Preparation of broccoli-like ferromagnetic cobalt microstructures with superior coercivity via an aqueous reduction strategy†
Abstract
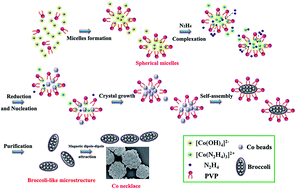
Controlled synthesis of novel hierarchical cobalt (Co) microstructures with extraordinary magnetic performances is a promising strategy for the development of magnetic metals for industrial purposes. Unlike the majority of experiments necessitating organic solvents at high temperatures or pressures, here broccoli-like Co microstructures were successfully prepared in aqueous solution under ambient temperature with the assistance of polyvinylpyrrolidone (PVP) for the first time. The synthetic process was associated with the chemical reduction of Co with pure water as solvent, hydrazine hydrate as reductant, and no complexant, nucleator, or external magnetic force was employed. The Co spheroids with broccoli shape were constructed by several beads with the length ranging from 0.67 μm to 1.22 μm, and they slightly self-assembled into a necklace with adjacent Co spheroids via spontaneous magnetic dipole–dipole attraction. The results indicated that the temperature, and the concentration of PVP and NaOH played an imperative role in the morphology and size of the ultrafine Co micro-aggregates in the present approach. More importantly, the broccoli-like Co entities exhibited a decreased saturation magnetization of 25.6 emu g−1 but an enhanced coercivity of 499.2 Oe mainly due to its anisotropic structure and smaller size, and could hold great potential for technological applications such as high-density data storage, and permanent magnetic materials.

 Please wait while we load your content...
Please wait while we load your content...