Natural eggshell membrane as separator for improved coulombic efficiency in air-cathode microbial fuel cells†
Abstract
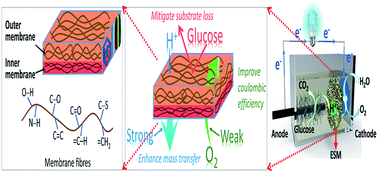
Natural eggshell membrane (ESM) is proposed as a separator to enhance electricity recovery in air-cathode microbial fuel cells (MFCs). The unique fibrous structure and surface chemistry of ESM can mitigate substrate and oxygen crossover, and biofouling of the cathode, achieving a remarkable coulombic efficiency of 67.14–95.03%, depending on the current density. This study demonstrates an effective, simple and green route for recovering electricity from organic matter in MFCs.

 Please wait while we load your content...
Please wait while we load your content...