Preparation of amino-functionalized magnetic nanoparticles for enhancement of bacterial capture efficiency†
Abstract
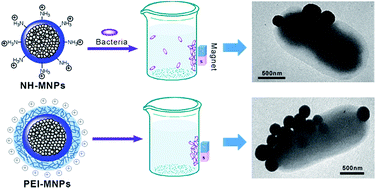
Pathogenic bacteria can lead to food poisoning and serious infectious diseases, and place a large burden on human health. A method for rapid capture and removal of the harmful bacteria from the contaminated source is essential and important. In this study, two types of amine group functionalized magnetic nanoparticles were successfully fabricated for effective capture of bacterial pathogens. These magnetic nanoparticles with a positive-charge surface strongly interacted with the negative-charge bacterial cell wall via electrostatic attractions to exhibit efficient adsorptive ability of a broad spectrum of bacterial pathogens. Owing to more cationic amine groups, the bacterial capture capacity of Fe3O4@sSiO2-PEI nanoparticles (PEI-MNPs) for E. coli BL21 was three times that of Fe3O4@sSiO2-NH2 nanoparticles (NH-MNPs), making them promising for practical applications. More importantly, the PEI-MNPs also showed excellent bacterial capture efficiency at a low concentration due to the multivalency effect that appeared in our system.

 Please wait while we load your content...
Please wait while we load your content...