Improving crystallization and processability of PBS via slight cross-linking†
Gui-Cheng Liu,
Wen-Qiang Zhang,
Shui-Lian Zhou,
Xiu-Li Wang* and
Yu-Zhong Wang*
Center for Degradable and Flame-Retardant Polymeric Materials (ERCPM-MoE), College of Chemistry, State Key Laboratory of Polymer Materials Engineering, National Engineering Laboratory of Eco-Friendly Polymeric Materials (Sichuan), Sichuan University, 29 Wangjiang Road, Chengdu 610064, China. E-mail: xiuliwang1@163.com; yzwang@scu.edu.cn; Fax: +86-28-85410755; Tel: +86-28-85410755
First published on 14th July 2016
Abstract
To improve the processability and crystallization of poly(butylene succinate) (PBS), a cross-linkable comonomer containing an alkynyl group, named 5-(2-(trimethylsilyl)ethynyl)isophthalate (DTS) was synthesized and copolymerized with dimethyl succinate and 1,4-butanediol to prepare a series of slightly cross-linked PBS copolyesters (PBDTSx). Due to the very low cross-linking degree, PBDTSx do not form gels when the DTS molar content is lower than 1.0%, and this made them keep good solubility and reprocessability. The cross-linking degree of DTS in PBDTSx is determined by NMR spectra. GPC data show that the Mw values of the copolyesters are higher than 23 × 104 g mol−1, and increase with the increase of DTS content. The thermal stability, crystallization, rheological behaviours, and mechanical properties of PBDTSx were investigated. Compared to neat PBS, PBDTSx have greatly increased crystallization rates because of promoted nucleation of the cross-linking domains. The elongation at break of PBDTSx drops slightly, while the Young's modulus increases. The rheological behaviours indicate that PBDTSx have higher melt viscosity than neat PBS even at a high shear rate, meaning that they will have better blow moulding processability. This work demonstrates that via controlling tiny cross-linking during the polymerization of PBS, the properties and processability of PBS can be improved, and this method overcomes the defects caused by traditional post cross-linking.
Introduction
Due to the increasing concerns on the environment and sustainable energy development, bio-based polymers derived from renewable resources have attracted much attention. Poly(butylene succinate) (PBS) is considered to be a promising bio-based polymer, which can compete with conventional polypropylene and polyethylene owing to its good thermal stability and mechanical properties.1 However, it still has some disadvantages, such as insufficient mechanical properties, low melt strength/viscosity and a narrow processing temperature window, which limit its wide applications. To resolve these problems, many methods, such as enhancing molecular weight by chain extension,2,3 physical blending4–7 and copolymerization,8–11 are used.Cross-linking is also an effective way to improve the properties of PBS. Usually the cross-linking reaction can occur under irradiation or thermal processing in the presence of peroxide or the compound containing more than one double bond.12–18 Bahari et al.13 found that the melt strength of PBS was improved by electron-beam radiation cross-linking and biodegradable PBS foam can be easily prepared. Via ultraviolet cross-linking the modified PBS shows improved thermal stability and dynamic mechanical property.14 The results reported by Kim et al.16 and Ma et al.17 revealed that the crystallization temperature and the mechanical property of cross-linked PBS were enhanced. The aforementioned cross-linking modification is achieved by post-processing, and extra equipment is needed. Furthermore, to prevent pollution caused by the electron beam, rigorous safeguarding is also needed. At the same time, in this way a lot of gels are formed, which will influence the reprocessability and biodegradability of PBS. If we make PBS slightly cross-linking at the polycondensation step instead of post-processing, its properties are accordingly improved, while the crosslinking degree is so low as not to affect PBS further thermal processing.
It has been demonstrated that alkynyl group is prone to cross-linking reaction around 200 °C to 350 °C, depending on its chemical structure. This cross-linking group has been introduced into a variety of polymers, such as polycarbosilane,19–21 thermosetting polyimide,22–24 polyphenylquinoxalines25,26 and poly(ethylene terephthalate),27,28 etc. to improve their thermal stability, adhesive strength and flame retardancy. As far as we know, alkynyl group has not been introduced into PBS yet.
In this study, dimethyl 5-(2-(trimethylsilyl)ethynyl)-isophthalate (DTS), a comonomer containing alkynyl group, is synthesized and introduced into PBS main chain via melt polymerization. The effect of DTS contents on thermal properties, crystallization properties, mechanical properties and rheological behaviours of PBS were investigated in detail. It is expected that the properties of PBS can be improved via changing its chemical structure.
Experimental section
Materials
1,4-Butanediol (BDO, AR grade), and zinc acetate (Zn(OAc)2, AR grade) were purchased from Kelong Chemical Corporation (Chengdu, China). Dimethyl succinate (DSA, AR grade) was purchased from Alfa Aesar Chemical Corporation (Tianjin, China). Tetrabutyl titanate (TBT), was also provided by Kelong Chemical Corporation, and dissolved in anhydrous toluene to prepare 0.2 g mL−1 solution. Iodine (I2, AR grade), sodium periodate (NaIO4, AR grade), dimethyl isophthalate (AR grade), ethynyltrimethylsilane (AR grade), copper iodide (CuI, AR grade) and bis(triphenylphosphine)-palladium(II)chloride (Pd(PPh3)2Cl2, AR grade) were purchased from Yinuokai Chemical Corporation (Beijing, China). Sulfuric acid (H2SO4, 96%), methylene chloride (CH2Cl2), sodium bicarbonate (NaHCO3), sodium sulfite (Na2SO3), triethylamine (TEA), tetrahydrofuran (THF), methanol and ethanol were purchased from Kelong Chemical Corporation.Characterization
Intrinsic viscosities of polyester were measured with an Ubbelohde viscometer at a concentration of 2.0 g dL−1 in chloroform at 30 ± 0.1 °C.The molecular weights of copolyesters were determined by GPC, using a Waters apparatus equipped with a model 1515 pump, a Waters model 717 auto samplers, and a 2414 refractive index detector. Chloroform and monodisperse polystyrene were used as the eluent and standard, respectively. The concentration of sample and the flow rate of eluent were 2.5 mg mL−1 and 1.0 mL min−1, respectively.
1H and 13C-NMR spectra were recorded using a Bruker AC-P 400 MHz spectrometer in CDCl3 solution using tetramethylsilane as an internal reference.
Thermogravimetric analysis for samples (5 mg) was performed on a NETZSCH Instrument (TG209 F1, Germany). The thermograms were recorded at a heating rate of 10 °C min−1 under a nitrogen flow of 50 mL min−1, within a temperature range of 40–550 °C.
The thermal transition behaviour of samples was examined by a TA Instrument (DSC-Q200). Samples (5 mg) were first heated to 130 °C (the first heating scan), and held 3 min to eliminate thermal history. After that, they were cooled to −70 °C at a cooling rate of 10 °C min−1 (cooling scan), and finally the samples were heated again to 130 °C at the heating rate of 10 °C min−1 (the second heating scan).
The self-nucleation experiments were carried out by a TA Instrument (DSC-Q200), according to the literature.29,30 The detailed procedures are shown as follows: the samples were kept at 140 °C for 5 min to eliminate the thermal prehistory, and then they were cooled at a rate of 10 °C min−1 to 40 °C to create a “standard thermal history”. After that, the samples were heated again at the same rate up to self-nucleation temperature (Ts, usually in the melting range) and kept at that temperature for 5 min to allow the samples to partially melt. At last, the samples were cooled at 10 °C min−1 from Ts down to 40 °C. During that final cooling step, the fraction of the samples initially molten at Ts will crystallize by using the unmelted crystal fragments as the self-nuclei.
The non-isothermal crystallization processes were examined by DSC. The samples were first heated to 140 °C and held for 5 min to eliminate the thermal history, and then cooled to 40 °C at different cooling rates of 20 °C min−1, 10 °C min−1, and 5 °C min−1.
The isothermal crystallization kinetic of samples was also studied by DSC. The treatment on the sample was as following: the thermal history was removed by heating the sample up to 140 °C and held for 5 min, and then it was quickly cooled to the predetermined crystallization temperature (94 °C), where it was left to crystallize until saturation.
Polarized optical microscope (NIKON ECLIPSELV100POL) equipped with a temperature controller (HSC621V) was used to observe the crystallization morphology of samples. The samples were first melted at 140 °C for 3 min to diminish any thermal history, and then quickly cooled to 94 °C to investigate their crystalline morphology.
WAXD measurements were carried out at room temperature on the Philips X'Pert X-ray diffractometer using the Cu-Kα radiation with a scan rate of 2° min−1 scanning from 5° to 40°.
Dumbbell-shaped samples with the thickness and width of 0.5 mm and 4 mm were prepared by hot pressing. Tensile testing for samples was measured on a Sansi Universal Testing Machine (CMT, Shenzhen, China) at room temperature at a stretching rate of 50 mm min−1. The average values of each sample were obtained from at least five measurements.
The dynamic rheological behaviour of samples was measured on a TADHR-2 rheometer equipped with a 25 mm parallel plate. The measurements were carried out at 130 °C, and dynamic oscillation frequency sweeps from 0.01 to 100 Hz, strain = 1%.
The capillary measurement was conducted by a RosandRH7D capillary rheometer. The diameter of the circular die was 1 mm and L/D was 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, with a 90° entrance angle. The measurements were performed at 130 °C and 140 °C. After being added into the barrel, the PBS pellets were compacted and preheated for 1 min.
1, with a 90° entrance angle. The measurements were performed at 130 °C and 140 °C. After being added into the barrel, the PBS pellets were compacted and preheated for 1 min.
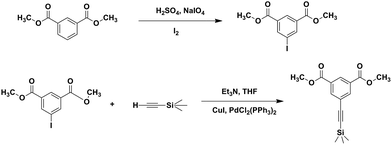
Synthesis of dimethyl 5-iodoisophthalate
Dimethyl 5-iodoisophthalate was synthesized according to the literature,31 and the detailed processes are shown as follows: 250 mL 96% sulfuric acid, iodine (46.66 g, 184 mmol) and sodium periodate (13.54 g, 63 mmol) were placed in a 500 mL round-bottom flask. After stirring 30 min at room temperature, the reaction mixture became black. After that, dimethyl isophthalate (55.70 g, 287 mmol) was added, and the reaction mixture was stirred at room temperature overnight. Finally, the reaction mixture was poured into crushed ice and the precipitates were obtained. The resulting precipitation was dissolved in 1 L methylene chloride, and washed with sodium bicarbonate. After neutralization was complete, sodium sulfite was added until the purple color of the organic layer disappeared. The organic layer was collected by rotary evaporation, and the white needle-like products with yields of 76% were obtained by recrystallized with methanol.1H-NMR (400 MHz, CDCl3) δ (ppm): 8.64 (t, J = 1.5 Hz, 1H), 8.55 (d, J = 1.5 Hz, 2H), 3.95 (s, 6H). 13C-NMR (101 MHz, CDCl3) δ (ppm): 164.7, 142.4, 132.1, 129.8, 93.5, 52.7.
Synthesis of dimethyl 5-(2-(trimethylsilyl)ethynyl)isophthalate (DTS)32
Scheme 1 presents the synthesis route for DTS, and the detailed procedures are shown here: dimethyl 5-iodoisophthalate (24.00 g, 75 mmol), CuI (0.45 g, 2.36 mmol), and Pd(PPh3)2Cl2 (0.53 g, 0.76 mmol) were added into a 500 mL round-bottom flask, and then the system was evacuated and purged with N2 for three times. After that, dry THF (50 mL), triethylamine (150 mL) and ethynyltrimethylsilane (7.37 g, 75 mmol) were added. The resulting solution was stirred under nitrogen at room temperature for 16 h. The mixture was then filtered, and the solution was dried by rotary evaporation. The obtained residue was recrystallized with ethanol. The yellow solid (16.55 g, 57 mmol) with yield of 76% was finally got.1H-NMR (CDCl3, 400 MHz) δ (ppm): 8.61 (t, J = 1.6 Hz, 1H), 8.30 (d, J = 1.6 Hz, 2H), 3.95 (s, 6H), 0.29 (s, 9H). 13C-NMR (101 MHz, CDCl3) δ (ppm): 165.7, 137.1, 131.0, 130.5, 124.4, 102.9, 96.9, 52.8, 0.24.
Synthesis of PBS copolyesters containing DTS (PBDTSx)
The copolyesters (PBDTSx) contain DTS were synthesized by a two-step melt polymerization (esterification and polycondensation), and their chemical structures are shown in Scheme 2. In the abbreviations, x refers to the molar percentage (mol%) of DTS units relative to the total diesters. In the first esterification step, diesters (the compositions of DSA and DTS mixtures are shown in Table 1) and BDO with the molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.06, as well as the transesterification catalyst Zn(OAc)2 (0.2 wt%, based on the total reactants) were added into a three-necked round-bottom flask equipped with a mechanical stirrer, water separator, and nitrogen inlet pipe. The reactor was heated to 175 °C and maintained for 4 h, and then the catalyst TBT (0.1 wt%, based on the total reactants) was added to the reaction system. The polycondensation was carried out at 230 °C for 4 h under vacuum of 20–70 Pa. The obtained products were purified by dissolving in chloroform and then precipitating in an excess methanol in order to remove oligomers and unreacted monomers. Finally, the filtered powder products were washed with methanol and dried under vacuum at 60 °C for 48 h.
1.06, as well as the transesterification catalyst Zn(OAc)2 (0.2 wt%, based on the total reactants) were added into a three-necked round-bottom flask equipped with a mechanical stirrer, water separator, and nitrogen inlet pipe. The reactor was heated to 175 °C and maintained for 4 h, and then the catalyst TBT (0.1 wt%, based on the total reactants) was added to the reaction system. The polycondensation was carried out at 230 °C for 4 h under vacuum of 20–70 Pa. The obtained products were purified by dissolving in chloroform and then precipitating in an excess methanol in order to remove oligomers and unreacted monomers. Finally, the filtered powder products were washed with methanol and dried under vacuum at 60 °C for 48 h.
| Sample | fa (mol%) | De (%) | Df (%) | (%) | (%) | Molecular weight | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| fDTS | fDSA | [η]b (dL g−1) | Mnc (g mol−1) | Mwc (g mol−1) | PDIc | |||||
| a Molar fraction of diesters in the feed.b Intrinsic viscosity measured in chloroform at 30 °C.c Obtained by GPC.d Not determined because of gel formed in chloroform. | ||||||||||
| PBS | 0 | 100 | 0 | 0 | 0 | 0 | 0.81 | 7.40 × 104 | 16.25 × 104 | 2.2 |
| PBDTS0.6 | 0.6 | 99.4 | 52.4 | 53.1 | 52.8 | 31.7 | 0.78 | 6.76 × 104 | 23.88 × 104 | 3.5 |
| PBDTS0.8 | 0.8 | 99.2 | 64.2 | 66.3 | 65.3 | 52.2 | 0.86 | 5.76 × 104 | 26.72 × 104 | 4.7 |
| PBDTS1.0 | 1.0 | 99.0 | 59.7 | 57.4 | 58.6 | 58.6 | 0.99 | 9.13 × 104 | 36.40 × 104 | 4.0 |
| PBDTS1.5 | 1.5 | 98.5 | d | d | d | d | d | d | d | d |
Results and discussion
Synthesis and chemical structure characterization
The monomer DTS can be successfully introduced into PBS main chain via melt polymerization. All the copolyesters show high molecular weight, whose intrinsic viscosities are in the range of 0.78–0.99 dL g−1 (Table 1). Except PBDTS1.5, PBDTSx have good solubility in chloroform, and their molecular weights can be determined by GPC, and the data are also shown in Table 1. From table we can see that the Mw values of PBDTSx are greater than that of PBS, which all are higher than 23 × 104 g mol−1. Their Mw values are enhanced with the increase of DTS content, indicating that introducing DTS favours improving the molecular weights of PBS. PBDTS1.5 does not dissolve well in chloroform, and some gels are obtained, which makes its molecular weight unable to be determined. Besides, their PDI values are also increased when DTS is introduced. It has been demonstrated that the higher molecular weights and PDI values will benefit enhancing the melt viscosity of polymer, indicating that PBDTSx has better processability, which will be discussed in the later part of this work.Fig. 1(a) shows 1H-NMR spectra of neat PBS and PBDTSx in the range of 0–6 ppm. From the figure, we can see that there is a little difference between them. For pure PBS, the peak appearing at 2.63 ppm (Ha) belongs to the methine proton of DSA, and the chemical shifts at 4.12 ppm (Hb) and 1.71 ppm (Hc) assign to the methine protons of BDO. For PBDTSx, besides the above peaks, some new peaks can be observed. The peak appearing at 0.28 ppm (Hd) is ascribed to the Si–CH3 of DTS. The chemical shifts for phenyl proton of DTS are found at 8.30 ppm (He), and 8.60 ppm (Hf) (Fig. 1(b)) after magnification. It is found that the peaks of He and Hf are splitted into two parts due to the different chemical environments.
It was found that DTS monomer can cross-link around at 225–255 °C (Fig. S1†), so these splits are caused by the cross-linking reaction of alkynyl group in DTS. It is the cross-linking reaction occurring during the preparation that makes PBDTSx show high PDI values. Based on the integral peak area of splitting peak, we can calculate the cross-linking degree of the comonomer DTS in the copolyesters according to the following eqn (1):
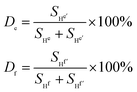 | (1) |
De or Df refers to the cross-linking degree of the comonomer DTS, SHf or SHe is the integral peak area of Hf or He (the uncross-linked part of DTS), and SHf′ or SHe′ is the integral peak area of Hf′ or He′ (the cross-linked part of DTS). Based on the results of De, and Df, we can get 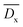 (eqn (2), the average values of De and Df), which refers to the average cross-linking degree of the comonomer DTS.
(eqn (2), the average values of De and Df), which refers to the average cross-linking degree of the comonomer DTS.
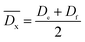 | (2) |
Besides, the relative average cross-linking degree 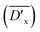 of DTS in the copolyesters can be obtained by eqn (3).
of DTS in the copolyesters can be obtained by eqn (3).
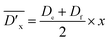 | (3) |
 , and
, and 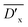 are listed in Table 1.
are listed in Table 1.
From the table we can see that the 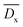 shows the highest value when 0.8 mol% DTS is introduced into PBDTSx. This means that DTS comonomer in PBDTS0.8 has the highest cross-linking degree, which made it have the highest PDI value. In view of DTS content in PBS, the relative average cross-linking degree
shows the highest value when 0.8 mol% DTS is introduced into PBDTSx. This means that DTS comonomer in PBDTS0.8 has the highest cross-linking degree, which made it have the highest PDI value. In view of DTS content in PBS, the relative average cross-linking degree 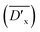 for copolyesters can be obtained, in which PBDTS1.0 shows the highest value. Because
for copolyesters can be obtained, in which PBDTS1.0 shows the highest value. Because 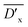 can reflect the cross-linking density of the copolyesters, PBDTS1.0 has the highest cross-linking density than the others.
can reflect the cross-linking density of the copolyesters, PBDTS1.0 has the highest cross-linking density than the others.
Thermal stability of PBDTSx copolyesters
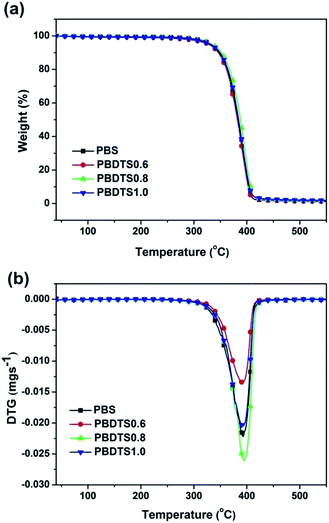
Fig. 2 shows the thermal stability of neat PBS and PBDTSx measured by TGA. All the TG curves almost overlap together, illustrating the thermal stability of PBDTSx is as same as PBS. The thermal decomposition temperatures such as the temperature at 5% weight loss (T5%), the temperature at the maximum decomposition rate (Tmax), as well as the residue weight at 550 °C are listed in Table 2. And all the data are almost as same as pure PBS, demonstrating that the introduction of a little DTS does not influence the thermal stability of PBS.| Sample | T5% (°C) | Tmax (°C) | Residue at 550 °C (%) |
|---|---|---|---|
| PBS | 332 | 393 | 1.5 |
| PBDTS0.6 | 328 | 392 | 1.1 |
| PBDTS0.8 | 334 | 395 | 1.6 |
| PBDTS1.0 | 332 | 392 | 2.0 |
Crystallization and thermal transition behaviours of PBDTSx
The thermal transition behaviour is very important for a certain polymer, which can influence its properties and determine the fields where the polymer can be used in. DSC was used to evaluate the thermal transition behaviours of PBDTSx. The heating and cooling curves are shown in Fig. 3, while the detailed thermal transition data are summarized in Table 3.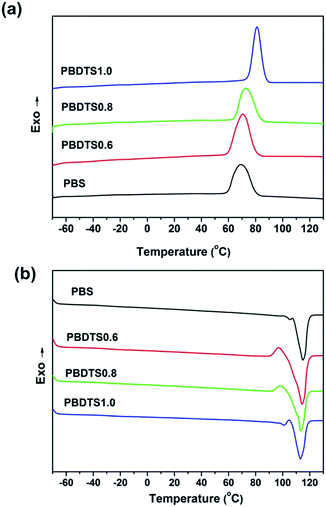 | ||
| Fig. 3 DSC curves of (a) second heating run and (b) cooling run of neat PBS and PBDTSx at a rate of 10 °C min−1. | ||
| Sample | Cooling | Second heating | NE | n | t1/2 (min) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Tc (°C) | ΔHc (J g−1) | Tg (°C) | Tm (°C) | ΔHm (J g−1) | Xca (%) | ||||
| a The degree of crystallinity (Xc) was calculated according to Xc = ΔHm/ΔH0m, where ΔH0m of perfectly (100%) crystalline PBS is 102 J g−1.35 | |||||||||
| PBS | 71.1 | 62.9 | −40.1 | 114.0 | 78.9 | 77.3 | 0 | 2.6 | 14.7 |
| PBDTS0.6 | 73.8 | 68.9 | −39.2 | 114.2 | 84.7 | 83.0 | 5.6 | 1.7 | 7.3 |
| PBDTS0.8 | 74.9 | 65.3 | −39.1 | 113.5 | 75.9 | 74.4 | 8.0 | 2.3 | 7.8 |
| PBDTS1.0 | 81.3 | 67.5 | −39.4 | 113.1 | 57.7 | 56.6 | 21.2 | 1.7 | 2.4 |
Due to the high crystallinity (Xc, discussed in later part), their glass transition temperatures (Tg) can be not see clearly from its DSC curves. So, the magnified DSC curves are provided as Fig. S2,† and from this figure, we can see that their Tg are almost as same as that of pure PBS. Besides, the melting temperatures (Tm) of PBDTSx are almost unchanged compared to pure PBS (Table 3). This means that although the introduction of DTS reduces the segment regularity of PBS chain, the content is so low to generate great effect on its chain mobility. Interestingly, the crystallization temperatures (Tc) of PBDTSx are higher than that of neat PBS. Especially for PBDTS1.0, its Tc is 10 degrees higher than that of PBS. The dependence of Tc on cooling rate was investigated by DSC. Fig. 4(a) shows the plots of non-isothermal crystallization temperature versus cooling rate of PBS and PBDTSx. From figure we can see that both the Tc of PBDTSx and PBS decrease with the cooling rate quickened up, and PBDTSx always have higher Tc than the neat PBS at the same cooling rate. It is widely known that the crystallization process of the melting polymer can be divided into two steps, nucleation and crystal growth. Though the cross-linking may prevent segment motion to crystal growth, the cross-link points can be regarded as crystal nucleus in the nucleation step. Therefore, it facilitates PBDTSx crystallization, and makes Tc increase.
 | ||
| Fig. 4 (a) Non-isothermal crystallization temperature versus cooling rate of neat PBS and PBDTSx, and (b) the neat PBS after partial or total melting at various temperatures. | ||
Based on the value of ΔHm, the crystallinity (Xc) is calculated and the detailed data are shown in Table 3. It can be seen that Xc is first increased and then decreased with the increase of DTS content. This states that although the cross-link points can be regarded as crystal nucleus favouring crystallization, with the increase of cross-linking degree, the segment integrity and mobility is reduced, which makes the Xc decreased.
To estimate the nucleating efficiency (NE) of PBDTSx, the following method put forward by Fillon et al. is used.29,30
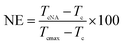 | (4) |
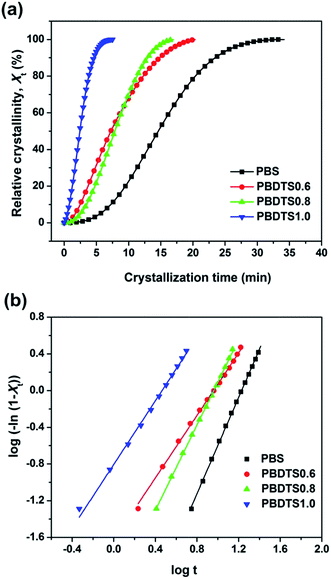
Fig. 4(b) shows the DSC cooling curves of neat PBS after partial or total melting at various temperatures. It is found that the crystallization peak shifts to high temperature as Ts decreases. When the temperatures decrease to 119 °C, the crystallization temperatures no longer move, thus, the optimum self-nucleation temperature Tcmax is determined as 119 °C. The detailed NE values of PBDTSx are also given in Table 3. With the increase of DTS content, the NE value rises from 5.6 of PBDTS0.6 to 21.2 of PBDTS1.0. Based on our previous NMR results, it is found via introducing alkynyl group into PBS main chain, some cross-linking reaction occurs. The cross-linking density 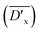 is enhanced with the increase of DTS content. NE value variation trend demonstrates that higher cross-linking density is beneficial to high nucleating efficiency, which will lead to a higher crystallization temperature and fast crystallization. To make clear how slightly cross-linking affects the crystallization process of PBDTSx, isothermal crystallization processes were investigated by DSC. The relative crystallinity vs. crystallization time plots are depicted in Fig. 5(a). From the figure, it can be seen clearly that the enhancing crystallization effect of DTS is pronounced. All the copolyesters complete 100% crystallization in a shorter time than PBS. Avrami equation was employed to analyze the isothermal crystallization kinetics:
is enhanced with the increase of DTS content. NE value variation trend demonstrates that higher cross-linking density is beneficial to high nucleating efficiency, which will lead to a higher crystallization temperature and fast crystallization. To make clear how slightly cross-linking affects the crystallization process of PBDTSx, isothermal crystallization processes were investigated by DSC. The relative crystallinity vs. crystallization time plots are depicted in Fig. 5(a). From the figure, it can be seen clearly that the enhancing crystallization effect of DTS is pronounced. All the copolyesters complete 100% crystallization in a shorter time than PBS. Avrami equation was employed to analyze the isothermal crystallization kinetics:
| 1 − Xt = exp(−ktn) | (5) |
log[−ln(1 − Xt)] = log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) k + n k + n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) t t
| (6) |
 | ||
| Fig. 5 (a) Relative crystallinity versus time plot, and (b) Avrami plots of PBS and PBDTSx at crystallization temperature of 94 °C. | ||
A plot of log[−ln(1 − Xt)] versus log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) t (Fig. 5(b)) gives a straight line from which both the Avrami exponent and the rate constant can be determined. By calculation, the n values of PBS and PBDTSx are 2.6, 1.7, 2.3, and 1.7 (shown in Table 3), suggesting that the crystallization of the neat PBS corresponds to a three dimensional growth with heterogeneous nucleation, while the PBDTSx corresponds to a two dimensional growth with heterogeneous nucleation.34 It may owe to the decreased segment integrity and mobility caused by the cross-linking, and the segments tend to be gathered on a two dimensional axial axe.
t (Fig. 5(b)) gives a straight line from which both the Avrami exponent and the rate constant can be determined. By calculation, the n values of PBS and PBDTSx are 2.6, 1.7, 2.3, and 1.7 (shown in Table 3), suggesting that the crystallization of the neat PBS corresponds to a three dimensional growth with heterogeneous nucleation, while the PBDTSx corresponds to a two dimensional growth with heterogeneous nucleation.34 It may owe to the decreased segment integrity and mobility caused by the cross-linking, and the segments tend to be gathered on a two dimensional axial axe.
Half-time of crystallization (t1/2) is another important parameter, which means the time needed to achieve 50% of the final crystallinity. The t1/2 values of PBS and PBDTSx are 14.7, 7.3, 7.8, and 2.4 min (shown in Table 3), respectively, illustrating PBDTSx crystallizes faster than PBS, especially for PBDTS1.0. This demonstrates again via promoting nucleation the slightly cross-linking can accelerate PBS crystallization greatly.
Meanwhile, POM was used to investigate the crystalline morphology of PBS and PBDTSx at 94 °C (Fig. 6). For PBDTS1.0, only after 90 s the small crystals are full of the view. For other PBDTSx their crystallizations are also speeded up compared to PBS, which is accord to the isothermal crystallization result. People can see completely spherical morphology from the POM image of pure PBS, however, the crystals for PBDTSx are tiny and imperfect, especially for PBDTS1.0.
Fig. 7 exhibits the WAXD patterns of neat PBS and PBDTSx. The crystal unit cell of PBS is monoclinic and it shows three strong characteristic diffraction peaks at around 19.6°, 21.7°, and 22.5°, which are ascribed to (020), (021) and (110) planes.11 For PBDTSx, the WAXD patterns share similar diffraction peak positions with the neat PBS, suggesting that they remain the same crystal structure. This indicates that although cross-linking enhanced the crystallization rate, it has no influence on crystallization structure of PBS.
Tensile properties of PBDTSx
The mechanical properties play an important role in the application of materials, and the tensile properties of the copolyesters are provided in Table 4, while the stress–strain curves of neat PBS and PBDTSx are given in Fig. S3.† The tensile strength of PBDTSx is close to neat PBS while the elongation at break decreases a little and the Young's modulus increases with the increase of DTS content. For PBDTS1.0, its Young's modulus is raised from 286 of PBS to 337 MPa, illustrating the rigidity of PBS is improved. This is ascribed to the networks formed between polymer chains, which are caused by cross-linking. And these networks reduce the segment mobility, so the elongation at break decreases and the rigidity increases. The networks are not strong enough when the molar percentage of DTS units is less than 1.0%, therefore, the tensile strengths of PBDTSx are close to neat PBS.| Sample | Tensile strength (MPa) | Elongation at break (%) | Young's modulus (MPa) |
|---|---|---|---|
| PBS | 33.7 ± 0.9 | 331 ± 16 | 286 ± 20 |
| PBDTS0.6 | 30.5 ± 1.0 | 221 ± 17 | 310 ± 25 |
| PBDTS0.8 | 32.0 ± 0.4 | 221 ± 24 | 333 ± 15 |
| PBDTS1.0 | 32.7 ± 2.1 | 263 ± 25 | 337 ± 10 |
Rheological behaviour of PBDTSx
The dynamic rheological behaviours of the neat PBS and slightly cross-linked PBDTSx were studied comparably, and their complex viscosity–frequency, storage modulus G′-frequency, loss modulus G′′-frequency and storage modulus G′-loss modulus G′′ curves are depicted in Fig. 8 and 9. The neat PBS acts as a Newtonian behaviour, and presents low complex viscosity. With the introduction of DTS, the complex viscosity increases significantly, which owed to the formation of network and an increase in molecular weight. The increase extent of complex viscosity has great relationship with the cross-linking degree of PBDTSx, and PBDTS1.0 always shows higher viscosity than the others.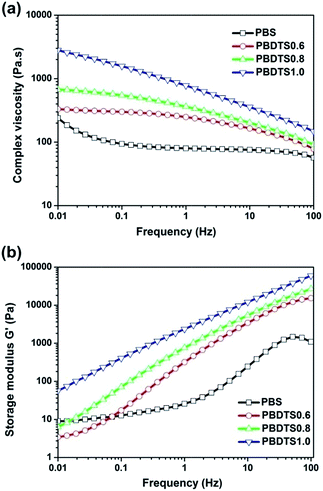 | ||
| Fig. 8 Dynamic rheological (a) complex viscosities vs. frequency and (b) storage modulus G′ vs. frequency for neat PBS and PBDTSx. | ||
 | ||
| Fig. 9 Dynamic rheological (a) loss modulus G′′ vs. frequency and (b) storage modulus G′ vs. loss modulus G′′ for neat PBS and PBDTSx. | ||
As we know, the storage modulus G′ is connected with the elasticity of microstructure, while the loss modulus G′′ is related to the molecular mobility and molecular interactions. From Fig. 8(b) and 9(a), it is clear that both G′ and G′′ increases with the increase of frequency, in which PBDTSx shows higher G′ and G′′ than the neat PBS.
This phenomenon means the PBDTSx have improved elastic response, molecular interactions and reduced chain mobility, which is due to the formation of cross-linking network caused by introducing DTS into its polymer chains.
The slopes of the G′ vs. G′′ curves can be regarded as an evidence of change in polymer microstructure.36–38 From Fig. 9(b), we can obviously see that the curves of PBDTSx are almost superimposed and deviated with that of neat PBS, suggesting that some microstructure change occurred in PBDTSx. This demonstrates again that with the introduction of DTS content, a small amount of cross-linking network are formed at polycondensation step.
The capillary rheological behaviours of the neat PBS and PBDTS1.0 are shown in Fig. 10. The capillary rheological behaviours are similar to the dynamic rheological behaviours, i.e. the shear viscosity of PBDTS1.0 is always higher than PBS at the whole shear region. The shear viscosity of PBDTS1.0 is enhanced from 200 Pa s of PBS to 1300 Pa s at a shear rate of 50 s−1 when the testing temperature is fixed at 130 °C. Further enhancing the shear rate to 3000 s−1, the shear viscosity of PBDTS1.0 and PBS are around 150 Pa s and 50 Pa s, respectively. Even the testing temperature is increased to 140 °C, PBDTS1.0 still remains higher shear viscosity than PBS. The high melt viscosity will benefit the blow moulding processing.
 | ||
| Fig. 10 Capillary rheological shear viscosities vs. shear rate (a) at 130 °C and (b) at 140 °C for neat PBS and PBDTS1.0. | ||
Conclusions
As we expected, the functional monomer containing alkynyl group can cross-link during the preparation of PBS, so a series of PBS copolyesters (PBDTSx) having slightly cross-linking are prepared successfully. PBDTSx show good solubility in chloroform and exhibit increased molecular weights when the molar percentage of DTS units is less than 1.0%. Based on NMR spectra, it is found that PBDTS1.0 has the highest cross-linking density than the others. PBDTSx still remain the similar thermal stability with the neat PBS. The cross-linking points in the PBS chains act as crystal nucleus in the nucleation step, so PBDTSx copolyesters have higher Tc and fast crystallization rates, especially for PBDTS1.0 with high relative cross-linking density. The elongation at break of PBDTSx decreases a little and the Young's modulus improves compared to pure PBS. With the introduction of DTS, the complex viscosity of PBDTSx increases significantly, and the elastic response is also enhanced. The capillary rheological behaviours of PBDTSx illustrate that the shear viscosity of PBDTS1.0 is always higher than PBS at the whole shear region and the testing temperatures. In this work, we provide a new feasible method to improve the processability and properties of PBS by adjusting its chemical chain structures during the preparation instead of post cross-linking modification.Conflict of interest
The authors declare no competing financial interest.Acknowledgements
This work was supported financially by the National Science Foundation of China (51273123 and 51121001), and the Program for Changjiang Scholars and Innovative Research Teams in University of China (IRT 1026).References
- J. Xu and B. Guo, Biotechnol. J., 2010, 5, 1149–1163 CrossRef CAS PubMed.
- J. B. Zeng, Y. D. Li, W. D. Li, Q. Y. Zhu, Z. Xiong, K. K. Yang and Y. Z. Wang, Acta Polym. Sin., 2009, 10, 1018–1024 CrossRef.
- Y. Han, S. Kim and J. Kim, Macromol. Res., 2002, 10(2), 108–114 CrossRef CAS.
- D. F. Coutinho, I. H. Pashkuleva and C. M. Alves, Biomacromolecules, 2008, 9, 1139–1145 CrossRef CAS PubMed.
- T. Ikehara, H. Kimura and Z. Qiu, Macromolecules, 2005, 38(12), 5104–5108 CrossRef CAS.
- J. B. Zeng, C. Liu, F. Y. Liu, Y. D. Li and Y. Z. Wang, Ind. Eng. Chem. Res., 2010, 49(20), 9870–9876 CrossRef CAS.
- Y. He, B. Zhu, W. H. Kai and Y. Inoue, Macromolecules, 2004, 37(21), 8050–8056 CrossRef CAS.
- Y. Tsai, L. Jheng and C. Hung, Polym. Degrad. Stab., 2010, 95, 72–78 CrossRef CAS.
- M. Nagata, H. Goto, W. Sakai and N. Tsutsumi, Polymer, 2000, 41(11), 4373–4376 CrossRef CAS.
- H. B. Chen, X. L. Wang, J. B. Zeng, L. L. Li, F. X. Dong and Y. Z. Wang, Ind. Eng. Chem. Res., 2011, 50, 2065–2072 CrossRef CAS.
- R. T. Duan, Q. X. He, X. Dong, D. F. Li, X. L. Wang and Y. Z. Wang, ACS Sustainable Chem. Eng., 2016, 4, 350–362 CrossRef CAS.
- D. The, F. Yoshii and N. Nagasawa, J. Appl. Polym. Sci., 2004, 91(4), 2122–2127 CrossRef CAS.
- L. Bahari, H. Mitomo and T. Enjoji, Polym. Degrad. Stab., 1998, 62(3), 551–557 CrossRef.
- X. Huang, C. Li, W. Zhu, D. Zhang, G. Guan and Y. Xiao, Polym. Adv. Technol., 2011, 22, 648–656 CrossRef CAS.
- M. Suhartini, H. Mitomo, N. Nagasawa, F. Yoshii and T. Kume, J. Appl. Polym. Sci., 2003, 88, 2238–2246 CrossRef CAS.
- D. Kim, W. Kim and D. Lee, J. Appl. Polym. Sci., 2001, 81(5), 1115–1124 CrossRef CAS.
- P. Ma, Z. Ma, W. Dong, Y. Zhang and P. J. Lemstra, Macromol. Mater. Eng., 2013, 298, 910–918 CrossRef CAS.
- N. Teramoto, M. Ozeki, I. Fujiwara and M. Shibata, J. Appl. Polym. Sci., 2005, 95, 1473–1480 CrossRef CAS.
- M. Kolel-Veetil, H. Beckham and T. Keller, Chem. Mater., 2004, 16(16), 3162–3167 CrossRef CAS.
- M. Yan, Y. Tan, Z. Zhang, J. Hu and Z. Xie, Eur. Polym. J., 2006, 42, 3068–3077 CrossRef CAS.
- J. Hu, Z. Zheng and T. Ma, J. Polym. Sci., Part A: Polym. Chem., 2004, 42(12), 2897–2903 CrossRef CAS.
- P. M. Hergenrother, R. G. Bryant, B. J. Jensen and S. J. Havens, J. Polym. Sci., Part A: Polym. Chem., 1994, 32, 3061–3067 CrossRef CAS.
- J. A. Johnston, F. M. Li, F. W. Harris and T. Takekoshi, Polymer, 1994, 35, 4865–4873 CrossRef CAS.
- J. G. Smith, J. W. Connell and P. M. Hergenrother, J. Compos. Mater., 2002, 36(19), 2255–2265 CrossRef CAS.
- P. M. Hergenrother, Macromolecules, 1981, 14(4), 898–904 CrossRef CAS.
- P. M. Hergenrother, Macromolecules, 1981, 14(4), 891–897 CrossRef CAS.
- H. B. Zhao, L. Chen, J. C. Yang, X. G. Ge and Y. Z. Wang, J. Mater. Chem., 2012, 22, 19849 RSC.
- H. B. Zhao, X. L. Wang, Y. Guan, X. L. Wang, L. Chen and Y. Z. Wang, Polymer, 2015, 70, 68–76 CrossRef CAS.
- B. Fillon, B. Lotz, A. Thierry and J. C. Wittmann, J. Polym. Sci., Part B: Polym. Phys., 1993, 31, 1395–1405 CrossRef CAS.
- B. Fillon, J. C. Wittmann, B. Lotz and A. Thierry, J. Polym. Sci., Part B: Polym. Phys., 1993, 31, 1383–1393 CrossRef CAS.
- G. E. Alliger, P. Muller, C. C. Cummins and D. G. Nocera, Inorg. Chem., 2010, 49, 3697–3699 CrossRef CAS PubMed.
- C. Song, Y. Ling, Y. Feng, W. Zhou, T. Yildirim and Y. He, Chem. Commun., 2015, 51, 8508–8511 RSC.
- M. A. Sabino, G. Ronca and A. J. Müller, J. Mater. Sci., 2000, 35, 5071–5084 CrossRef CAS.
- X. L. Wang, K. K. Yang, Y. Z. Wang, D. Y. Wang and Z. Yang, Acta Mater., 2004, 52, 4899–4905 CrossRef CAS.
- C. L. Huang, L. Jiao, J. B. Zeng, M. Zhang, L. P. Xiao, K. K. Yang and Y. Z. Wang, Polymer, 2012, 53, 3780–3790 CrossRef CAS.
- M. H. Al-Saleh and U. Sundararaj, Polymer, 2010, 51, 2740–2747 CrossRef CAS.
- S. J. Chin, S. Vempati, P. Dawson, M. Knite, A. Linarts, K. Ozols and T. McNally, Polymer, 2015, 58, 209–221 CrossRef CAS.
- R. T. Zeng, W. Hu, M. Wang, S. D. Zhang and J. B. Zeng, Polym. Test., 2016, 50, 182–190 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: DSC curves of first heating scan and the second heating scan for monomer DTS. See DOI: 10.1039/c6ra13488b |
| This journal is © The Royal Society of Chemistry 2016 |