Effect of alkyl chain topology on the structure, optoelectronic properties and solar cell performance of thienopyrroledione-cored oligothiophene chromophores†
Abstract
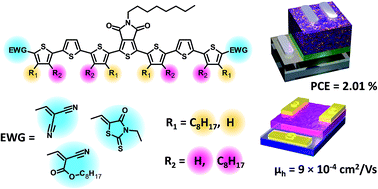
We have investigated a series of oligothiophenes containing a central thienopyrroledione group with rhodanine, dicyanovinyl and octylcyanoacrylate end-capping groups. For each end capping group, two alkyl chain configurations were explored by appending n-octyl chains to the oligothiophenes in both proximal and distal topologies. Substitution of different alkyl topologies and end-capping groups altered not only intramolecular conformations but also intermolecular interactions, thus affecting frontier molecular orbitals and bulk properties such as optical, thermal transitions, solid state packing, and device properties. The electronic properties of the materials were probed in field effect transistors (FETs), single carrier diodes and bulk heterojunction (BHJ) solar cell devices. FET devices revealed that all materials behaved as p-type semiconductors with mobilities in the range of 10−5 to 10−3 cm2 V−1 s−1. In solar cell devices, we observed that the optimal end capping group was rhodanine, while the optimal alkyl chain configuration was the proximal configuration. The rhodanine capped molecule with proximal alkyl chain isomerism led to a power conversion efficiency of ∼2%. Grazing-incidence wide-angle X-ray scattering studies of this series of molecules revealed a strong tendency to undergo edge-on packing with no π–π stacking in the vertical direction, which may limit their performance in BHJ solar cells.

 Please wait while we load your content...
Please wait while we load your content...