Particle adsorption on a polyether sulfone membrane: how electrostatic interactions dominate membrane fouling†
D. Breite,
M. Went,
I. Thomas,
A. Prager and
A. Schulze*
Leibniz Institute of Surface Modification, Permoserstraße 15, Leipzig, D-04318, Germany. E-mail: agnes.schulze@iom-leipzig.de; Tel: +49 341 235 2400
First published on 4th July 2016
Abstract
This study presents a new method focussing on electrostatic interactions during fouling of microfiltration membranes. Therefore, a new fouling test system was developed. To investigate the impact of surface charge on membrane fouling charged polystyrene beads were used. Also, the tested polyethersulfone membranes were modified with differently charged functional groups. Zeta potential measurements were conducted to describe the charged state of the membranes and particles. Fouling was investigated by filtration of the particle suspensions through the different membranes. It was found that oppositely charged surfaces of the membrane and particle lead to membrane fouling due to electrostatic attraction. Accordingly, no fouling occurred for the combination of evenly charged surfaces due to electrostatic repulsion. Additional experiments with a zwitterionic membrane surface revealed that fouling significantly depends on the pH value. Possessing an isoelectric point of pH 7 this membrane can exists in a positively charged, uncharged, or negatively charged state. Again, fouling occurred when electrostatic attraction was present while electrostatic repulsion resulted in antifouling. In the case of an uncharged surface fouling occurred in general due to the absence of electrostatic interactions. These new findings allowed us to establish a new standard fouling test that reveals the influence of electrostatic interactions in a fouling problem. To further demonstrate the advantages of this system standard protein fouling experiments were conducted and compared. It was found that different types of interactions and changes in the tertiary structure of proteins have to be considered. Thus, the gained results were difficult to interpret and false assumptions may occur. In contrast, electrostatic forces are sufficient to explain the fouling of polystyrene beads. The fouling test system presented by the authors is solely focused on electrostatic interactions and has, therefore, a clear advantage compared to the common protein test system.
1. Introduction
1.1 Membrane fouling and surface interactions
The problem of membrane fouling affects nearly all membrane applications and technologies. Attractive interactions between the membrane and fouling reagents (e.g. biomolecules, salts, bacteria, viruses) present in the surrounding solution lead to adsorption of these foulants. Subsequently, the membrane pores get blocked and the membrane performance decreases.1,2 In addition, the fouling process depends on solution properties like temperature, pH, or ionic strength.3So far membranes are often evaluated by their surface hydrophilicity.4 A hydrophilic membrane surface can build up a water film that prevents hydrophobic interactions. Hydrophobic interactions take place between the hydrophobic parts of the membrane and fouling reagents like proteins.5 Focusing on hydrophobic interactions is a consequence of the fact that most used membrane materials are hydrophobic polymers.6–13 These polymers are more durable, solvent resistant, and long-term stable than other materials. Typical methods to hydrophilize membranes are surface grafting reactions,6,7,9,11,13–15 electron-beam (EB) modifications,16,17 or plasma treatments.8,12,18–23
According to the Derjaguin–Landau–Verwey–Overbeek (DLVO) theory24,25 and its extended version by Van Oss (XDLVO),26,27 the total interfacial interaction of a membrane surface and a fouling reagent is composed of Lifshitz–van der Waals, Lewis acid–base, and electrostatic double layer interactions. All three types of interactions contribute to the total interfacial interaction, but to a different extent.
In recent literature, the research focus was shifted towards the influence of electrostatic interactions.28–35 Electrostatic interactions are observed due to the ionized functionalities present on both membrane and foulant. Depending on the charged state of these moieties electrostatic forces occur following Coulomb's law. Evenly charged functionalities repel each other while attractive forces are found for oppositely charged surfaces. According to the XDLVO theory, electrostatic interactions contribute to the total interfacial interaction between a membrane and a fouling reagent. The zeta potential can be determined to estimate the impact of electrostatic interactions. This potential is measured at the slipping plane of the electrochemical double layer between a surface and the surrounding electrolyte. Therefore, the zeta potential represents an extenuated surface potential. Unfortunately, diverse studies known from literature came to different conclusions regarding the impact of electrostatic interactions on membrane fouling. Hadidi and Zydney showed the effect of using differently charged membranes for fouling with differently charged proteins.30 They found reduced fouling for combinations of equal charges. This proves that electrostatic interactions are important. On the other hand, Xiao et al. developed a semi-empirical model which describes the combination of surface charge and hydrophilicity. They concluded that membrane fouling is only influenced by surface charge to a minor extent.33 A possible explanation for the different conclusions was stated by Cai et. al. Calculations with plane membrane surfaces showed minor contribution to electrostatic interactions. In contrast, when surface roughness was considered the contribution of electrostatic interactions to the total interfacial interaction was increased.36
Nevertheless, using the current existing test systems it is not possible to distinguish if fouling decreased due to increased hydrophilicity or due to electrostatic repulsive interactions. Therefore, the new approach presented by the authors might help to differentiate electrostatic interactions from other interfacial interactions.
1.2 Investigation of electrostatic interactions
As mentioned above, a commonly used method to characterize membrane fouling is fouling by proteins. Here, proteins like albumin (from bovine serum, BSA) are added to the solution surrounding the membrane. Depending on the total interfacial interaction between membrane surface and protein the latter might be adsorbed to the membrane surface.37 Unfortunately, proteins like BSA can interact with surfaces in many different ways. Hydrophobic parts in the protein structure cause hydrophobic interactions, and specific binding sites form e.g. hydrogen bonds. Proteins are also overall charged leading to electrostatic interaction with the charged membrane surface. In addition, a lot of proteins change their tertiary structure or even denaturate when they approach an artificial surface. Thus, size and shape may change during the fouling experiment. Accordingly, the already complex fouling process gets even more difficult to control, and a determination of electrostatic interaction alone is impossible.38The fouling test system presented by the authors uses different polystyrene (PS) beads with tailored surface charge. Bacchin, Aimar, and Sanchez developed a model for colloidal fouling of membranes,39 and a series of different publications previously reported the usability of PS beads for membrane fouling.40–52 Also, in prior studies the authors already presented how differently charged PVDF membranes are fouled by charged PS beads.53 The benefits of using charged PS beads in fouling experiments are their spherical shape and the adjustable surface charge. Hydrophobic interactions are nearly impossible and the fouling process is dominated by electrostatic interactions.
In this study we show that charged PS beads can be used to solely identify electrostatic interactions undisturbed by other types of interactions. Furthermore, the test system using PS beads is compared with a common protein fouling test. For the latter various interactions and structural changes of the used proteins have to be considered. This complicates the explanation of the gained results. In contrast, fouling of PS beads can be described by electrostatic interactions alone proving a clear advantage of the test system invented by the authors.
2. Methods and materials
2.1 Reagents and materials
The used PES flat sheet membranes were prepared using non-solvent induced phase separation. The following chemicals were obtained from Sigma Aldrich (St. Louis, USA): styrene, potassium persulfate (KPS), 2,2′-azobis(2-methylpropion-amidine) dihydrochloride (AIBA), lauryldimethylammonia acetate, tetraethylpentamine (TEPA), lysine, polystyrene sulfonate (PSS, 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1), phosphate buffered saline (PBS, pH 7.0), albumin from bovine serum (BSA, 67 kDa), lysozyme from chicken egg white (14.6 kDa), citric acid, 1-methyl-2-pyrrolidone (NMP). Other purchased chemicals: aluminum oxide (Brockmann activity I, Fluka, Sigma Aldrich, St. Louis, USA), n-hexane (Merck, Darmstadt, Germany), 2-aminoethyl methacrylate hydrochloride (AEMA, Acros Organics, Thermo Fisher Scientific, Geel, Belgium), glutaraldehyde (Merck, Darmstadt, Germany), hydrochloric acid solution (0.1 M, VWR, Radnor, USA), sodium hydroxide solution (0.1 M, VWR, Radnor, USA), sodium carbonate (anhydrate, VWR, Radnor, USA), sodium bicarbonate (Thermo Fisher Scientific, Geel, Belgium), polyethylene glycol (PEG, 400 g mol−1, Acros Organics, Thermo Fisher Scientific, Geel, Belgium), polyether sulfone (PES, Ultrason E2010, BASF, Ludwigshafen, Germany). Bicinchoninic acid (BCA, Pierce, IL, USA) protein assay reagent A + B was provided by Thermo Fisher Scientific (Geel, Belgium).
000 g mol−1), phosphate buffered saline (PBS, pH 7.0), albumin from bovine serum (BSA, 67 kDa), lysozyme from chicken egg white (14.6 kDa), citric acid, 1-methyl-2-pyrrolidone (NMP). Other purchased chemicals: aluminum oxide (Brockmann activity I, Fluka, Sigma Aldrich, St. Louis, USA), n-hexane (Merck, Darmstadt, Germany), 2-aminoethyl methacrylate hydrochloride (AEMA, Acros Organics, Thermo Fisher Scientific, Geel, Belgium), glutaraldehyde (Merck, Darmstadt, Germany), hydrochloric acid solution (0.1 M, VWR, Radnor, USA), sodium hydroxide solution (0.1 M, VWR, Radnor, USA), sodium carbonate (anhydrate, VWR, Radnor, USA), sodium bicarbonate (Thermo Fisher Scientific, Geel, Belgium), polyethylene glycol (PEG, 400 g mol−1, Acros Organics, Thermo Fisher Scientific, Geel, Belgium), polyether sulfone (PES, Ultrason E2010, BASF, Ludwigshafen, Germany). Bicinchoninic acid (BCA, Pierce, IL, USA) protein assay reagent A + B was provided by Thermo Fisher Scientific (Geel, Belgium).
All chemicals named above are used as received. The used water was ultrapure water taken from a MilliQ-System (Merck Millipore, Billerica, USA). Dialysis membranes used for particle purification were obtained from Carl Roth (cellulose acetate, Nadir, molecular weight cut-off (MWCO): 10–20 kDa, Wiesbaden, Germany).
2.2 Synthesis and characterization of PS beads
PS beads were synthesized by a dispersion polymerization method. Before usage the styrene monomer was purified by passing it through an aluminum oxide column. For synthesis the reaction system (three-necked flask equipped with a reflux condenser, a mechanicals stirrer, and a septum) was filled with water and heated to 70 °C by an oil bath. The system was degassed and kept under nitrogen atmosphere until the end of reaction. Styrene monomer was added via syringe to gain a 5 wt% emulsion and the reaction was started by addition of the initiator (8 mmol L−1 in final solution). KPS54 or AIBA55 were used to achieve anionic or cationic PS beads, respectively. After 2 h of reaction time the system was vented with air. The particle suspension was washed with n-hexane to remove leftover monomer and was then dialyzed three times using a dialysis membrane and MilliQ water.Particle size, zeta potential, and isoelectric point (IEP) of the different PS bead suspensions were determined using the Malvern Zetasizer (Zetasizer Nano ZS with multipurpose titrator MPT-2, Malvern Instruments, Worcestershire, UK). To take scanning electron microscopy (SEM) pictures (Ultra 55 SEM, Carl Zeiss Ltd, Goettingen, Germany) the particles were spin-coated on a silica wafer. The samples were coated with a thin (30 nm) chromium film using a Z400 sputter system (Leybold, Hanau, Germany).
2.3 Preparation of PES membranes
The used PES membranes were prepared using a non-solvent induced phase separation process (NIPS). A solution containing 14 wt% PES, 65 wt% PEG and 21 wt% NMP was casted on a glass plate using a casting knife (ZWA 2121 Wasag Applicator, Zehntner Testing Instruments, Sissach, Switzerland) with a 200 μm gap and a custom-made automated casting system. The polymer solution film was then kept in humidified air at room temperature for 5 min, followed by precipitation in cooled water (∼10 °C) for 10 min. Afterwards, the membranes were rinsed three times with water for 30 min, respectively. Until further usage, the membranes were stored in water. In the following, the pristine PES membranes will be referred to as PES-REF.2.4 Membrane modification and characterization
To modify membranes the PES-REF membranes were dipped into an aqueous solution of the chosen reagent followed by EB irradiation in wet state. Irradiation was performed in a N2 atmosphere with O2 quantities <10 ppm using a custom-made electron accelerator. The voltage and the current were set to 160 kV and 10 mA, respectively. The absorbed dose was adjusted by the speed of the sample transporter.To modify membranes with PSS a PSS solution (2 wt%)56 was used, and the irradiation dose was adjusted to 200 kGy.
An aqueous solution of AEMA (0.5 wt%) and an irradiation dose of 150 kGy were used to generate the primary stage of the alkaline PES-TEPA membrane. The membranes were rinsed with water three times for 30 min, respectively. To gain the final TEPA modification the membranes dipped into an aqueous solution of GA (2 wt%) at pH 9.2 (NaHCO3/Na2CO3 buffer system) for 2 h. The GA solution was removed and the membrane was roughly rinsed before it was immersed into an aqueous solution of TEPA (2 wt%) at pH 9.2 (same buffer) for another 2 h. The reactions with GA and TEPA were repeated as described before to create a 2nd generation of dendrimeric structures.57
To generate the zwitterionic lysine modification the membranes were dipped into an aqueous solution of AEMA (2 wt%), followed by EB irradiation (200 kGy), and rinsing with water three times for 30 min, respectively. The subsequent reaction with GA was performed as described for the TEPA modification, but in the next step lysine (2 wt%) was used instead of TEPA.53
Finally, all modified membranes were rinsed three times for 30 min, respectively, and were subsequently dried at room temperature.
SEM imaging (Ultra 55 SEM, Carl Zeiss Ltd., Goettingen, Germany) was used to characterize the morphology of the modified membranes. The samples were coated with a thin (30 nm) chromium film using a Z400 sputter system (Leybold, Hanau, Germany).
Porosity and pore size distribution of the membranes were investigated with a mercury porosimeter (PoreMaster 30, Quantachrome Instruments, Odelzhausen, Germany). Values of at least three different samples were averaged.
Permeability was determined using a stainless steel pressure filter holder (16249, Sartorius, Goettingen, Germany) for dead-end filtration. A volume of 200 mL of deionized water was passed through a membrane sample (active area: 17.3 cm2) at 1 bar and the time of flow-through was recorded. Values of at least three different samples were averaged. Pure water permeation flux J was calculated following eqn (1).
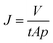 | (1) |
The chemical composition was investigated using X-ray photoelectron spectroscopy (XPS, Kratos Axis Ultra, Kratos Analytical Ltd., Manchester, UK).
The surface wettability of the membranes was determined using a static water contact angle measurements system (DSA 30E, Krüss, Hamburg, Germany) with the sessile drop method. Values of at least three different samples were averaged.
Membrane zeta potentials were investigated using streaming potential measurements carried out with the adjustable gap cell in the SurPASS system (Anton Paar, Graz, Austria), where the zeta potential ζ can be calculated based on the Smoluchowski eqn (2). Values of at least three different samples were averaged.
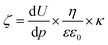 | (2) |
2.5 Membrane fouling with PS beads
Filtration tests with PS beads were performed in dead-end filtration mode using a 50 mL stirring cell (Amicon, Merck Millipore, Billerica, USA) to determine membrane fouling. A membrane disk (active area: 15.9 cm2) was mounted into the stirring cell and a volume of 140 mL water was filtered at 0.1 bar to check water permeability. Afterwards, a volume of 140 mL PS bead suspension (∼50 mg L−1) at pH 7 was filtered through the membrane under the same conditions. After every 20 mL a filtrate fraction was gathered. Membranes with zwitterionic moieties were also investigated at pH 4 and 9. The pH of every suspension was checked prior to use with a pH electrode system (HI 3220, Hanna Instruments, Kehl, Germany). The pH was adjusted to 4 or 9 using hydrochloric acid solution (0.1 M) or sodium hydroxide solution (0.1 M), respectively. The time of flow-through was recorded for each fraction. The concentration of PS beads was measured spectrometrically (Infinite M200, Tecan, Männedorf, Switzerland) monitoring the UV absorption of polystyrene at 290 nm. Values of at least three different samples were averaged.2.6 Membrane fouling with proteins
Protein fouling of the PES membranes was investigated with a bicinchoninic acid (BCA) based assay37,58 using BSA and lysozyme as proteins. The membrane samples (1 cm in diameter, fitting into the wells of 48-well microtiter plate) were first put into 250 μL of ethanol. The microtiter plate was placed on a mechanical shaker for 10 min followed by rinsing the probes three times with water. To each membrane sample 200 mL of a protein solution (2.0 mg mL−1) in 50 mM PBS (pH 7.0) was added. The plates were shaken for 1 h at ambient temperature followed by rising three times with 1 mL of PBS. The BCA reagent was added and the plate was incubated for 25 min at 37 °C. The plate was then shaken for 5 min at ambient temperature and the solution was transferred to a 96-well microtiter plate. The light adsorption at 562 nm was measured with a microtiter plate reader (Infinite M200, Tecan, Männedorf, Switzerland). For calibration, several protein concentrations, ranging between 0 and 30 mg mL−1, were used per the BCA test instructions.3. Results and discussion
In this work we want to describe how charged PS beads can be used to identify electrostatic interactions during membrane fouling. First of all, to properly comprehend the conducted fouling experiments and the occurring interactions the used PS beads and membranes will be characterized in the following sections.3.1 Characteristics of PS beads
As already mentioned, using PS beads as fouling reagents has a crucial advantage because of their uniform size, shape, and tunable surface charge.The particles used in this work were prepared with either negative or positive surface charges depending on the used polymerization initiator. The zeta potential curves of both particle species are shown in Fig. 1a. All error bars shown represent 95% confidence values. The charged functional groups present on the particle surface are shown in Fig. 1b. The cationic particles are positively charged (∼+74 mV at pH 7) over a broad range of pH and have an isoelectric point (IEP) at pH 10.8 due to the present amino functions. Contrary, the anionic PS beads are negatively charged (∼−90 mV at pH 7) over a broad range of pH and have an IEP at pH 2.5 because of the sulfonic acid groups. The size of the particles was determined be around 200 nm. Therefore, the particles can permeate through the membranes (average pore size: ∼0.8 μm) in case of repulsive interactions and do not block the pores due to size exclusion.
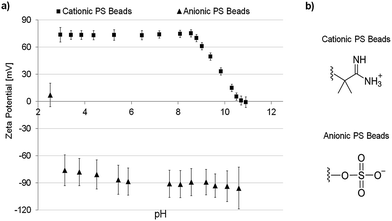 | ||
| Fig. 1 (a) Zeta potential of charged PS beads vs. pH; (b) charged moieties of the particular particles. | ||
Further descriptions of the polymerization mechanism, structures of initiator molecules and data regarding particle size and dispersity are shown in the ESI Section S3.1.†
3.2 Characteristics of PES membranes
To show that differently charged PS beads can be used to identify electrostatic interactions during membrane fouling a set of differently charged membranes is also needed. Therefore, PES membranes were prepared using the NIPS process. Subsequently, electron beam-based modifications were applied to the membranes to introduce differently charged moieties (Fig. 2b). The different modification reactions which lead to the desired surface charge are well known from literature and will not be discussed here. Nevertheless, detailed descriptions of the reaction mechanisms are given in Section S3.2 of the ESI.† | ||
| Fig. 2 (a) Zeta potential of charged PES membranes vs. pH; (b) charged moieties of the particular membrane surface modification. | ||
The zeta potential vs. pH curves of the different membranes are presented in Fig. 2a. All error bars shown represent 95% confidence values. The PES-REF membrane (circles) shows a trend which is typical for uncharged polymeric materials. Due to the adsorption of hydroxide ions originating from the self-ionization of water the membrane surface appears to be negatively charged with a zeta potential of −43 mV at pH 7.59,60 The isoelectric point (IEP) appears at ∼2.2 what can be calculated by extrapolation. This is slightly more acidic than expected for a pristine polymer material and can be explained by sulfonate groups present in the PES polymer.
A similar trend is found for the PES-PSS membrane (triangles), where the IEP is neither recorded nor calculable by extrapolation using the existing data. However, the PES-PSS membrane has a nearly stable negative zeta potential of around −44 mV over the measured range of pH, confirming the presence of additional sulfonic acid groups on the membrane surface.
The PES-lysine membrane (diamonds) represents a group of modifications with zwitterionic moieties that are often found in recent literature.32 As a zwitterionic structure the lysine moiety contains both: acidic (carboxylic acid function) and alkaline groups (amino function). Accordingly, the PES-lysine membrane's IEP was found to be at a nearly neutral pH of 6.2. Therefore, the membrane's zeta potential strongly depends on the pH of the surrounding solution. In the acidic range of pH (protonated amino groups) the potential is highly positive and in an alkaline environment (deprotonated acid groups) the membrane surface is negatively charged.
The PES-TEPA membrane (squares) is highly positively charged over a broad range of pH and has a zeta potential of +34 mV at pH 7. Here, an IEP of 8.9 is reached. This immense shift of IEP (compared to the PES-REF membrane) could only be reached by growing a hyperbranched dendrimer structure (Fig. S4.2†) containing a high density of protonated amino groups61 to the membrane surface.
Beside the membrane's surface charge the surface hydrophilicity might also influence the fouling tendencies of the different membranes. Surface hydrophilicity can be well estimated by water contact angle determination. The water contact angle of the PES-REF membrane was found to be 103 ± 1° which shows that the PES-REF membrane is highly hydrophobic. Contrary, the modified membranes are more hydrophilic due to the charged groups present on the membrane surfaces. The water contact angles were measured to be 42 ± 1° for the PES-PSS membrane, 40 ± 2° for the PES-lysine membrane, and 31 ± 2° for the PES-TEPA membrane.
Further descriptions of the membrane preparation, the involved reaction mechanisms, and data regarding membrane characteristics (water permeation flux, bubble point, SEM images, porosity, average pore size and XPS data) are shown in the ESI Section S3.2.†
3.3 Membrane fouling with PS beads
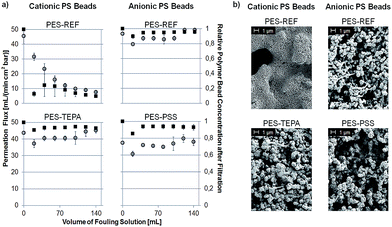
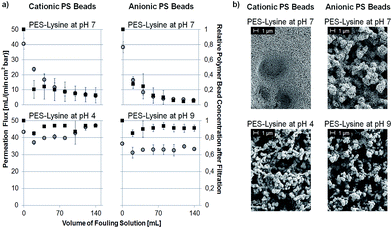
First of all, it should be noted that this study is focused on interactions between membrane and particles. The PS bead suspensions are stable under storage conditions and therefore interactions between particles can be excluded prior to the experiment. The initial fouling is determined by interactions between membrane and particles. If particles are adsorbed to the membrane surface the membranes pores will be blocked. The following cake layer formation and the respective interactions between particles are merely consequences of this size exclusion effect. Without the initial fouling interactions between particles will also not occur. Therefore, the discussion in the following will focus on interactions between membrane and particles instead of interactions between the particles itself.To determine if a membrane is prone to fouling by electrostatic interactions fouling experiments were conducted as described in Section 2.5. Each membrane was tested by filtering suspensions of negatively and positively charged PS beads in a dead-end filtration set-up. Chosen results are shown in Fig. 3a. During the experiment the permeation flux of the filtered fouling suspension (left axis, open circles) was monitored as well as the concentration of PS beads in the filtrate (right axis, filled squares). All error bars shown represent 95% confidence values. A complete overview of all conducted fouling experiments is shown in Fig. S6.1 in the ESI.† The corresponding SEM images of the fouled membranes are shown in Fig. 3b as well as in Fig. S6.2 in the ESI.†
The PES-REF membrane is heavily fouled by cationic PS beads which is detected by a rapid decline in permeation flux as well as by a decreasing concentration of PS beads in the filtrate. The reason is electrostatic attraction between the negatively charged membrane surface (−43 mV at pH 7) and the positively charged PS beads (+74 mV at pH 7). In contrast, no fouling occurred using negatively charged PS beads (−90 mV at pH 7) due to electrostatic repulsion between the evenly charged membrane surface and PS beads. These results are supported by the corresponding SEM images (Fig. 3b). The PES-REF membrane is completely blocked by cationic PS beads. On the contrary, no negatively charged PS beads are adsorbed.
The PES-PSS membrane shows the same fouling characteristics as the PES-REF membrane. No permeation flux decline or decreasing PS bead concentration is found using negatively charged particles (Fig. 3a). Also, no pore blocking is visible in the corresponding SEM image (Fig. 3b). This is in agreement with the expected result because the zeta potential of the PES-PSS membrane (−44 mV at pH 7) was just slightly changed compared to the PES-REF membrane (−43 mV at pH 7). Contrary, strong fouling occurs using cationic PS beads. The rapidly declining flux and decreasing PS bead concentration (Fig. S6a†) as well as the corresponding SEM image (Fig. S6b†) are presented in the ESI.† The reasons are the electrostatic attractive forces between the cationic particles and the negatively charged membrane surface.
The property mostly affected by the PSS-modification is the membrane's surface hydrophilicity. As shown in Section 3.2 the PES-REF membrane is highly hydrophobic (water contact angle: 103 ± 1°) while the PES-PSS membrane is highly hydrophilic (water contact angle: 42 ± 1°). Nevertheless, both membranes exploit the same fouling characteristic. This shows the importance of electrostatic interactions and zeta potential measurements as well as the need for a method to identify electrostatic interactions.
The fouling tendency of the PES-TEPA membrane is in complete opposition to the fouling of the PES-PSS membrane. Here, the highly positively charged membrane (+34 mV at pH 7) repels the evenly charged cationic PS beads (Fig. 3a) and attracts the oppositely charged anionic particles (Fig. S6a†). The corresponding SEM images support these results. No pore blocking is visible using cationic particles (Fig. 3b) while anionic PS beads lead to a completely covered membrane surface (Fig. S6b†). The reasons are, again, electrostatic repulsive and attractive forces.
The PES-lysine membrane's surface interactions require a more detailed explanation. As already mentioned the zeta potential of the PES-lysine membrane is strongly pH dependent. At pH 4 the membrane is positively charged (+40 mV) while being negatively charged (−43 mV) at pH 9 and nearly uncharged (−5 mV) at pH 7. Therefore, the fouling tendencies and electrostatic interactions are pH depended, too. The results of the conducted fouling experiments are presented in Fig. 4a as well as in Fig. S6.1 in the ESI.† All error bars shown represent 95% confidence values. The SEM images of the fouled PES-lysine membranes at different pH values are shown in Fig. 4b as well as in Fig. S.6b in the ESI.†
For both, anionic and cationic PS beads the permeation flux and the concentration of PS beads in the filtrate decrease when the PES-lysine membrane is investigated at pH 7. Accordingly, the corresponding SEM images show complete pore blocking (Fig. 4b). In this unique case both kinds of charged particle species tend to foul the membrane. A possible explanation is the small zeta potential of −5 mV of the PES-lysine membrane at this pH. Comparable to particle suspensions with small zeta potentials62 the membrane surface is destabilized and PS beads from the surrounding solution easily adsorb. Despite the membrane's hydrophilicity the PES-lysine membrane fouls due to the absence of electrostatic repulsive forces. This means if there is a membrane that shows fouling with both, anionic and cationic PS beads the IEP of the used membrane is most probably in the same range as the pH of the surrounding solution. This leads to a membrane surface which is nearly uncharged, and therefore, unable to execute electrostatic interactions.
The problem of an uncharged membrane surface can be prevented by shifting the pH of the surrounding solution. As shown in Fig. 4a the PES-lysine membrane shows no permeation flux decline or decreased PS bead concentration in the filtrate when cationic PS beads are filtered at pH 4. Also, no pore blocking is visible in the corresponding SEM image (Fig. 4b). Here, the membrane is highly positively charged (+40 mV, as shown in Fig. 2). The same is true for the filtration of anionic particles at pH 9 where the membrane is highly negatively charged (−43 mV, as shown in Fig. 2). In both cases, electrostatic repulsion can take place resulting in an anti-fouling characteristic of the PES-lysine membrane at the specific pH.
In contrast, heavy fouling of the PES-lysine membrane is observed for the filtration of cationic PS beads at pH 9 and for the filtration of anionic particles at pH 4 (Fig. S6 in the ESI†). Here, the particles and membrane surfaces are oppositely charged leading to electrostatic attractive interactions.
3.4 Membrane fouling with proteins
In the last part of this work, the fouling characteristics of the different PES membranes were also tested using two different proteins. This allows a direct comparison between the fouling with PS beads and the commonly used proteins leading to further insight into the advantages and disadvantages of both methods.The proteins chosen are bovine serum albumin (BSA) and lysozyme. BSA is negatively charged over a broad range of pH (IEP: 4.7) and can be directly compared to the fouling with anionic PS beads. Lysozyme has a high IEP of 11.1 and is positively charged over a broad range of pH. Therefore, it is comparable with cationic PS beads.
The fouling tendencies of the different PES membranes were tested as described in Section 2.6. Fig. 5 shows the relative change of protein adsorption referenced to the PES-REF membrane at pH 7. All error bars shown represent 95% confidence values.
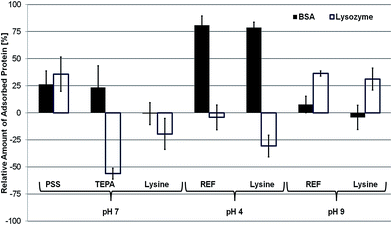 | ||
| Fig. 5 Relative amount of protein (filled: BSA, open: lysozyme) adsorbed to the differently charged membrane surfaces, normalized to the PES-REF membrane at pH 7. | ||
The PES-PSS membrane shows relatively higher protein adsorption for both tested proteins (+23% for BSA and +36% for lysozyme). The adsorption of the positively charged lysozyme to the negatively charged PES-PSS membrane surface can be addressed to electrostatic attractive forces. Nevertheless, BSA is also adsorbed to the surface while electrostatic repulsion would have been expected. The reason for the unforeseen behavior of BSA can be explained with its protein characteristics. Due to the acidic groups present on the PES-PSS membrane BSA unfolds and changes its tertiary structure63,64 leading to surface adsorption. This leads to false assumptions regarding the electrostatic interactions that might occur showing a clear advantage of the distinct fouling test system using charged PS beads.
Furthermore, it should be pointed out that the PES-PSS membrane is more hydrophilic than the PES-REF membrane. Nevertheless, fouling increased for both proteins. This shows the importance of electrostatic interactions and zeta potential analysis as they can partially explain the found fouling tendencies.
The PES-TEPA membrane shows increased fouling by BSA (+23%) while the fouling with lysozyme is drastically decreased (−56%). This is in agreement with the fouling tendencies found for the adsorption of charged PS beads. Here, electrostatic forces are considered as the dominant interactions. The highly positively charged PES-TEPA membrane repels the cationic lysozyme while the anionic BSA is attracted.
The PES-lysine membrane adsorbs nearly the same amount of BSA as the PES-REF membrane, while lysozyme adsorption is reduced by 20%. This is unexpected considering the small zeta potential of −5 mV of the PES-lysine membrane at pH 7. According to the fouling experiments using charged PS beads an unspecific fouling of both, BSA and lysozyme would be expected. This is true for BSA as the fouling remains the same compared to the PES-REF membrane. However, despite the small negative surface charge present on the PES-lysine membrane, the cationic lysozyme is repelled. This can neither be explained by electrostatic interactions nor by improved hydrophilicity, as this would have to impact the fouling of both proteins.
Similar to the experiments with PS beads fouling with BSA and lysozyme was also investigated at different pH. To maintain comparability the fouling of proteins was first tested with the PES-REF membrane at pH 4 or 9 to see the influence of pH alone. Afterwards, the fouling of the PES-lysine membrane at pH 4 and 9 was investigated.
At pH 4 BSA (IEP: 4.7) is slightly positively charged and has an increased size due to a changed tertiary structure.63,64 Nevertheless, it is still stable in the used solution. The fouling of BSA is increased by 81% for the PES-REF membrane and by 79% for the PES-lysine membrane (normalized to the fouling of the PES-REF membrane at pH 7). The zeta potential of the PES-REF membrane at pH 4 (−37 mV) has not changed much compared to pH 7 (−43 mV). Therefore, the increased fouling can be directly addressed to the changed tertiary structure and the nearly uncharged state of BSA at pH 4. The same is true for the fouling of the PES-lysine membrane which is highly positively charged at pH 4 (+40 mV). Due to the changed tertiary structure of BSA fouling is increased especially if compared to the BSA fouling of the PES-lysine membrane at pH 7 (−1%).
Lysozyme is less influenced by the lower pH of 4 and remains highly positively charged.65 The fouling of the PES-REF membrane (−4%) is nearly the same as at pH 7 due to the still existing electrostatic attractive interactions. Contrary, the fouling of the PES-lysine membrane is significantly reduced by 30% (referenced to the fouling of the PES-REF membrane at pH 7) which is even better than the fouling reduction of the PES-lysine membrane at pH 7 (−20%). This further improved fouling reduction can be clearly addressed to the now existing electrostatic repulsive interactions between the cationic lysozyme and the positively charged membrane.
A different situation can be found for the fouling at pH 9. Now, BSA is negatively charged and has an increased size due to a changed tertiary structure.63,64 Compared to the fouling of the PES-REF membrane at pH 7 the fouling of the PES-REF membrane at pH 9 (−43 mV) is slightly increased (+8%) and the fouling of the PES-lysine membrane (−43 mV) is slightly decreased (−4%). Due to the opposing effects of increased electrostatic repulsion and increased size the overall effect is just marginal, and the resulting fouling remains nearly unchanged.
Lysozyme is still positively charged at pH 9 but has also a changed tertiary and increased size.65 This way, the fouling for both, the PES-REF membrane (+36%) and the PES-lysine membrane (+31%) is increased at pH 9. The reasons are the increased electrostatic attractive forces as well as the changed tertiary structure of lysozyme.
All in all, we could show that protein fouling tests lead to very complex and sometimes inexplicable results. BSA and lysozyme are not stable in size and shape when the chemical environment changes. Therefore, many different effects overlap during the filtration experiments, and the dominating interaction cannot be identified. The fouling test system presented by the authors is solely focused on electrostatic interactions and has, therefore, a clear advantage compared to the common protein test system.
4. Conclusion
The aim of this work was to present a new method using differently charged PS beads to identify electrostatic interactions while fouling of microfiltration membranes. We successfully showed that anionic and cationic PS beads can be used to identify the electrostatic interactions which occurred with a representative set of differently charged membrane surfaces. It was revealed that:• Fouling occurs due to electrostatic attraction. This was demonstrated for the combination of cationic PS beads and negatively charged membrane surfaces as well as for anionic particles and membranes with positively charged surface functions.
• In contrast, electrostatic repulsion leads to anti-fouling. Anionic PS beads are repelled by negatively charged surfaces and cationic particles do not adsorb to membranes with positively charged surface functions.
• In case of uncharged, zwitterionic surfaces electrostatic interactions cannot be detected. This leads to unspecific fouling with both, anionic and cationic PS beads.
Furthermore, we could show that the fouling of zwitterionic membranes is highly pH dependent. At lower pH the membrane is positively charged, and cationic PS beads are repelled while anionic ones are attracted. Contrary, in its negatively charged state at higher pH anionic PS beads are repelled and cationic ones are attracted.
Given the fact, that all modified membranes are very hydrophilic electrostatic interactions have to be the dominating forces to explain the different fouling tendencies towards the charged polystyrene beads.
Furthermore, the fouling tendencies towards charged PS beads were compared to fouling by comparable charged proteins, BSA and lysozyme. We could show that in some cases similar trends are found. Nevertheless, most of the gained results needed additional explanations or even contradicted the fouling tendencies expected when electrostatic forces are considered as the dominant interactions. Therefore, the method to analyze exclusively electrostatic interactions in fouling experiments presented by the authors has a clear advantage compared to the common protein fouling test system.
Acknowledgements
The authors thank N. Schönherr for measuring particle size, zeta potential and isoelectric point of the PS beads. Financial support by the Federal State of Germany and the Free State of Saxony is gratefully acknowledged.Notes and references
- C. A. Haynes and W. Norde, J. Colloid Interface Sci., 1995, 169, 313–328 CrossRef CAS.
- M. Wahlgren and T. Arnebrant, Trends Biotechnol., 1991, 9, 201–208 CrossRef CAS PubMed.
- W. J. C. van de Ven, K. v. t. Sant, I. G. M. Pünt, A. Zwijnenburg, A. J. B. Kemperman, W. G. J. van der Meer and M. Wessling, J. Membr. Sci., 2008, 308, 218–229 CrossRef CAS.
- T. Young, Philos. Trans. R. Soc. London, 1805, 95, 65–87 CrossRef.
- I. Lundström, Phys. Scr., 1983, T4, 5–13 CrossRef.
- Y. Chang, C.-Y. Ko, Y.-J. Shih, D. Quémener, A. Deratani, T.-C. Wei, D.-M. Wang and J.-Y. Lai, J. Membr. Sci., 2009, 345, 160–169 CrossRef CAS.
- H. Chen and G. Belfort, J. Appl. Polym. Sci., 1999, 72, 1699–1711 CrossRef CAS.
- P. K. Chu, J. Y. Chen, L. P. Wang and N. Huang, Mater. Sci. Eng., R, 2002, 36, 143–206 CrossRef.
- J. R. Du, S. Peldszus, P. M. Huck and X. Feng, Water Res., 2009, 43, 4559–4568 CrossRef CAS PubMed.
- K. C. Khulbe, C. Feng and T. Matsuura, J. Appl. Polym. Sci., 2010, 115, 855–895 CrossRef CAS.
- S. X. Liu, J. T. Kim and S. Kim, J. Food Sci., 2008, 73, E143–E150 CrossRef CAS PubMed.
- N. Saxena, C. Prabhavathy, S. De and S. DasGupta, Sep. Purif. Technol., 2009, 70, 160–165 CrossRef CAS.
- B. Van der Bruggen, J. Appl. Polym. Sci., 2009, 114, 630–642 CrossRef CAS.
- Q. Shi, Y. Su, W. Chen, J. Peng, L. Nie, L. Zhang and Z. Jiang, J. Membr. Sci., 2011, 366, 398–404 CrossRef CAS.
- M. Ulbricht, POLYMER, 2006, 47, 2217–2262 CrossRef CAS.
- A. Schulze, B. Marquardt, S. Kaczmarek, R. Schubert, A. Prager and M. R. Buchmeiser, MACROMOL. RAPID. COMM., 2010, 31, 467–472 CrossRef CAS PubMed.
- A. Schulze, B. Marquardt, M. Went, A. Prager and M. R. Buchmeiser, Water science and technology : a journal of the International Association on Water Pollution Research, 2012, 65, 574–580 CrossRef CAS PubMed.
- K. Asfardjani, Y. Segui, Y. Aurelle and N. Abidine, J. Appl. Polym. Sci., 1991, 43, 271–281 CrossRef CAS.
- A. Boulares-Pender, I. Thomas, A. Prager and A. Schulze, J. Appl. Polym. Sci., 2013, 128, 322–331 CrossRef CAS.
- M. G. Buonomenna, L. C. Lopez, P. Favia, R. d’Agostino, A. Gordano and E. Drioli, Water Res., 2007, 41, 4309–4316 CrossRef CAS PubMed.
- K. C. Khulbe and T. Matsuura, J. Membr. Sci., 2000, 171, 273–284 CrossRef CAS.
- K. R. Kull, M. L. Steen and E. R. Fisher, J. Membr. Sci., 2005, 246, 203–215 CrossRef CAS.
- N. Vandencasteele and F. Reniers, J. Electron Spectrosc. Relat. Phenom., 2010, 178–179, 394–408 CrossRef CAS.
- E. J. W. Verwey and J. T. G. Overbeek, Theory of the Stability of Lyophobic Colloids, lsevier Publishing Company, Inc., Amsterdam, 1948 Search PubMed.
- B. V. Derjaguin and L. Landau, Acta Physicochim. URSS, 1941, 14, 633–662 Search PubMed.
- C. J. Van Oss, R. J. Good and M. K. Chaudhury, J. Colloid Interface Sci., 1986, 111, 378–390 CrossRef CAS.
- C. J. van Oss, Colloids Surf., B, 1995, 5, 91–110 CrossRef CAS.
- A. E. Childress and M. Elimelech, Environ. Sci. Technol., 2000, 34, 3710–3716 CrossRef CAS.
- A. V. Dudchenko, J. Rolf, K. Russell, W. Duan and D. Jassby, J. Membr. Sci., 2014, 468, 1–10 CrossRef CAS.
- M. Hadidi and A. L. Zydney, J. Membr. Sci., 2014, 452, 97–103 CrossRef CAS.
- S. Hong and M. Elimelech, J. Membr. Sci., 1997, 132, 159–181 CrossRef CAS.
- M. Kumar and M. Ulbricht, Polymer, 2014, 55, 354–365 CrossRef CAS.
- K. Xiao, X. Wang, X. Huang, T. D. Waite and X. Wen, J. Membr. Sci., 2011, 373, 140–151 CrossRef CAS.
- Q. Zhang and C. D. Vecitis, J. Membr. Sci., 2014, 459, 143–156 CrossRef CAS.
- K. W. Trzaskus, W. M. de Vos, A. Kemperman and K. Nijmeijer, J. Membr. Sci., 2015, 496, 174–184 CrossRef CAS.
- H. Cai, H. Fan, L. Zhao, H. Hong, L. Shen, Y. He, H. Lin and J. Chen, J. Colloid Interface Sci., 2016, 465, 33–41 CrossRef CAS PubMed.
- P. K. Smith, R. I. Krohn, G. T. Hermanson, A. K. Mallia, F. H. Gartner, M. D. Provenzano, E. K. Fujimoto, N. M. Goeke, B. J. Olson and D. C. Klenk, Anal. Biochem., 1985, 150, 76–85 CrossRef CAS PubMed.
- E. Arkhangelsky, I. Levitsky and V. Gitis, Water Sci. Technol., 2008, 58, 1955–1961 CrossRef CAS PubMed.
- P. Bacchin, P. Aimar and V. Sanchez, AIChE J., 1995, 41, 368–376 CrossRef CAS.
- A. Abdelrasoul, H. Doan, A. Lohi and C.-H. Cheng, Sep. Purif. Technol., 2014, 135, 199–210 CrossRef CAS.
- Z. Adamczyk, Adv. Colloid Interface Sci., 2003, 100, 267–347 CrossRef.
- Z. Adamczyk and P. Warszyhski, Adv. Colloid Interface Sci., 1996, 63, 41–149 CrossRef CAS.
- C. M. Bondy and C. Santeufemio, J. Membr. Sci., 2010, 349, 12–24 CrossRef CAS.
- A. Gordano, V. Arcella and E. Drioli, Desalination, 2004, 163, 127–136 CrossRef CAS.
- M. J. Han, G. N. B. Baroña and B. Jung, Desalination, 2011, 270, 76–83 CrossRef CAS.
- C. Henry, J.-P. Minier and G. Lefèvre, Adv. Colloid Interface Sci., 2012, 185, 34–76 CrossRef PubMed.
- M. O. Lamminen, H. W. Walker and L. K. Weavers, J. Membr. Sci., 2004, 237, 213–223 CrossRef CAS.
- M. O. Lamminen, H. W. Walker and L. K. Weavers, Sep. Sci. Technol., 2006, 41, 3569–3584 CrossRef CAS.
- M. O. Lamminen, H. W. Walker and L. K. Weavers, J. Membr. Sci., 2006, 283, 225–232 CrossRef CAS.
- Y. Lanteri, P. Fievet, C. Magnenet, S. Déon and A. Szymczyk, J. Membr. Sci., 2011, 378, 224–232 CrossRef CAS.
- H. Ma, L. F. Hakim, C. N. Bowman and R. H. Davis, J. Membr. Sci., 2001, 189, 255–270 CrossRef CAS.
- B. Teychene, P. Loulergue, C. Guigui and C. Cabassud, J. Membr. Sci., 2011, 370, 45–57 CrossRef CAS.
- D. Breite, M. Went, A. Prager and A. Schulze, Polymer, 2015, 7, 2017–2030 CAS.
- S. Inukai, T. Tanma, S. Orihara and M. Konno, Chem. Eng. Res. Des., 2001, 79, 901–905 CrossRef CAS.
- D. Duracher, F. Sauzedde, A. Elaissari, A. Perrin and C. Pichot, Colloid Polym. Sci., 1998, 276, 219–231 CAS.
- A. Schulze, M. F. Maitz, R. Zimmermann, B. Marquardt, M. Fischer, C. Werner, M. Went and I. Thomas, RSC Adv., 2013, 3, 22518–22526 RSC.
- A. Boulares-Pender, A. Prager, C. Elsner and M. R. Buchmeiser, J. Appl. Polym. Sci., 2009, 112, 2701–2709 CrossRef CAS.
- Journal.
- R. Zimmermann, S. Dukhin and C. Werner, J. Phys. Chem. B, 2001, 105, 8544–8549 CrossRef CAS.
- R. Zimmermann, U. Freudenberg, R. Schweiß, D. Küttner and C. Werner, Curr. Opin. Colloid Interface Sci., 2010, 15, 196–202 CrossRef CAS.
- S. E. Burke and C. J. Barrett, Langmuir, 2003, 19, 3297–3303 CrossRef CAS.
- J. Jiang, G. Oberdörster and P. Biswas, J. Nanopart. Res., 2008, 11, 77–89 CrossRef.
- T. Estey, J. Kang, S. P. Schwendeman and J. F. Carpenter, J. Pharm. Sci., 2006, 95, 1626–1639 CrossRef CAS PubMed.
- A. Michnik, K. Michalik and Z. Drzazga, J. Therm. Anal. Calorim., 2005, 80, 399–406 CrossRef CAS.
- F. Makki and T. D. Durance, Food Res. Int., 1996, 29, 635–645 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra13787c |
| This journal is © The Royal Society of Chemistry 2016 |