Inhibitory effect of MnCr2O4 spinel coating on coke formation during light naphtha thermal cracking
Abstract
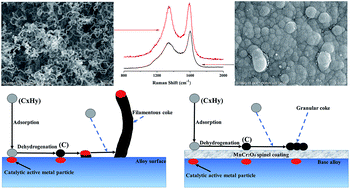
In this paper, MnCr2O4 spinel coating was fabricated on a HP40 alloy by pack cementation and subsequent thermal oxidation. The spinel coating was dense and uniform, and was applied to inhibit coke formation during light naphtha thermal cracking. Coking behaviors on the HP40 alloy and spinel coated alloy were compared under the same conditions. The anti-coking rate of the MnCr2O4 spinel coating exceeded 60% during light naphtha thermal cracking. Lots of filamentous coke formed on the uncoated HP40 alloy, but catalytic coke was completely inhibited on the MnCr2O4 spinel coated alloy resulting in a granular appearance of coke. Raman spectra analyses indicated that coke deposited on the surface of the spinel coating had a larger proportion of disordered carbon and amorphous carbon compared with that on the uncoated HP40 alloy.

 Please wait while we load your content...
Please wait while we load your content...