was reported that some of the ester internal donors could be removed by TEA, internal donors that strongly coordinated with the support could not be removed,31 which could influence the active center in the catalyst. It was suggested that the different types of internal donors remaining in the catalyst might have different impacts on the regioselectivity of the active center in the Ziegler–Natta catalyst, which led to SID and DIBP catalysts producing polypropylene with different molecular weights.
. In addition, it was found that the molecular weight of the polypropylene prepared by each kind of active center in the DIBP catalyst was obviously different with that in the SID catalyst, which indicated that the active centers in the SID catalyst were different with that in the DIBP catalyst. The molecular weight of polypropylene mainly depended on the hydrogen response, as the chain transfer to hydrogen was more sensitive than the chain transfer to a monomer or TEA.27 The sensitivity of the chain transfer to hydrogen was primarily dependent on the regioselectivity of the active center, which had a great relationship with the proportion of secondary (2,1-) monomer insertion.30 Taking into account the same addition amount of hydrogen for SID and DIBP systems, the different molecular weights of polypropylene prepared by active centers in SID and DIBP catalysts were ascribed to the different internal donors in the catalyst. Although it was reported that some of the ester internal donors could be removed by TEA, internal donors that strongly coordinated with the support could not be removed,31 which could influence the active center in the catalyst. It was suggested that the different types of internal donors remaining in the catalyst might have different impacts on the regioselectivity of the active center in the Ziegler–Natta catalyst, which led to SID and DIBP catalysts producing polypropylene with different molecular weights.
| Samples | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Fra | Mwb | Fr | Mw | Fr | Mw | Fr | Mw | Fr | Mw | |
| a Fr was the weight percentage of the fraction produced by a certain active center in catalyst.b Weight average molecular weight, in 104 g mol−1. | ||||||||||
| Cat-1 | 16.3 | 56.4 | 33.9 | 19.7 | 32.3 | 8.2 | 12.9 | 2.9 | 4.4 | 0.9 |
| Cat-2 | 18.1 | 56.5 | 35.0 | 19.8 | 31.1 | 8.0 | 11.6 | 3.0 | 3.9 | 1.2 |
| Cat-3 | 18.3 | 57.9 | 35.7 | 19.8 | 30.7 | 7.7 | 11.4 | 2.9 | 3.6 | 1.1 |
| Cat-4 | 19.0 | 57.6 | 35.9 | 19.2 | 30.1 | 8.1 | 11.3 | 3.3 | 3.5 | 1.2 |
| Cat-5 | 19.8 | 58.4 | 37.9 | 18.5 | 29.3 | 7.3 | 10.2 | 2.5 | 2.7 | 0.7 |
| Cat-D | 8.2 | 138.9 | 30.7 | 51.2 | 38.8 | 17.9 | 18.0 | 6.7 | 4.3 | 2.1 |
The stereo-defect in polypropylene chain
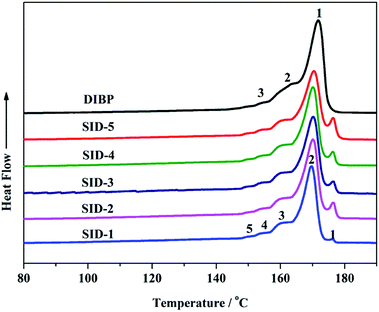
The stereo-defect of the molecular chain could significantly impact the thermal and mechanical properties of polypropylene; therefore, detailed characterization of the stereo-defect in the molecular chain was important to obtain polypropylene with better properties. The fraction of heptane-insoluble crystalline polypropylene was defined as the isotactic index (I.I.) of polypropylene, which could be used to roughly analyze the stereo-defect in the polypropylene chain. Table 4 shows the isotactic index of the polypropylene prepared by different internal donors. The isotactic index of polypropylene increased from 96.3% to 98.6% as the internal donor successively changed from SID-1 to SID-4, which indicated that the volume of the SID internal donor could impact the stereoregularity of polypropylene. In addition, the isotactic index of polypropylene produced by SID-4 was close to that prepared by the DIBP internal donor. In order to obtain more information on the stereo-defect in the molecular chain, successive self-nucleation and annealing (SSA) and nuclear magnetic resonance spectroscopy (13C-NMR) were both used to study the microstructure of polypropylene.32,33| Donor | ΔHma (J g−1) | I.I.b (%) | Tm1c (°C) | Tm2 (°C) | Tm3 (°C) | Tm4 (°C) | Tm5 (°C) |
|---|---|---|---|---|---|---|---|
| a The isotactic index (I.I.) was tested by extraction with boiling n-heptane for 6 h.b The value of the endothermic enthalpy was determined from the SSA curve.c The melting temperature was determined from the peak value in the SSA curves. | |||||||
| SID-1 | 104.2 | 96.3 | 175.9 | 170.2 | 165.8 | 160.6 | 154.8 |
| SID-2 | 99.1 | 96.9 | 175.9 | 169.4 | 165.1 | 160.3 | 154.7 |
| SID-3 | 109.9 | 98.0 | 176.0 | 169.9 | 165.6 | 160.5 | 155.0 |
| SID-4 | 118.1 | 98.6 | 176.3 | 169.9 | 165.5 | 160.3 | 153.5 |
| SID-5 | 106.2 | 97.7 | 176.1 | 169.8 | 165.2 | 160.0 | 154.0 |
| DIBP | 121.0 | 98.5 | 171.4 | 166.0 | 160.2 | — | — |
As an effective method for the microstructure features of polypropylene, SSA can give lamellar thicknesses and thickness distribution of the polypropylene, which could be connected with the isotactic sequence length of the polymer. The melting curves of polypropylene prepared by different internal donors after SSA thermal fractionation are shown in Fig. 2, and the melting enthalpy ΔHm of each polypropylene is listed in Table 4. Comparing the melting enthalpies ΔHm of polypropylene prepared by SID-1, SID-3 and SID-4, it was found that the melting enthalpy ΔHm of polypropylene increased from SID-1 to SID-4, while the melting enthalpy ΔHm of polypropylene prepared by SID-5 was lower than those prepared by SID-4, which reflected a trend in the crystallinity degree of polypropylene.
Each melting peak of polypropylene in the SSA melting curves was related to a different thickness of the lamellar crystallites, which formed and annealed at each self-nucleation temperature.34 The Thomson–Gibbs equation35 can give the information about the lamellar thickness of polypropylene:
| Tm = T0m(1 − 2σ/ΔH0Li) |
The lamellar thicknesses of polypropylene after SSA thermal fractionation were calculated and are listed in Table 5. It was shown that the polypropylene prepared by SID internal donors had five kinds of lamellar thicknesses but polypropylene prepared by DIBP internal donors had only three kinds of lamellar thicknesses, which indicated that the distribution of the lamellar thickness in polypropylene produced by SID internal donors might be different than that prepared by DIBP internal donors. It was also found that all of the SID internal donors produced polypropylene with similar lamellar thicknesses, which differed from those prepared by DIBP internal donors, revealing that the type of internal donor could affect the thickness of each kind of lamellae in polypropylene.
| Donor | L1 | L2 | L3 | L4 | L5 |
|---|---|---|---|---|---|
| SID-1 | 22.65 | 13.81 | 11.78 | 9.45 | 7.74 |
| SID-2 | 22.65 | 13.57 | 11.52 | 9.34 | 7.71 |
| SID-3 | 22.68 | 13.66 | 11.71 | 9.42 | 7.82 |
| SID-4 | 22.79 | 13.66 | 11.67 | 9.34 | 7.44 |
| SID-5 | 22.71 | 13.62 | 11.56 | 9.23 | 7.55 |
| DIBP | 16.04 | 11.89 | 9.30 | — | — |
Moreover, it was found that the isotactic sequence length of polypropylene was closely related with lamellar thickness. The following equations38 were used to calculated the average lamellar thickness, thickness distribution (arithmetic average Ln, weighted average Lw and broadness index I) and average isotactic sequence length (arithmetic average MSLn, weighted average MSLw). ni is the content of each fraction on the SSA curve, and Li is the thickness of lamella for each fraction, Lhelix = 0.65 nm.
| I = Lw/Ln MSL = 3L/Lhelix |
Comparing the average isotactic sequence length MSLn of polypropylene prepared by different internal donors in Table 6, the isotactic sequence length MSLn of polypropylene prepared by SID internal donors ranged from 57.04 to 59.82, which was close to that produced by DIBP internal donors. Meanwhile, the distribution of the lamella thickness I of polypropylene prepared by SID internal donors ranged from 1.056 to 1.074, which was broader than that prepared by DIBP internal donors. It was suggested that SID internal donors in the catalyst were more conducive to producing polypropylene with a broad distribution of lamella thickness than DIBP. In addition, comparing SID-4 and DIBP, it was found that the average lamella thickness of polypropylene prepared by SID-4 was similar to that produced by DIBP, but the thickness and thickness distribution of each lamella in polypropylene for SID-4 and DIBP internal donors were different.
| Donor | Ln (nm) | Lw (nm) | MSLn | MSLw | I |
|---|---|---|---|---|---|
| SID-1 | 12.36 | 13.27 | 57.04 | 61.25 | 1.074 |
| SID-2 | 12.77 | 13.37 | 58.93 | 61.71 | 1.057 |
| SID-3 | 12.62 | 13.39 | 58.24 | 61.80 | 1.061 |
| SID-4 | 12.96 | 13.52 | 59.82 | 62.40 | 1.056 |
| SID-5 | 12.66 | 13.48 | 58.43 | 62.22 | 1.065 |
| DIBP | 13.11 | 13.76 | 60.50 | 63.09 | 1.043 |
Nuclear magnetic resonance spectroscopy (13C-NMR) was always used to study the macrostructure of polypropylene.39 The methyl regions' results from the 300 MHz 13C-NMR spectrum of the polypropylene are listed in Table 7. It was found that the content of the regular triads “mm” of polypropylene prepared by SID-1–SID-4 internal donors increased gradually from 85.46% to 90.98%, while the amount of “mr” decreased from 7.81% to 3.74% and the amount of “rr” also decreased from 6.66% to 4.47%. In addition, comparing polypropylene prepared by SID-4 and DIBP internal donors, the content of the regular triads' “mm” of polypropylene prepared by an SID-4 internal donor was slightly lower than that prepared by a DIBP internal donor. It suggested that both type and volume of the internal donors could impact the regular triads' “mm” of polypropylene. 13C-NMR results could also be used to analyze the average meso sequence length of polypropylene. Average isotactic sequence length calculated from 13C-NMR results was defined as MSL, which could be calculated by the following equation:40
| Donor | mm | mr | rr | MSL |
|---|---|---|---|---|
| SID-1 | 85.46 | 7.81 | 6.66 | 15.56 |
| SID-2 | 88.08 | 6.11 | 5.81 | 22.97 |
| SID-3 | 89.61 | 5.25 | 5.15 | 19.16 |
| SID-4 | 90.98 | 3.74 | 4.47 | 28.58 |
| SID-5 | 88.88 | 5.50 | 5.63 | 22.76 |
| DIBP | 91.67 | 3.82 | 4.51 | 30.66 |
Table 7 showed the average isotactic sequence length MSL calculated from the results of 13C-NMR. Comparing polypropylene prepared by SID-1, SID-3, and SID-4, it was found that the average meso sequence length MSL calculated from 13C-NMR data increased from SID-1 to SID-4; however, SID-5 gives polypropylene with a lower meso sequence length than SID-4, which was in good agreement with the trend of MSLn calculated from SSA. Moreover, the trend of the average isotactic sequence length was also similar for the isotacticity index and regular triads' “mm” of polypropylene prepared by different SID internal donors, which suggested that the appropriate volume size for SID internal donors in the catalyst would benefit a decrease in the content of stereo-defect in the polypropylene chain. However, further increasing the volume of SID internal donors in the catalyst might lead to a polypropylene chain containing more stereo-defect. It was supposed that SID internal donors with a different volume might have a different effect on the stereoselectivity of the catalyst, which led to the production of polypropylene with different stereo-defects.
The thermal properties of polypropylene
Fig. 2 shows that the SSA curves of polypropylene prepared by SID internal donors were all broader and had more peaks than that prepared by DIBP internal donors, which represented more types and a broader distribution of lamellar thickness for polypropylene prepared by SID internal donors. Therefore, it was assumed that SID internal donors might produce polypropylene with broad DSC curves. Fig. 3 displays that the DSC curves prepared by SID internal donors were indeed broader than DIBP. Many researchers found that the broad melting curve of the polymer was beneficial for expanding the crystallization temperature range for expanded polypropylene, which was conducive to control the foaming of polypropylene.41 Hence, that polypropylene produced by SID internal donors might possibly be used as the material for expanded polypropylene.Active centers with different stereoselectivity
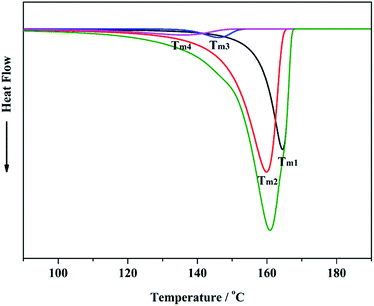
It was reported that there were multiple active centers with different stereoselectivity in heterogeneous Ziegler–Natta catalysts42 that could produce polypropylene chains with varying distributions of stereo-defects. The results of 13C-NMR and SSA confirmed that internal donors could significantly impact the relative content and distribution of stereo-defects in the molecular chain of polypropylene. Different internal donors in the catalyst might have different influences on the stereoselectivity of active centers in the Ziegler–Natta catalyst. Combining the results of 13C-NMR and DSC, a modeling of the DSC melting curve23 could be used to analyze the stereoselectivity of active centers in the Ziegler–Natta catalyst. If all of the active centers in the catalyst were perfectly isospecific, which means that the probability of meso linking of adjacent propylene monomer units was highest (stereospecificity parameter piso was the highest and defined as 1), polypropylene prepared by the active centers would have very narrow DSC curves and the Tm of the DSC curves would be highest (T0m); the “mmmm” value of the polymer would be close to 1. There are certain relationships between Tm, “mmmm” value and stereospecificity parameter piso that have been reported by Kissin.42 Fig. 4 is the straight fitting of Tm from the DSC curve and “mmmm” value from 13C-NMR. It was found that the highest melting temperature T0m was 171.2 °C, which was obtained by extrapolating the “mmmm” value to 1. The highest melting temperature T0m can be used to calculate the stereospecificity parameter piso of the active center.43 Fig. 5 is the modeling of the DSC curve of polypropylene prepared by SID catalyst. It shows that there are four fitting curves to fit the DSC melting curve of polypropylene, which corresponds to four active centers with different stereospecificity parameters, piso. Combining the melting temperature of the fitting curve from Fig. 5 with T0m from Fig. 4, the stereospecificity parameter piso for each active center could be obtained.43 The stereospecificity parameter piso and the relative content of the active centers Istereo and IIstereo for different catalysts are listed in Table 8, and correspond to Tm1 and Tm2 in the DSC curve model and had high stereoselectivity in the catalyst. Comparing active centers Istereo and IIstereo for Cat-1, Cat-3 and Cat-4, the relative content of active Istereo and IIstereo both increased from Cat-1 to Cat-4, while Cat-5 had a lower content of active centers Istereo and IIstereo than Cat-4. Meanwhile, the trends for stereospecificity parameters piso1 and piso2 were similar with the rule of the relative content of the active centers Istereo and IIstereo. It was indicated that the stereoselectivity of the catalyst increased as the volume of the SID internal donor in the catalyst increased, but a SID internal donor with an excessively large volume could decrease the stereoselectivity of the catalyst. It was reported that Ti active centers attach to the surface of the (110) facets of MgCl2 crystallites by coordination of Cl− on TiCl4 with Mg on MgCl2.44 Meanwhile, ester internal donors can also coordinate with Mg, which would lead to dispersed Ti on the surface of MgCl2 crystallites and decrease the space of the Ti active centers.27 It was also confirmed that although some of the ester internal donors could be exchanged by external donors during polymerization, the ester internal donors remaining in the catalyst could significantly impact the active centers of the catalyst.31 Therefore, it was speculated that as the SID internal donors with increased volume remained in the catalyst, the space of the active centers around the internal donors became smaller, which favors improving the stereoselectivity of the catalyst. However, an excessively large volume of the SID internal donors would result in an excessively small space for active centers, which might be harmful to meso-insertion of propylene units in the polymer chain. Therefore, the stereoselectivity of the catalyst decreased. In addition, the structures of SID and DIBP were different, which might lead to the different stereoselectivities of the SID and DIBP catalysts.| Samples | Istereo | IIstereo | ||||
|---|---|---|---|---|---|---|
| piso1 (°C) | ΔEact(si/re)1 | Yield (%) | piso2 (°C) | ΔEact2(si/re)2 | Yield (%) | |
| Cat-1 | 99.09 | −3.14 | 30.2 | 98.62 | −2.86 | 56.2 |
| Cat-2 | 99.21 | −3.23 | 31.8 | 98.66 | −2.88 | 58.0 |
| Cat-3 | 99.26 | −3.28 | 32.2 | 98.77 | −2.93 | 58.2 |
| Cat-4 | 99.27 | −3.29 | 33.9 | 98.81 | −2.95 | 60.5 |
| Cat-5 | 99.23 | −3.25 | 32.5 | 98.76 | −2.93 | 59.6 |
| Cat-D | 99.49 | −3.45 | 25.6 | 99.06 | −3.12 | 64.8 |
In addition, stereospecificity parameter piso, defined as ksi/(ksi + kre) in kinetics (Scheme 3), can be used to analyze the activation energy ΔEact(si/re), as the following equation: ΔEact(si/re) = RT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln(ksi/kre) = RT
ln(ksi/kre) = RT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln[(1 − piso)/piso].23 Table 8 shows that the law of ΔEact(si/re)1 and ΔEact(si/re)2 values for active centers in the SID catalyst was contrary to the trend of the isotactic sequence length (from SSA and 13C-NMR), regular triads “mm” (from 13C-NMR) and isotacticity index of polypropylene. This trend further confirmed that the internal donor in the catalyst can impact the stereoselectivity of active centers in the Ziegler–Natta catalyst, which led to changing of the activation energy ΔEact(si/re) and further influencing the distribution and content of stereo-defects of propylene during polymerization. Finally, it showed the general trend of isotactic sequence lengths and thermal properties of polypropylene for different internal donors.
ln[(1 − piso)/piso].23 Table 8 shows that the law of ΔEact(si/re)1 and ΔEact(si/re)2 values for active centers in the SID catalyst was contrary to the trend of the isotactic sequence length (from SSA and 13C-NMR), regular triads “mm” (from 13C-NMR) and isotacticity index of polypropylene. This trend further confirmed that the internal donor in the catalyst can impact the stereoselectivity of active centers in the Ziegler–Natta catalyst, which led to changing of the activation energy ΔEact(si/re) and further influencing the distribution and content of stereo-defects of propylene during polymerization. Finally, it showed the general trend of isotactic sequence lengths and thermal properties of polypropylene for different internal donors.
Conclusions
Five salicylates with different size hydrocarbon substituents on phenyl groups were firstly synthesized and employed as ecofriendly internal donors of the Ziegler–Natta catalyst for propylene polymerization. The effect of these salicylates and traditional industrial internal donor DIBP on the microstructure of polypropylenes was studied by GPC, SSA and NMR. Meanwhile, the active centers in the catalyst were studied by deconvolution of GPC curves and DSC model. GPC results showed that the molecular weights of polypropylene prepared by SID internal donors were all lower than that prepared by DIBP, which indicated that the polypropylene chains produced by SID internal donors were easier to transfer than those prepared by DIBP internal donors. Deconvolution of GPC curves showed that as the volume of the SID internal donors increased, the relative contents of the active centers for high molecular weights increased, while the relative contents of the active centers for low molecular weights decreased. The isotactic sequence lengths from 13C-NMR and SSA both gradually increased from SID-1 to SID-4, while SID-5 gave polypropylene with a lower meso sequence length than SID-4, which was similar with the trend of the isotacticity index and regular triads “mm” of polypropylene. The general trend of stereo-defects in the polypropylene chain was explained by the stereoselectivity of the active centers in the catalyst for different internal donors, indicating that appropriately increasing the volume of SID internal donors could improve the stereoselectivity of the catalyst. However, excessively large volumes of SID internal donors would reduce the stereoselectivity of the catalyst. In addition, it was found that the catalyst activities of catalysts containing SID internal donors with proper volume were higher than that of catalysts containing DIBP internal donors. The isotactic sequence length and isotacticity index of polypropylene prepared by isobutyl 2-benzyloxy-3,5-isopropyl benzoate were close to that produced by DIBP internal donors.Acknowledgements
The authors express thanks for the supports of the National Natural Science Foundation of China (NO. 51073170) and Innovation Program of CAS Combination of Molecular Science and Education.References
- J. Qiao, M. Guo, L. Wang, D. Liu, X. Zhang, L. Yu, W. Song and Y. Liu, Polym. Chem., 2011, 2, 1611–1623 RSC.
- T. Taniike and M. Terano, in Polyolefins: 50 Years after Ziegler and Natta I: Polyethylene and Polypropylene, ed. W. Kaminsky, 2013, vol. 257, pp. 81–97 Search PubMed.
- J. Brandrup and E. H. Immergut, Polymer Handbook, Wiley, New York, 3rd edn, 1989 Search PubMed.
- H. Youliang, C. Hefei, L. Huayi and Z. Liaoyun, Petrochem. Technol., 2013, 11, 1189–1196 Search PubMed.
- V. Busico, MRS Bull., 2013, 38, 224–228 Search PubMed.
- K. Soga, T. Shiono and Y. Doi, Macromol. Chem. Phys., 1988, 189, 1531–1541 CrossRef CAS.
- M. L. Ferreira and D. E. Damiani, J. Mol. Catal. A: Chem., 1999, 150, 53–69 CrossRef CAS.
- D. M. Xu, Z. Y. Liu, J. Zhao, S. M. Han and Y. L. Hu, Macromol. Rapid Commun., 2000, 21, 1046–1049 CrossRef CAS.
- Z. L. Ma, L. Wang, W. Q. Wang, L. F. Feng and X. P. Gu, J. Appl. Polym. Sci., 2005, 95, 738–742 CrossRef CAS.
- S. Tanase, K. Katayama, N. Yabunouchi, T. Sadashima, N. Tomotsu and N. Ishihara, J. Mol. Catal. A: Chem., 2007, 273, 211–217 CrossRef CAS.
- Y. Yang, X. F. Dang, J. Y. Yao, H. Y. Li and Y. L. Hu, Acta Polym. Sin., 2013, 511–517, DOI:10.3724/sp.j.1105.2013.12268.
- N. Cui, Y. Ke, H. Li, Z. Zhang, C. Guo, Z. Lv and Y. Hu, J. Appl. Polym. Sci., 2005, 99, 1399–1404 CrossRef.
- K. W. Choi, K. J. Chu, J. H. Yim and S. K. Ihm, Eur. Polym. J., 1998, 34, 739–745 CrossRef CAS.
- D. R. Burfield and P. J. T. Tait, Polymer, 1974, 15, 87–96 CrossRef CAS.
- X. F. Dang, Q. Li, H. Y. Li, Y. Yang, L. Y. Zhang and Y. L. Hu, J. Polym. Res., 2014, 21(619), 1–8 CAS.
- R. K. Madan and J. Levitt, J. Am. Acad. Dermatol., 2014, 70, 788–792 CrossRef CAS PubMed.
- M. Chang, US Pat., 8003558, 2011.
- A. G. Potapov, V. A. Zakharov and G. D. Bukatov, Kinet. Catal., 2007, 48, 403–408 CrossRef CAS.
- A. J. Muller, Z. H. Hernandez, M. L. Arnal and J. J. Sanchez, Polym. Bull., 1997, 39, 465–472 CrossRef CAS.
- M. L. Arnal, Z. H. Hernandez, M. Matos, J. J. Sanchez, G. Mendez, A. Sanchez and A. Muller, Use of the SSA technique for polyolefin characterization, Soc Plastics Engineers, Brookfield Center, 1998 Search PubMed.
- G. Morini, E. Albizzati, G. Balbontin, I. Mingozzi, M. C. Sacchi, F. Forlini and I. Tritto, Macromolecules, 1996, 29, 5770–5776 CrossRef CAS.
- V. Busico, R. Cipullo, G. Monaco, G. Talarico, M. Vacatello, J. C. Chadwick, A. L. Segre and O. Sudmeijer, Macromolecules, 1999, 32, 4173–4182 CrossRef CAS.
- Y. V. Kissin, Q. Zhou, H. Li and L. Zhang, J. Catal., 2015, 332, 156–163 CrossRef CAS.
- R. Ghosh, S. Maiti and A. Chakraborty, Tetrahedron Lett., 2004, 45, 6775–6778 CrossRef CAS.
- A. J. Müller, A. T. Lorenzo and M. L. Arnal, Macromol. Symp., 2009, 277, 207–214 CrossRef.
- T. F. J. Pijpers, V. B. F. Mathot, B. Goderis, R. L. Scherrenberg and E. W. van der Vegte, Macromolecules, 2002, 35, 3601–3613 CrossRef CAS.
- J. C. Chadwick, G. M. M. Van Kessel and O. Sudmeijer, Macromol. Chem. Phys., 1995, 196, 1431–1437 CrossRef CAS.
- Z. Chen, Master Degree of Science, Tianjin University, 2008 Search PubMed.
- P. Y. Li, S. T. Tu, T. Xu, Z. S. Fu and Z. Q. Fan, J. Appl. Polym. Sci., 2015, 132(41689), 1–10 Search PubMed.
- J. C. Chadwick, F. van der Burgt, S. Rastogi, V. Busico, R. Cipullo, G. Talarico and J. J. R. Heere, Macromolecules, 2004, 37, 9722–9727 CrossRef CAS.
- A. G. Potapov, G. D. Bukatov and V. A. Zakharov, J. Mol. Catal. A: Chem., 2010, 316, 95–99 CrossRef CAS.
- J. Chau and J. Teh, J. Therm. Anal. Calorim., 2005, 81, 217–223 CrossRef CAS.
- M. L. Arnal, V. Balsamo, G. Ronca, A. Sanchez, A. J. Muller, E. Canizales and C. U. de Navarro, J. Therm. Anal. Calorim., 2000, 59, 451–470 CrossRef CAS.
- V. Virkkunen, P. Laari, P. Pitkänen and F. Sundholm, Polymer, 2004, 45, 4623–4631 CrossRef CAS.
- U. W. Gedde, Polymer physics, Chapman & Hall, London, 1995 Search PubMed.
- M. Iijima and G. Strobl, Macromolecules, 2000, 33, 5204–5214 CrossRef CAS.
- A. Wlochowicz and M. Eder, Polymer, 1984, 25, 1268–1270 CrossRef CAS.
- M. Keating, I. H. Lee and C. S. Wong, Thermochim. Acta, 1996, 284, 47–56 CrossRef CAS.
- N. Tan, L. Q. Yu, Z. Tan and B. Q. Mao, J. Appl. Polym. Sci., 2015, 132, 42487 Search PubMed.
- R. Paukkeri, E. Iiskola, A. Lehtinen and H. Salminen, Polymer, 1994, 35, 2636–2643 CrossRef CAS.
- Y. Zou, Master Degree of Science, Harbin Institute of Technology, 2015.
- Y. V. Kissin, J. C. Chadwick, I. Mingozzi and G. Morini, Macromol. Chem. Phys., 2006, 207, 1344–1350 CrossRef CAS.
- Y. V. Kissin, J. Res. Updates Polym. Sci., 2013, 2, 8 CAS.
- U. Giannini, Die Makromolekulare Chemie, 1981, 5, 216–229 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |