Theoretical insights into the alkaline metal M (M = Na and Cs) promotion mechanism for CO2 activation on the Cu(111) surface†
Lihui Ou*,
Yuandao Chen and
Junling Jin
College of Chemistry and Chemical Engineering, Hunan University of Arts and Science, Changde 415000, China. E-mail: oulihui666@126.com; Tel: +86-736-7186115
First published on 1st July 2016
Abstract
A systematic study on the alkaline metal M (M = Na and Cs) promotion mechanism for CO2 activation on the Cu(111) surface is presented for the first time based on self-consistent density functional theory calculations. The results show that CO2 adsorbs weakly and molecularly on a clean Cu(111) surface, whereas a drastic influence on the bonding, structure, and reactivity of adsorbed CO2 through the formation of CO2δ− radical anions was exerted by the presence of alkaline metals Na and Cs adatoms on the Cu(111) surface. This is the main physical origin of alkaline metals Na and Cs promotion in CO2 activation. The Na and Cs adatoms lower the dissociation activation barrier of CO2, in which the effect of Cs on CO2 dissociation is significantly larger than that of Na. Thus, the promotion effect of alkaline metals for the CO2 reduction into hydrocarbons on Cu catalysts can be attributed to a reduction of the dissociation activation barrier of CO2. The results also show that the origin of this promotion effect is predominately a direct electronic interaction between the Na and Cs-promoted Cu(111) surface and CO2 molecules. The presence of Na and Cs on the Cu(111) resulted in a decrease in the work function of the surface. The reduced amount of the work function on the Cs-promoted Cu(111) surface is more than that on the Na-promoted Cu(111) surface, explaining why the dissociation activation barrier of CO2 is lower on the Cs-promoted Cu(111) surface. The strong work function decrease of Na and Cs-promoted Cu(111) surface is corroborated by the presence of charge transfer. The charge transfer leads to the formation of a partially negative species, CO2δ−, which can be ascribed to the enhancement of back-donation of electrons from Na and Cs-promoted Cu(111) surface into an empty π orbital of CO2 compared to that on the clean Cu(111) surface. However, the back-donation mechanism of electrons is different between the Na and Cs-promoted Cu(111) surfaces, in which Na is an effective electron donor, whereas Cs is an electron acceptor, thus leading to the difference between promoting mechanisms of alkaline metals Na and Cs on CO2 activation. Cu as an electron donor in the Cs-promoted Cu(111) surface may result in a more reduced amount of the work function of Cs-promoted Cu(111) surface.
1. Introduction
Activation of the rather inert and stable carbon dioxide (CO2) molecule is deemed to be an interesting field of research on Cu electrodes since CO2 is low-cost raw material and metallic Cu is able to convert CO2 into hydrocarbons such as CH4 and C2H4 with high Faraday efficiency.1–9 Currently, a greatly challenging task is to activate CO2 molecules and to form intermediates suitable for hydrogenation since CO2 adsorbs weakly and nondissociatively on a clean Cu single-crystal electrode.10In the past few decades, alkaline metal promotion effects in electrochemistry and heterogeneous catalysis have been a hot area of research11–19 since alkaline adatoms such as Na, K, and Cs can significantly enhance many important catalytic reaction rates, for example, CO2 electrochemical reduction, ammonia synthesis11,12 and Fischer–Tropsch synthesis, etc.13,14 Currently, on some well-defined single-crystal surfaces, coadsorption of alkaline metals and other species has been studied extensively due to the difficulty in directly observing microscopic processes of catalytic reactions.19–21 For example, an adsorbed N2 molecule on Fe(111) could be stabilized and the dissociation barrier of N2 could be lowered by preadsorbed alkaline metal K.20 On Au(111), adsorption of alkaline metal K could lead to a decrease of the work function of Au(111), enhancement of the binding energy of CO2, and the formation of carbon dioxide anion radical ˙CO2−.21 Simultaneously, the study also showed that the reaction pathway of ˙CO2− depends on the coverage of alkaline metal K, dissociation of ˙CO2− occurs at low K coverage, whereas at high K coverage ˙CO2− is converted into stable carbonate. Some more recent theoretical work exceeds the abovementioned coadsorption studies. Alkaline-promoted N2 dissociation reaction pathways have been reported on Ru(0001) by Nørskov and co-workers and the dissociation barrier of N2 is lowered due to the presence of adsorbed Na and Cs by using density functional theory (DFT).22 In particular, Nørskov and co-workers have suggested a classical electrostatic interaction model, which plays an important role in the alkaline metal promotion effect. Most recently, using DFT, CO dissociation was studied on clean Rh(111) and K-covered Rh(111) surfaces by Liu and co-workers,23 in which the changes of the reaction pathways and reaction barriers induced by alkaline metal K had been determined, in particular, a significant decrease of the activation barrier for CO dissociation had been identified. In their analysis, both electronic and geometrical factors were considered to dominate the alkaline metal promotion effect. During the course of CO2 electrochemical reduction, alkaline metal promotion has also been reported experimentally to greatly enhance the adsorption ability of CO2 on a Cu electrode due to its character of electron-donation, the neutrally adsorbed CO2 with a stable linear structure could be transformed into the more reactive negatively charged CO2 by a promotion effect24 and carbon dioxide anion radical ˙CO2− was detected on alkaline metal modified polycrystalline Cu surfaces,25–29 indicating that the alkaline metal adatoms on Cu promoted formation of the more reactive ˙CO2− with bent form. Thus, it can be speculated that the formation of ˙CO2− may play a major role in the synthesis of hydrocarbons and it is a key step during the course of CO2 activation.30–32 Although the more reactive ˙CO2− has now been undoubtedly better identified, the O–C–O bond angle is still unknown and it is not clear whether the chemisorbed CO2 is bent upwards or downwards, and whether the chemisorbed CO2 is coordinated to a single metal atom as a monodentate or two atoms as a bidentate on alkaline metal promoted Cu(111). So far, no experimental and theoretical studies have been presented about the origin of the alkaline metal promotion effect on the CO2 reduction process on a Cu electrode. Therefore, there are some key questions remaining to be answered in this research field, such as, how are the CO2 activation and the dissociation barrier modified by the alkaline metal? Which factors influence the formation of chemisorbed CO2? What is the origin of the modifications by the alkaline metal? Aiming at answering these questions, we have performed a systematic DFT study on the mechanism of Na- and Cs-promoted CO2 activation and dissociation on the Cu(111) surface in the present paper since on the (111) facet, the formation of hydrocarbon CH4 is favored.
In our present paper, self-consistent DFT calculations are used to investigate the effect of alkaline metal adatoms Na and Cs on the adsorption and dissociation of CO2 molecule on the Cu(111) surface. The results show that adsorbed alkaline metal atoms Na and Cs on the Cu(111) surface facilitate the adsorption of CO2 molecules, and they lower the activation barriers for dissociation of CO2 molecules significantly. The results also show that the effect of alkaline metal Cs is notably larger than that of Na. Based on the present study, we can clearly show that the origin of the promotion effect is predominately a direct electronic interaction between the Na and Cs-promoted Cu(111) surface and CO2 molecules.
2. Computational method and modeling
In the framework of DFT, calculations were performed by using the generalized gradient approximation (GGA) of Perdew–Burke–Ernzerhof (PBE).33 Ultrasoft pseudopotentials were used to described the nuclei and core electrons.34 Using periodic super-cells with the Cu electrodes modeled by large four-layer (111) slabs with a 3 × 3 surface, we performed calculations on the most stable geometry structure of various adsorption and co-adsorption intermediates. A vacuum region of 16 Å was placed above the slabs and only one of the two surfaces exposed is allowed to adsorb. The Kohn–Sham orbitals were expanded in a plane-wave basis set, and the plane-wave cutoff energy was optimized at 26 Ry. The smearing technique of Methfessel and Paxton was used to treat the Fermi-surface effects with a value of smearing parameter35 of 0.02 Ry. For the (3 × 3) supercell, a (3 × 3 × 1) uniformly shifted k-mesh was used to perform the surface Brillouin zone (BZ) integrations. The alkaline metal Na- and Cs-modified Cu(111) surface is modeled by adding adsorbed Na and Cs atoms on Cu(111) (1/9, 2/9, 3/9, 4/9, and 5/9 ML (monolayer)). All calculations were implemented by using the PWSCF codes included in the Quantum ESPRESSO distribution,36 while the XCRYSDEN37–39 graphical package was used to produce figures of the chemical structures. The calculated equilibrium lattice constant of Cu was 3.66 Å, which agreed well with theoretical and experimental values (3.66 and 3.62 Å, respectively).40,41 During the course of calculations, the two atomic layers from the bottom of the Cu(111) slab were fixed at the theoretical bulk positions, whereas the top two layers and the adsorbates were relaxed to their lowest energy configurations. The convergence criteria for the total energy was set to within 10−5 Ry and the ionic relaxation loop for the Cartesian force components acting on each atom was limited to below 10−3 Ry bohr−1.The minimum energy paths (MEPs) for the dissociation reaction of CO2 were determined by using the climbing-image nudged elastic band (CI-NEB) method.42,43 The image of highest energy was used to approximate the transition state of the optimized reaction coordinate. Using the quasi-Newton method, the transition state images from the CI-NEB calculations were optimized, which minimizes the forces to find the saddle point. For each intermediate point in MEPs, geometry optimization was also performed, in which a four-layer Cu(111) slab with a large 3 × 3 surface unit cell and the most stable geometry structure obtained on (3 × 3) Cu(111) were used. Zero point energy corrections were applied to the adsorption energies and activation energy barriers. In order to consider the zero point energy contributions to these energies, the vibrational properties are studied with density functional perturbation theory with the linear response.44 The PHONONS code36 was used in our calculations for the zero point energies. The dynamical matrices are first obtained for each adsorption state. The dynamical matrix is then Fourier-transformed to real space and the force-constant matrices are constructed, which are used to obtain phonon density of states. Finally, zero point energies are evaluated by phonon density of states.
3. Results and discussion
3.1 Adsorption and reactivity of CO2 on alkaline metal M (M = Na and Cs) promoted Cu(111)
To investigate the promotion effect of alkaline metals Na and Cs on CO2 activation, we first examined the adsorption and reactivity of a CO2 molecule on clean and Na and Cs-promoted Cu(111). Cu(111) surfaces with different coverage of Na and Cs were considered for comparison purposes. The direct comparison proved to be very enlightening among these systems. The adsorption energy (Ead) of adsorbate CO2 was calculated according to Ead(CO2) = E[Cu(111)–Mx(x = 0–5)–CO2] − E[Cu(111)–Mx] − E(CO2), where E[Cu(111)–Mx–CO2], E[Cu(111)–Mx] and E(CO2) refer to the total energy of alkaline metal M (M = Na and Cs) promoted Cu(111) with an adsorbed CO2, the total energy of alkaline metal M (M = Na and Cs) promoted Cu(111), and the total energy of free CO2, respectively. The optimized geometry structures of CO2 on clean and alkaline metals Na and Cs-promoted Cu(111) surface are given in Fig. S1 and S2,† respectively. As observed in Tables 1 and 2, the adsorption energy of CO2 over the clean Cu(111) surface was found to be −0.0027 eV. The weak interaction between CO2 and the surface is also indicated by the rather long distance (3.793 Å) between the CO2 and Cu(111) surface in the optimized structure, almost unchanged C–O bond lengths (1.172 Å), and O–C–O angle (179.982°) compared with that in the isolated CO2 molecule, namely, CO2 adsorbed with the molecular axis oriented almost parallel to the clean Cu(111) surface as linear, electronically undistorted molecules (see Fig. S1a†). Thus, the results showed unequivocally that the CO2 adsorbs very weakly on the clean Cu(111) surface, and we can conclude that CO2 may not bind to the clean Cu(111) surface. The adsorption of CO2 on evaporated Cu films at 195 and 273 K was also not found in the early classical experimental work from Collins and Trapnell,45 which was ascribed to the full occupation of d-bands. Using tools of modern surface science, a number of studies confirmed this feature.28,31,46–51 For example, at 110 or 250 K, Rodriguez et al.28 found that no measurable amount of CO2 was able to be reflected on the clean Cu surface at exposure up to 350 L, as confirmed by the complete absence of an oxygen signal in the XPS and AES after exposure. From studies of AES, TPD, UPS and XPS, Solymosi31 also reached the same conclusion for the clean Cu(111) surface.| Eada (eV) | rC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (Å) O (Å) |
rC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (Å) O (Å) |
rC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (Å)/average O (Å)/average |
θO![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (degree) O (degree) |
dCO2-surface (Å) | |
|---|---|---|---|---|---|---|
| a The values in parentheses are the adsorption energies with zero point energy corrections. It can be observed that the effects of zero point energies are not significant, and these corrections do not alter the change trends of the adsorption energies. | ||||||
| Isolated CO2 | 1.172 | 1.172 | 1.172 | 180.000 | ||
| 0 Na | −0.0027(−0.0026) | 1.172 | 1.172 | 1.172 | 179.982 | 3.793 |
| 1/9 Na | −0.2992(−0.2732) | 1.176 | 1.167 | 1.172 | 177.489 | 3.750 |
| 2/9 Na | −0.5508(−0.5318) | 1.235 | 1.288 | 1.262 | 128.596 | 2.092 |
| 3/9 Na | −0.8296(−0.8136) | 1.292 | 1.285 | 1.289 | 119.654 | 1.961 |
| 4/9 Na | −1.0390(−1.0306) | 1.308 | 1.284 | 1.296 | 118.048 | 2.049 |
| 5/9 Na | −1.8306(−1.7356) | 1.310 | 1.309 | 1.310 | 115.479 | 2.071 |
| Eada (eV) | rC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (Å) O (Å) |
rC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (Å) O (Å) |
rC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (Å)/average O (Å)/average |
θO![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (degree) O (degree) |
dCO2-surface (Å) | |
|---|---|---|---|---|---|---|
| a The values in parentheses are the adsorption energies with zero point energy corrections. It can be observed that the effects of zero point energies are not significant, and these corrections do not alter the change trends of the adsorption energies. | ||||||
| Isolated CO2 | 1.172 | 1.172 | 1.172 | 180.000 | ||
| 0 Cs | −0.0027(−0.0026) | 1.172 | 1.172 | 1.172 | 179.982 | 3.793 |
| 1/9 Cs | −0.2856(−0.2756) | 1.238 | 1.289 | 1.264 | 127.448 | 1.924 |
| 2/9 Cs | −1.1234(−1.1136) | 1.268 | 1.269 | 1.269 | 125.160 | 2.052 |
| 3/9 Cs | −0.9860(−0.9634) | 1.281 | 1.285 | 1.283 | 122.046 | 2.012 |
| 4/9 Cs | −1.6388(−1.6101) | 1.248 | 1.302 | 1.275 | 124.159 | 1.897 |
| 5/9 Cs | −2.5405(−2.5303) | 1.258 | 1.254 | 1.256 | 129.554 | 2.078 |
In the presence of alkaline metal Na and Cs adatoms, a significant promotion effect on CO2 adsorption could be observed, in which a drastic influence was exerted on the adsorption of CO2 on the Cu(111) surface. It was found that the presence of alkaline metals Na and Cs increased the binding energy of CO2 to the Cu(111) surface as observed in Tables 1 and 2, and the adsorption energies of CO2 increase gradually with alkaline metal coverage on the Cu(111) surface. The distances between CO2 and alkaline metal Na and Cs-promoted Cu(111) surfaces are shorter compared with that between CO2 and the clean Cu(111) surface, at ca. 2.00 Å, C–O bond lengths in adsorbed CO2 are stretched significantly, and O–C–O angles are significantly smaller on alkaline metals Na and Cs-promoted Cu(111) surfaces, at ca. 120.00°. It may be imagined that electron transfer from a substrate into the CO2 molecule is involved in the adsorption processes at surfaces which in turn would lead to the formation of a CO2 anion. A significant effect was also found experimentally in the presence of preadsorbed alkaline metals on the Cu and other metal surfaces.31,45 For example, Egawa et al.52 observed experimentally the stabilizing effect of alkaline metal adatoms on the adsorption of CO2 on Pd(100), in which Na was deposited on Pd. The presence of adsorbed Na atoms greatly increased the adsorbed energy of CO2. For the effect of alkaline metal Na, Wambach et al. also observed similar features on Pd(111).53 It was also found experimentally that addition of alkaline metal Cs on the Cu surface could enhance the amount of adsorbed CO2 and the adsorption probability of CO2.28,31,45 In the meantime, based on the optimized geometry structures in Fig. S1 and S2,† the formation of a Cu–C bond in a monodentate form, i.e. a pure carbon coordination, such as Fig. S1c–f and S2c, d and f† or bidentate structures, i.e. a mixed carbon–oxygen coordination, such as Fig. S2b and e† can be proposed. In bidentate form, an elongation of the interacting C–O bond is found to be longer with respect to the non-coordinated bond. For example, in Fig. S2b and e,† the interacting C–O bond lengths are 1.289 and 1.302 Å, respectively, which are longer than other non-coordinated C–O bonds in Fig. S2.† The longer C–O bond may lead to an easier dissociation of adsorbed CO2, which will be corroborated by following minimum energy analysis (MEP). The coordination mode in which only the oxygen atoms are involved in the bonding has not been observed in our present study. This is the main physical origin of alkaline metal Na and Cs promotion in CO2 activation. At low Na coverage (1/9 Na), it can be observed that CO2 adsorbed as electronically only slightly distorted molecules, the O–C–O angle is 177.489°, with the molecular axis oriented almost parallel to the surface plane, and the C–O bond length is almost unchanged (see Fig. S1b†). However, a significantly distorted CO2 molecule is observed at 1/9 Cs coverage (see Fig. S2b†), the corresponding bond angle is 127.448°, and C–O bond lengths at 1/9 Cs are also significantly longer than that at 1/9 Na coverage. At 2/9 coverage, the change of O–C–O bond angle and C–O bond length are also larger on Cs-promoted Cu(111) than that on Na-promoted Cu(111). Notably particularly, further increasing the coverage of alkaline metals, we observe that the distortion of CO2 reaches a maximum at 3/9 Cs, and then decreases gradually, whereas they increase gradually in the presence of alkaline metal Na. Thus, although the adsorption of CO2 is stronger on Cs-promoted Cu(111), the distortion of CO2 molecule is smaller than on Na-promoted Cu(111) at higher coverages. This may be due to different electronic interaction mechanisms among alkaline metals Na, Cs, Cu(111) and CO2 molecule, which will be confirmed through subsequent population analyses. The shorter distances between CO2 and Cs-promoted Cu(111) surfaces compared with that between CO2 and Na-promoted Cu(111) surface may be able to explain the stronger adsorption of CO2.
3.2 CO2 dissociation on clean and alkaline metal M (M = Na and Cs) promoted Cu(111)
Alkaline metal promotion leads to the chemisorption of CO2 molecules, an anion radical ˙CO2δ− may be formed, which can dissociate to form CO species during the course of reduction of CO2 into hydrocarbons. The presence of alkaline metals may lower the activation barrier of CO2 dissociation. However, on the Cu(111) surface, there is no direct measurement of the effect of alkaline metals on the dissociation of CO2, so it is not clear if the promotion of CO2 reduction is due to a reduction of the activation barrier for the CO2 dissociation on the alkaline metal promoted Cu(111) surface or due to some other effect. Thus, in order to compare the degree of difficulty of CO2 dissociation on clean Cu(111) and on alkaline metal M (M = Na and Cs) promoted Cu(111), in the present paper, we use self-consistent DFT calculations to investigate the effect of adsorbed alkaline metals Na and Cs on CO2 dissociation to form CO and O on the Cu(111) surface by performing MEP analysis, and the kinetic energetics of CO2 dissociation are examined. Only the coverage of 1/9 of a monolayer (ML) of Na and Cs is considered in this section since we only focus on the promotion effect of alkaline metal on CO2 dissociation and CO2 adsorbed on the Cu(111) surface in bidentate form at 1/9 Cs coverage.Our calculated activation barrier for the CO2 dissociation reaction is 1.41 eV (1.40 eV with zero point energy corrections) on a clean Cu(111) surface, whereas in the presence of Na and Cs, the results show a decrease in the dissociation barrier by 0.22 and 0.61 eV (0.20 and 0.60 eV with zero point energy corrections), respectively (see Fig. 1). The reason for reduction of the activation barrier may be that the weak CO2–Cu interaction on clean Cu(111) is an obstacle to CO2 dissociation. Thus, the calculated results suggested that the adsorbed alkaline metals lower the activation barrier for dissociation of CO2 significantly even though only a coverage of 1/9 ML of Na and Cs is considered, and also suggested that the effect of Cs is significantly larger than that of Na since the reduction of the dissociation barrier is even larger when Cs is added to the Cu(111) surface, and the adsorbed CO2 is in bidentate form which leads to an easier cleavage of the C–O bond. Simultaneously, we observe that the effects of zero point energies on activation energy barriers are not significant, and the trends of the reaction pathways shown above are not altered through these corrections. Our recent theoretical studies have shown that the relatively slow step on the clean Cu(111) surface is CO formation from the thermodynamic and kinetic viewpoint during the course of CO2 reduction into hydrocarbons.54 Therefore, the results clearly describe the experimentally observed promoting effect of alkaline metal Na and Cs on CO2 reduction on the Cu surface, and show that it can be ascribed to a reduction of the dissociation activation barrier of CO2.
 | ||
| Fig. 1 Minimum energy paths that include transition state and final state for CO2 dissociation on clean Cu(111) and on alkaline metals Na and Cs-promoted Cu(111). | ||
3.3 The origin of the promoting effect of alkaline metals Na and Cs on CO2 activation on Cu(111)
Now that the DFT calculations can describe at least qualitatively the promoting effect of alkaline metals on CO2 dissociation has been established, we turn to the problem of the origin of the promotion effect. By looking at the effect of two different alkaline metals Na and Cs, and the effect of their coverage, we investigate the promotion effect. The changes of work function of clean and Na and Cs-promoted Cu(111) surface with different Na and Cs coverage are examined first, and should play an important role since a relatively low work function can favor electron transfer, the computational details of work function are demonstrated in the ESI.† The calculated vacuum level, Vvacuum and Fermi level, EFermi for the clean Cu(111) and Na and Cs-promoted Cu(111) are summarized in Table S1.† It can be observed that the Fermi levels of Na and Cs-promoted Cu(111) are all increased compared with that of clean Cu(111) surface. Our present results show that the adsorption of Na and Cs on the Cu(111) surface resulted in a decrease in the work function in the absence of CO2 molecule. It is 4.68 eV on a clean Cu(111) surface, whereas it is 4.48 and 4.36 eV at 1/9 Na and Cs, respectively. When increasing the Na coverage, it can be observed that the work function increases first to a maximum, 4.57 eV at 3/9 Na coverage, and then decreases gradually to 4.48 eV at 5/9 Na coverage, which is the same as that at 1/9 Na coverage. Simultaneously, on the Cs-promoted Cu(111) surface, the work function increases to 4.48 eV, and then decreases and returns to 4.36 eV at 4/9 and 5/9 Cs coverage as observed at 1/9 Cs coverage (see Table 3). Namely, the work function can pass through a maximum when achieving higher coverages. Thus, we can speculate that the change of the work function depended on the coverage of alkaline metals Na and Cs, and no influence was exerted on the work function of the Na and Cs-promoted Cu(111) surface when further increasing the Na and Cs coverage. In the meantime, it can be observed that the reduced amount of the work function on Cs-promoted Cu(111) is more than that on Na-promoted Cu(111), which explains why the activation barrier for CO2 dissociation is lower on Cs-promoted Cu(111) surface. Thus, it was concluded that the reactivity of Na and Cs-promoted Cu surfaces towards the adsorption of CO2 is dominated by their work function. Clean Cu(111) surface of high work function (4.68 eV) does not adsorb CO2, whereas the formation of CO2δ− anion may occur on Na and Cs-promoted Cu(111) surfaces of lower work function.| Alkaline metal | Work function (eV) | Alkaline metal | Work function (eV) |
|---|---|---|---|
| 0 | 4.68 | 0 | 4.68 |
| 1/9 Na | 4.48 | 1/9 Cs | 4.36 |
| 2/9 Na | 4.51 | 2/9 Cs | 4.48 |
| 3/9 Na | 4.57 | 3/9 Cs | 4.43 |
| 4/9 Na | 4.49 | 4/9 Cs | 4.36 |
| 5/9 Na | 4.48 | 5/9 Cs | 4.36 |
An electronic interaction between CO2 and the Na and Cs-promoted Cu(111) surface, a large negative charge on the chemisorbed CO2 and the possible formation of a partially negative species, CO2δ− were suggested by the work function decreasing. These features may be ascribed to enhancement of electron back-donation from Na and Cs-promoted Cu(111) surface into an empty π orbital of CO2. As a consequence of the enhanced electron donation, the adsorption ability of CO2 to Na and Cs-promoted Cu(111) surface is increased, as exhibited in Tables 1 and 2. To determine the promoting mechanism of alkaline metals Na and Cs on CO2 activation on the Cu(111) surface and electron interaction between Na and Cs and the Cu(111) surface, we carried out the Löwdin population analysis in the present study. The computational details of Löwdin population analysis are demonstrated in the ESI.† The analysis indicates that s and d orbitals of surface Cu atoms gain electrons, whereas s and p orbitals of surface Na atoms lose electrons in Na-promoted Cu(111) (see Table S2†), in which Na is an effective electron donor and lowers the work function of the surface. However, it was noticed in Table S3† that the nature of the electron interaction on the Cs-promoted Cu(111) surface were practically not the same as those observed for Na-promoted Cu surface. On the Cs-promoted Cu(111) surface, the s and d orbitals of surface Cu atoms lose electrons, whereas the total net electrons of surface Cs atoms is positive, in which s orbitals of the surface Cs atoms lose electrons, p and d orbitals gain electrons, i.e. Cs is totally an electron acceptor. Thus, we can speculate that the promoting mechanism of alkaline metals Na and Cs on CO2 activation may be different. In the meantime, Cu as an electron donor in Cs-promoted Cu(111) may lead to a more reduced amount of work function of Cs-promoted Cu(111) surface.
To obtain further insight into the promoting effect of alkaline metals Na and Cs on CO2 activation on the Cu(111) surface and the electronic interaction between CO2 and clean and Na and Cs-promoted Cu(111) surfaces, the Löwdin population analysis was also carried out in the presence of CO2. The analysis indicates that s and d orbitals of surface Cu atoms gain an electron in Na-promoted Cu(111), the net result of electron gain or loss of C and O atoms in CO2 is almost charge neutrality at 1/9 Na coverage. However, s and p orbitals of C and O atoms in CO2 also gain an electron at higher Na coverage (see Table S4†). In Na-promoted Cu(111), s and p orbitals of surface Na atom also lose electrons as observed on free CO2 adsorbed Cu(111) surface. However, it was found in Table S5† that s and d orbitals of surface Cu atom lose electrons, s orbitals of surface Cs atom lose electrons, p, and d orbitals gain electrons, and the total net electrons of surface Cs atoms is positive on Cs-promoted Cu(111) surface in the presence of CO2, which is consistent with that on free adsorbed CO2 Cu(111) surface. For adsorbed CO2 molecules on the Cu(111) surface with different Cs coverage, s and p orbitals of C atoms gain electrons, s orbitals of O atoms lose electrons, whereas p orbitals of O atoms gain electrons and the total net electrons of CO2 molecule is positive. Thus, the electronic interaction between CO2 and the Na and Cs-promoted Cu(111) surface leads to the formation of a partially negative species, CO2δ− with a negative charge on the chemisorbed CO2. These features can be ascribed to an enhanced back-donation of electrons from the Na and Cs-promoted Cu(111) surface into an empty π orbital of CO2 compared to that on a clean Cu(111) surface. However, the back-donation mechanism of electrons is different between the Na and Cs-promoted Cu(111) surfaces. On the Na-promoted Cu(111) surface, surface Cu atoms gain electrons, alkaline metal Na lose electrons, whereas on the Cs-promoted Cu(111) surface, surface Cu atoms lose electrons, alkaline metal Cs gain electrons, thus leading to the difference of CO2 activation mechanism. Because of the difference between alkaline metal promotion mechanisms, CO2 can gain more electrons on Na-promoted Cu(111) than Cs-promoted Cu(111) at higher coverages (see Tables S4 and S5†), which may explain why a smaller CO2 distortion occurs on Cs-promoted Cu(111) in spite of the stronger adsorption. Although the precise nature of the strongly adsorbed CO2 species is less clear, the charge transfer to an empty π orbital of CO2 is a reasonable suggestion particularly since alkaline metals are anticipated to reduce the work function of the surface. The interaction with a surface Na or Cs atom suggests the direct formation of a CO2δ− anion.
In order to understand the electron transfer between alkaline metals Na and Cs and the Cu(111) surface, the local density of states (LDOS) analyses of systems are performed in the present study since it can describe the number of electrons per interval of energy at each energy level that are available to be occupied. A high LDOS at a specific energy level means that there are many electrons available for occupation. Only the coverage of 1/9 ML of Na and Cs is considered since we only focus on the electron transfer between alkaline metals and Cu. Fig. 2 and 3 give the LDOS of Na- and Cs-promoted Cu(111) surfaces, respectively. For comparison, the LDOS of pure Na, Cs, and free alkaline metal adsorbed Cu(111) surface are also included. As shown in Fig. 2a, the s state of Na in Na-promoted Cu(111) shifts down into a lower energy level compared with that of pure Na, implying that there are fewer electrons in s states of Na on Na-promoted Cu(111) surface. The d states of Cu between pure and Na-promoted Cu(111) are almost unchanged, see Fig. 2b, suggesting that the number of electrons in d states of Cu will be not changed, and the s states of Cu will gain electrons in Na-promoted Cu(111). Thus, the results from the LDOS are consistent with the above corresponding Löwdin population analysis. In Cs-promoted Cu(111), the d state of Cs shifts up into a higher energy level and a significantly higher LDOS of d states in Cs is observed in contrast with that of pure Cs, as shown in Fig. 3a, meaning that there are electrons transferred to d states of Cs on the Cs-promoted Cu(111) surface. Similarly, d states of Cu in Cs-promoted Cu(111) are also almost consistent with that in pure Cu(111). Noticeably, s states of Cu in Cs-promoted Cu(111) shift down into a lower energy level compared with that in pure Cu(111), as shown in Fig. 3b, indicating that s states of Cu will lose electrons, which is also in agreement with the above corresponding Löwdin population analysis. Therefore, the present LDOS analyses likewise indicate that Na is an effective electron donor and Cs is an electron acceptor, and further confirmed the above Löwdin population analysis.
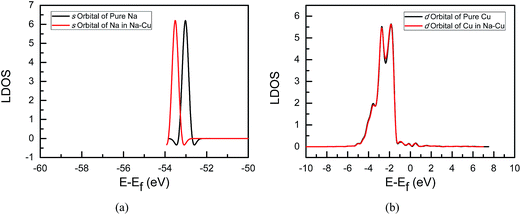 | ||
| Fig. 2 Local density of states of alkaline metal Na-promoted Cu(111) surface: (a) s orbital of Na; (b) d orbital of Cu. | ||
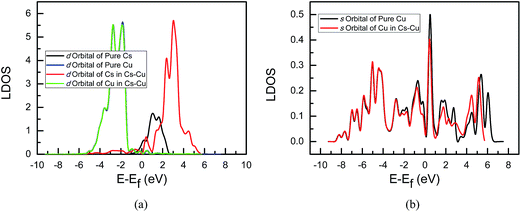 | ||
| Fig. 3 Local density of states of alkaline metal Cs-promoted Cu(111) surface: (a) d orbital of Cs and d orbital of Cu; (b) s orbital of Cu. | ||
Based on the above analysis, a basic change in the bonding and structure of the adsorbed CO2 can be caused by the extended electron donation. In the case of weak CO2 adsorption on a clean Cu(111) surface, it was assumed that CO2 is bonded via an oxygen lone electron pair, with a vertical molecular bond, whereas on Na and Cs-promoted Cu(111) surface, the formation of a Cu–C bond has been proposed in the form of a monodentate or bidentate structure, as shown in Fig. S1 and S2.† The mixed carbon–oxygen leads to more stable adsorption of CO2 than the pure carbon coordination, yielding more stabilization than the single carbon–Cu bond. Thus, we can come to the conclusion that a bent anionic adsorption state, CO2δ− on the Na and Cs-promoted Cu(111) surface take part in the surface chemistry of CO2, this being the precursor of the further reduction of the CO2 molecule. The bent CO2δ− anion is produced by the transfer of electrons from the Na and Cs-promoted Cu(111) surface to the CO2 molecule, which is in favor of breaking of the C–O bond. The strong work function decrease of the Na and Cs-promoted Cu(111) surface is corroborated by the presence of charge transfer to the CO2.
In our present study, the self-consistent DFT calculations based on plane wave basis sets have been used to study the alkaline metal M (M = Na and Cs) promotion mechanism for CO2 activation on the Cu(111) surface. Currently, DFT has become the most widely used and successful method for simulating systems of interacting electrons. The great success of DFT is that simple approximations perform remarkably well for a wide range of problems in chemistry, particularly for prediction of the structure and thermodynamic properties of molecules and solids. However, its application can still suffer from large pervasive errors in predicted adsorption properties despite the widespread popularity and success of DFT. The major errors may include underestimation of the barriers of chemical reactions, the energies of dissociating molecular ions, charge transfer excitation energies, and overestimation of binding energies of charge transfer complexes. These errors are not breakdowns of the theory itself but are only due to deficiencies of the currently used approximate exchange–correlation functionals.55,56 Because of the fundamental limitations of DFT, our present calculated energies at T = 0 K may be not adequately accurate. However, our present calculations are qualitatively in agreement with previous experimental studies. For example, our results show that CO2 adsorbs weakly and molecularly on a clean Cu(111) surface, whereas a drastic influence on the bonding, structure, and reactivity of adsorbed CO2 was exerted on the Cu(111) surface by the presence of alkaline metals Na and Cs adatoms, leading to the formation of surface CO2δ− radical anions. In the early classical experimental work, the strong adsorption of CO2 on evaporated Cu films at 195 and 273 K was also not found, and this feature was confirmed in a number of studies by using tools of modern surface science.28,31,45–51 In the presence of preadsorbed alkaline metals, the significant promotion effect on CO2 activation was observed experimentally on the Cu surface.28,31,45 For example, it was found experimentally that the amount of adsorbed CO2 and the adsorption probability of CO2 in the gas phase could be enhanced through addition of alkaline metals on the Cu surface. Additionally, during the course of CO2 electrochemical reduction, alkaline metals Na and Cs promotion has also been reported experimentally to greatly enhance the adsorption ability of CO2 on Cu electrode and carbon dioxide anion radical ˙CO2− was detected on alkaline metal modified polycrystalline Cu surfaces.24–29 Thus, although the real experimental conditions for CO2 activation on alkaline metals Na and Cs promoted Cu(111) are not simulated in this paper, we would expect the conclusions in trends to be reasonably accurate.
4. Conclusions
A systematic study on the alkaline metal M (M = Na and Cs) promotion mechanism for CO2 activation on a Cu(111) surface is presented for the first time using computational techniques based on self-consistent DFT calculations. The results show that CO2 adsorbs weakly and molecularly on a clean Cu(111) surface, whereas a drastic influence on the bonding, structure, and reactivity of adsorbed CO2 was exerted on the Cu(111) surface by the presence of alkaline metals Na and Cs adatoms, leading to the formation of surface CO2δ− radical anions. This is the main physical origin of alkaline metal Na and Cs promotion in CO2 activation. The Na and Cs adatoms lower the dissociation activation barrier of the CO2 molecule on the Cu(111) surface, in which the effect of Cs is significantly larger than that of Na since the reducing of the barrier for dissociation is even larger when adding Cs to the surface and the adsorbed CO2 in bidentate form leads to an easier cleavage of the C–O bond. In this way we explain the experimental observation that the promotion effect of alkaline metals on CO2 reduction into hydrocarbons on Cu catalysts can be ascribed to a reducing of the dissociation activation barrier of CO2 since CO2 activation is a crucial step in hydrocarbon synthesis reactions on a Cu single-crystal electrode surface.The studies also show that the origin of this promotion effect is predominately a direct electronic interaction between the Na and Cs-promoted Cu(111) surfaces and CO2 molecule. The adsorption of Na and Cs resulted in a decrease in the work function of the surface, and the reduced amount of the work function on the Cs-promoted Cu(111) surface is more than that on the Na-promoted Cu(111) surface, explaining why the activation barrier for CO2 dissociation is lower on the Cs-promoted Cu(111) surface. In the meantime, it can be observed that the change of the work function depended on the coverage of alkaline metals Na and Cs, and no influence was exerted on the work function of the surface by a further increase of the Na and Cs coverage. The strong work function decrease of the Na and Cs-promoted Cu(111) surface is corroborated by the presence of charge transfer to the CO2, which leads to formation of a partially negative species, CO2δ− due to an enhancement of back-donation of electrons from Na and Cs-promoted Cu(111) surface into an empty π orbital of CO2. Thus, the charge transfer to an empty π orbital of CO2 is a reasonable suggestion particularly for CO2 activation. However, the back-donation mechanism of electrons is different between Na and Cs-promoted Cu(111) surfaces, in which surface Cu atoms gain electrons, alkaline metal Na loses electrons in Na-promoted Cu(111), i.e. Na is an effective electron donor, whereas surface Cu atoms lose electrons, alkaline metal Cs gains electrons in Cs-promoted Cu(111), i.e. Cs is an electron acceptor, thus leading to the difference of promoting mechanism of alkaline metals Na and Cs on CO2 activation. In the meantime, Cu as an electron donor in Cs-promoted Cu(111) may result in the more reduced amount of the work function of the Cs-promoted Cu(111) surface.
Acknowledgements
This work is financially supported by the National Natural Science Foundation of China (Grant No. 21303048), the Project supported by Hunan Provincial Natural Science Foundation of China (Grant No. 13JJ4101), the Construct Program of the Key Discipline in Hunan Province (Applied Chemistry) and Doctoral Start-up Fund of Hunan University of Arts and Science.References
- Y. Hori, K. Kikuchi, A. Murata and S. Suzuki, Chem. Lett., 1986, 15, 897–898 CrossRef.
- Y. Hori, A. Murata, R. Takahashi and S. Suzuki, J. Am. Chem. Soc., 1987, 109, 5022–5023 CrossRef CAS.
- M. Gattrell, N. Gupta and A. Co, J. Electroanal. Chem., 2006, 594, 1–19 CrossRef CAS.
- R. L. Cook, R. C. MacDuff and A. F. Sammells, J. Electrochem. Soc., 1989, 136, 1982–1984 CrossRef CAS.
- D. W. DeWulf, T. Jin and A. J. Bard, J. Electrochem. Soc., 1989, 136, 1686–1691 CrossRef CAS.
- Y. Hori, A. Murata and R. Takahashi, J. Chem. Soc., Faraday Trans. 1, 1989, 85, 2309–2326 RSC.
- Y. Hori, H. Wakebe, T. Tsukamoto and O. Koga, Electrochim. Acta, 1994, 39, 1833–1839 CrossRef CAS.
- H. De Jesús-Cardona, C. del Moral and C. R. Cabrera, J. Electroanal. Chem., 2001, 513, 45–51 CrossRef.
- C. Delacourt, P. L. Ridgway, J. B. Kerr and J. Newman, J. Electrochem. Soc., 2008, 155, B42–B49 CrossRef CAS.
- H. J. Freund and M. W. Roberts, Surf. Sci. Rep., 1996, 25, 225–273 CrossRef.
- S. R. Tennison, Catalytic Ammonia Synthesis, ed. J. R. Jenniings, Plenum, New York, 1991, p. 303 Search PubMed.
- O. Hinrichsen, F. Rosowski, M. Mulhler and G. Ertl, Chem. Eng. Sci., 1996, 51, 1683–1690 CrossRef CAS.
- M. P. Kiskinova, Poisoning and Promotion in Catalysis Based on Surface Science Concepts and Experiments; Studies in Surface Science and Catalysis 70, Elsevier, Amsterdam, 1992 Search PubMed.
- G. A. Somorjai, Introduction to surface Chemistry and Catalysis, Wiley, New York, 1994, p. 442 Search PubMed.
- R. L. Toomes and D. A. King, Surf. Sci., 1996, 349, 19–42 CrossRef CAS.
- S. Wilke and M. H. Cohen, Surf. Sci., 1997, 380, L446–L454 CrossRef CAS.
- F. Solymosi, A. Berko and Z. Toth, Surf. Sci., 1993, 285, 197–208 CrossRef CAS.
- P. J. Feibelman and D. R. Hamann, Phys. Rev. Lett., 1984, 52, 61–64 CrossRef CAS.
- K. Wandelt, Physics and Chemistry of Alkali Metal Adsorption, ed. H. P. Bonzel, A. M. Bradshaw and G. Ertl, Elsevier, Amsterdam, 1989, p. 25 Search PubMed.
- G. Ertl, S. B. Lee and M. Weiss, Surf. Sci., 1982, 114, 527–545 CrossRef CAS.
- A. P. Farkas and F. Solymosi, J. Phys. Chem. C, 2009, 113, 19930–19936 CAS.
- J. J. Mortensen, B. Hammer and J. K. Nørskov, Phys. Rev. Lett., 1998, 80, 4333–4336 CrossRef CAS.
- Z. P. Liu and P. Hu, J. Am. Chem. Soc., 2001, 123, 12596–12604 CrossRef CAS PubMed.
- J. Onsgaard, L. Bech, C. P. Svensgaard, J. Godowski and S. V. Hoffmann, Prog. Surf. Sci., 2001, 67, 205–216 CrossRef CAS.
- S. Kaneco, N. Hiei, Y. Xing, H. Katsumata, H. Ohnishi, T. Suzuki and K. Ohta, Electrochim. Acta, 2002, 48, 51–55 CrossRef CAS.
- J. Onsgaard, S. V. Hoffmann, P. Moller, P. J. Godowski, J. B. Wagner, G. Paolucci, A. Baraldi, G. Comelli and A. Groso, ChemPhysChem, 2003, 4, 466–473 CrossRef CAS PubMed.
- S. Kaneco, K. Iiba, N. H. Hiei, K. Ohta, T. Mizuno and T. Suzuki, Electrochim. Acta, 1999, 44, 4701–4706 CrossRef CAS.
- J. A. Rodriguez, W. D. Clendening and C. T. Campbell, J. Phys. Chem., 1989, 93, 5238–5248 CrossRef CAS.
- W. Akemann and A. Otto, Surf. Sci., 1993, 287–288, 104–109 CrossRef CAS.
- H. J. Freund and R. P. Messmer, Surf. Sci., 1986, 172, 1–30 CrossRef CAS.
- F. Solymosi, J. Mol. Catal., 1991, 65, 337–358 CrossRef CAS.
- R. G. Copperthwaite, P. R. Davies, M. A. Morris, M. W. Roberts and R. A. Ryder, Catal. Lett., 1988, 1, 11–19 CrossRef CAS.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865–3868 CrossRef CAS PubMed.
- D. Vanderbilt, Phys. Rev. B: Condens. Matter Mater. Phys., 1990, 41, 7892–7895 CrossRef.
- M. Methfessel and A. T. Paxton, Phys. Rev. B: Condens. Matter Mater. Phys., 1989, 40, 3616–3621 CrossRef CAS.
- S. Baroni, A. Dal Corso, S. de Gironcoli and P. Giannozzi, PWSCF and PHONON: plane-wave pseudo-potential codes, http://www.pwscf.org, 2001 Search PubMed.
- A. Kokalj, J. Mol. Graphics Modell., 1999, 17, 176–179 CrossRef CAS PubMed.
- A. Kokalj and M. Causà, Scientific visualization in computational quantum chemistry, in Proceedings of High Performance Graphics Systems and Applications European Workshop, CINECA-Interuniversity Consortium, Bologna, Italy, 2000 Search PubMed.
- A. Kokalj and M. Causà, XCrySDen: (X-Window) CRYstalline Structures and DENsities, http://www-k3.ijs.si/kokalj/xc/XCrySDen.html, 2001 Search PubMed.
- J. Greeley, A. A. Gokhale, J. Kreuser, J. A. Dumesic, H. Topsøe, N. Y. Topsøe and M. Mavrikakis, J. Catal., 2003, 213, 63–72 CrossRef CAS.
- W. M. Haynes, CRC Handbook of Chemistry and Physics, Internet Version 2012, CRC Press/Taylor and Francis, Boca Raton, FL, 93rd edn, 2011 Search PubMed.
- G. Henkelman and H. Jonsson, J. Chem. Phys., 2000, 113, 9978–9985 CrossRef CAS.
- G. Henkelman, B. P. Uberuaga and H. Jonsson, J. Chem. Phys., 2000, 113, 9901–9904 CrossRef CAS.
- S. Baroni, S. D. Gironcolli, A. D. Corso and P. Giannozzi, Rev. Mod. Phys., 2000, 73, 515–562 CrossRef.
- A. C. Collins and B. M. W. Trapnell, Trans. Faraday Soc., 1957, 53, 1476–1482 RSC.
- P. R. Norton and R. L. Trapping, Chem. Phys. Lett., 1976, 38, 207–212 CrossRef CAS.
- A. B. Anderson, Surf. Sci., 1977, 62, 119–132 CrossRef CAS.
- N. K. Ray and A. B. Anderson, Surf. Sci., 1982, 119, 35–45 CrossRef CAS.
- F. Solymosi and J. Kiss, Surf. Sci., 1981, 108, 368–380 CrossRef CAS.
- F. Solymosi and J. Kiss, Surf. Sci., 1981, 104, 181–198 CrossRef CAS.
- F. Solymosi and A. Berkó, Surf. Sci., 1982, 122, 275–291 CrossRef CAS.
- C. Egawa, I. Doi, S. Naito and K. Tamaru, Surf. Sci., 1986, 176, 491–504 CrossRef CAS.
- J. Wambach, G. Odörfer, H. J. Freund, H. Kuhlenbeck and M. Neumann, Surf. Sci., 1989, 209, 159–172 CrossRef CAS.
- L. H. Ou, RSC Adv., 2015, 5, 57361–57371 RSC.
- N. Schuch and F. Verstraete, Nat. Phys., 2009, 5, 732–735 CrossRef CAS.
- A. J. Cohen, P. Mori-Sánchez and W. Yang, Science, 2008, 321, 792–794 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra10321a |
| This journal is © The Royal Society of Chemistry 2016 |
