Non-interacting, sp2 carbon on a ferroelectric lead zirco-titanate: towards graphene synthesis on ferroelectrics in ultrahigh vacuum†
Abstract
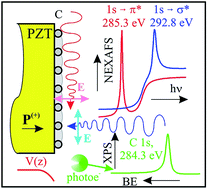
Carbon layers grown on lead zirco-titanate (PZT) weakly interact with the substrate and exhibit a nearly two dimensional character, up to a carbon surface density approaching that of graphene. The first feature is evidenced by X-ray photoelectron spectroscopy, and the second by angle resolved near-edge-absorption spectroscopy (NEXAFS). The binding energies and lineshape parameters of C 1s are similar to that of graphene. The dichroism of C K-edge NEXAFS shows the prevalence of in-plane sp2 bonds for layers whose effective coverage is below the graphene surface density. The polarization state of the substrate, oriented outwards, is preserved upon carbon deposition. The surface Pb content is strongly affected by the carbon ad-layers.

 Please wait while we load your content...
Please wait while we load your content...