DOI:
10.1039/C6RA08715A
(Paper)
RSC Adv., 2016,
6, 67888-67897
Simple combination of humic acid with biogenic hydroxyapatite achieved highly efficient removal of methylene blue from aqueous solution†
Received
5th April 2016
, Accepted 12th July 2016
First published on 13th July 2016
Abstract
Biogenic hydroxyapatite (bHAP) derived from eggshell waste impregnated with humic acid (HA) was utilized as an adsorbent to remove methylene blue (MB) from aqueous solution. bHAP simply coupled with HA resulted in highly efficient MB removal due to the introduction of negatively charged groups, which could enhance the adsorption of cationic dyes through electrostatic interactions as confirmed by Fourier transform infrared spectroscopy (FTIR). Adsorption experiment data indicated that MB adsorption by HA-impregnated bHAP (HA-bHAP) obeyed a pseudo-second-order equation, and the adsorption amount of MB increased with both the increases in solution pH and temperature. The adsorption isotherm agreed well with the Sips model, and the maximum adsorption capacity at the given conditions was 393.47 mg g−1. Regeneration studies exhibited that HA-bHAP could be recyclable for a long time. Overall, the results reported herein demonstrated the potential of HA-bHAP for the removal of MB and other cationic dyes from aqueous solution.
1. Introduction
The release of colored wastewater into the ecosystem is a dramatic source of aesthetic pollution, eutrophication, and perturbation in aquatic life.1 Methylene blue (MB) is an important cationic dye, which is most commonly used for coloring among all other dyes of its category. Although MB is not strongly hazardous, acute exposure of MB to human beings will cause skin and eye irritation, carcinogenicity, reproductive and developmental toxicity, neurotoxicity and chronic toxicity.2 Therefore, the removal of such a dye from process effluent becomes environmentally important. Among several chemical and physical methods, adsorption is known to be a promising technique, which has gained great importance due to its ease of operation, simplicity of design, high efficiency, energy saving, and comparable low cost of application in decoloration processes.3
At present, the search for cost-effective and easily available adsorbents has led many researchers to explore more economic and efficient techniques of using the natural and synthetic materials as adsorbents. Hydroxyapatite [HAP, Ca10(PO4)6(OH)2] is such an ideal material for the adsorption of various contaminants due to its special chemical composition and crystal structure, together with the excellent biocompatibility and high adsorption capacity. Synthetic HAP has been widely reported not only for biomedical applications but also as an environmental benign functional material for adsorption of heavy metals and organic pollutants from contaminated soil and water.4,5 However, some challenges still remain in the preparation and application of HAP. One serious issue is that most of the laboratory-produced HAP as well as commercially-available HAP are relatively expensive due to the use of high purity reagents. Another drawback is the low efficiency to remove MB by HAP as reported in our previous work.6 On the one hand, alternative procedures to produce HAP from cheap and recyclable or waste materials have been pursued. For example, the use of waste eggshells has been widely reported for the preparation of biogenic HAP (bHAP) because of the fact that it is cheap and readily available.7 Eggshell waste is widely produced from houses, restaurants, and bakeries. As a natural biomaterial, eggshell has a little developed porosity and pure CaCO3 as an important constituent and behaves good biocompatibility.8 The utilization of eggshell waste as a calcium source for the preparation of HAP can reduce the amount of waste to be disposed of and reduce the costs from the requirement of using expensive and high purity calcium reagents to prepare HAP. On the other hand, exploring a simple, effective and environmentally friendly method to significantly improve the adsorption ability of HAP toward MB is urgently needed.
Keeping this in view, more and more efforts have been paid to enhance the adsorption affinity of HAP towards dyes. Currently, surface modification, including physical coating and covalent binding, has often been explored to promote the removal efficiency of dyes. For example, covalent attachment of carboxylic groups to the surface of magnetic nanoparticles was achieved by reacting magnetite with polyacrylic acid, and the resulting material showed marked ability in adsorbing MB.9 Humic acid-coated Fe3O4 nanoparticles prepared by the coprecipitation method were found to effectively adsorb rhodamine B10 and MB11 from water. It is considered that the surface modification can not only alter the surface property and environmental behavior of adsorbent, but also affect the adsorption capacity for adsorbate.12 However, to the best of our knowledge, there exist only few reports on the surface modification of HAP to improve the adsorption property towards dyes. In one of these reports, Nguyen and Pho5 modified magnetic HAP nanoparticles with chitosan for the removal of Reactive blue 19 from aqueous solution. Adsorption of malachite green by organic surfactant EHDAB modified HAP has been reported by El-Zahhar and Awwad.13 The possibility of using humic acid (HA) modified bHAP as an adsorbent material to remove dyes from water is, however, not explored. Humic substances such as HA are the most predominant reactive fractions of natural organic matter. HA usually contains many functional groups, including carboxyl, phenolic hydroxyl, carbonyl, hydroxyl, aldehyde acid, and methoxyl groups. The existence of carboxylic and phenolic groups results in HA predominantly carrying negative charges, which can enhance the adsorption of cations through electrostatic interaction.14 It is expected that the surface modification of bHAP with HA could significantly enhance the adsorption capacity of bHAP towards the cationic dyes such as MB.
In light of this research background, the aim of this work was to demonstrate that the combination of HA with bHAP can be used as a highly efficient and cost-effective technology for cationic dyes removal from aqueous solution. The HA impregnated bHAP adsorbent was carefully characterized and the adsorption kinetics and isotherms were also investigated in detail.
2. Materials and methods
2.1 Materials
MB (Basic blue 9: CI-52015, C16H18ClN3S·3H2O), obtained from Sinopharm Chemical Reagent Company (Shanghai, China), were used without any further purification. HA was obtained as a commercial reagent-grade solid from Aldrich Chemical Company. All other chemicals were the analytic grade reagents commercially available and used without further purification.
2.2 Preparation and characterization of HA impregnated bHAP
The bHAP sample was prepared according to the literature7 with a little modification. However, a brief description is provided as follows: appropriate amounts of eggshells were washed thoroughly and heated in a box furnace at 1100 °C for 24 h to decompose organic matters and convert the calcium carbonate to calcium oxide. The white product was finely ground in an agate pestle and mortar. The calcium hydroxide suspension was prepared by slurring calcium oxide powder into freshly double distilled water. The suspension was then exposed to an ultrasonic irradiation source of 50 W (30 kHz, Model UP50H, Hielscher Ultrasonics, Germany) at maximum amplitude for 1 h. Then a required amount of H3PO4 was added dropwise to calcium hydroxide suspension while undergoing a second hour of ultrasonic irradiation. Intensive stirring and slow reagent addition were applied for the purpose of avoiding a local inhomogeneity. After complete addition, the resultant white slurry (pH = 7.0) was centrifuged at 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 × g for 30 min. Then the product was washed twice with distilled water and twice with ethanol and dried at 80 °C with a vacuum dryer for 24 h to obtain fine white powder which was subsequently sintered at 100 °C in an oven for 6 h to finally produce bHAP material. This powdered sample was then used for the preparation of HA impregnated bHAP (HA-bHAP). HA was impregnated onto bHAP at pH 5.0 using the batch adsorption techniques reported by our previous work.15 Briefly, the initial pH of HA solution (pH 5.0, 2000 mg L−1) was adjusted and 2.0 g of bHAP was added in the HA solution and shaken at 200 rpm for 24 h. After attaining equilibrium, the suspension was centrifuged and the residue was washed with 0.01 mol L−1 NaClO4 solution followed by deionized water. The obtained HA-bHAP sample was then freeze-dried, ground, and stored for future experiments. The amount of unadsorbed HA was determined using UV-visible spectrophotometer (UV-2450, Shimadzu, Japan) at a wavelength of 254 nm. The amount of HA loaded onto bHAP was calculated and found to be 118.7 mg g−1.
000 × g for 30 min. Then the product was washed twice with distilled water and twice with ethanol and dried at 80 °C with a vacuum dryer for 24 h to obtain fine white powder which was subsequently sintered at 100 °C in an oven for 6 h to finally produce bHAP material. This powdered sample was then used for the preparation of HA impregnated bHAP (HA-bHAP). HA was impregnated onto bHAP at pH 5.0 using the batch adsorption techniques reported by our previous work.15 Briefly, the initial pH of HA solution (pH 5.0, 2000 mg L−1) was adjusted and 2.0 g of bHAP was added in the HA solution and shaken at 200 rpm for 24 h. After attaining equilibrium, the suspension was centrifuged and the residue was washed with 0.01 mol L−1 NaClO4 solution followed by deionized water. The obtained HA-bHAP sample was then freeze-dried, ground, and stored for future experiments. The amount of unadsorbed HA was determined using UV-visible spectrophotometer (UV-2450, Shimadzu, Japan) at a wavelength of 254 nm. The amount of HA loaded onto bHAP was calculated and found to be 118.7 mg g−1.
Phase composition of the samples was determined by the X-ray diffraction (XRD) method in the range of 2θ from 20 to 60° using Cu Kα radiation (λ = 1.5405 Å) on a Rigaku D/max-IIIB X-ray diffraction equipment (Rigaku Co., Japan). The surface morphology of the HA-bHAP adsorbent was investigated by scanning electron microscopy (SEM, Model S-3000N, Hitachi, Japan). Fourier transform infrared spectra (FTIR) spectra of the adsorbent before and after dye adsorption were recorded on a Thermo Scientific Nicolet iS5 FTIR spectrometer (Nicolet Instrument, Thermo Company, USA) with the KBr pellet technique.
2.3 Batch experimental program
The MB adsorption capacity of HA-bHAP was investigated in terms of variation in pH, adsorbent dosage, contact time, ionic strength and temperature. Typically, 100 mg of prepared HA-bHAP was added into a 100 mL of mixed solution containing 100 mg L−1 MB, the mixture was adjusted to pH 7.0 with HCl or NaOH and stirred for 4 h. After the completion of experiment, the absorbance of the supernatant dye solution was analyzed using UV-vis spectrophotometer at 663 nm. A calibration plot of absorbance against concentration was used to determine the concentration of the MB solution. From the initial and final concentration, dye removal (%) was calculated as:| |
 | (1) |
where C0 and Ce are the initial and equilibrium concentrations in mg L−1. The amount of dye adsorbed on the HA-bHAP adsorbent was calculated as:| |
 | (2) |
where qe (mg g−1) is the amount of MB adsorbed per unit mass of the adsorbent; V (L) is the volume of the MB solution; and m (g) is the mass of the adsorbent. As mentioned above, C0 and Ce are the initial and equilibrium concentrations in mg L−1. All the experiments were performed in triplicate and average values were taken into account.
For batch desorption study, the adsorbent utilized for the adsorption was separated from the dye solution. The MB-loaded HA-bHAP (0.2 g) was agitated in a 250 mL Erlenmeyer flask containing 50 mL of aqueous solution of KCl and KH2PO4 of known concentration at 298 K at 150 rpm for 6 h in the orbital shaker. Thereafter, the supernatant was analyzed for MB released into the eluent.
2.4 Mathematical models
The adsorption kinetics of MB on HA-bHAP were described with the pseudo-first-order, pseudo-second-order and intra-particle diffusion models, while the Langmuir, Freundlich and Sips models were used to fit measured adsorption isotherms. Detailed descriptions about these models can be found in the ESI.†
3. Results and discussion
3.1 Characterization of HA-bHAP
Fig. 1 showed the XRD patterns of prepared bHAP, HA-bHAP, and reference pattern of pure HAP (JCPDS no. 09-0432), as well as the non-biogenic HAP, which was synthesized by a chemical precipitation method as described in our previous work.6 The characteristic peaks for HAP at 2θ = 25.8°, 28.7°, 32.1°, 33.6°, 40°, 46.7°, 49.5°, and 53.3° were observed clearly. They related to their corresponding indices (002), (210), (211), (300), (310), (222), (213), and (004), respectively. This revealed that the resultant sample was pure HAP with a hexagonal crystal structure and the modification of the surface of bHAP with HA did not result in the phase change of bare bHAP. However, the bHAP and HA-bHAP exhibited relatively broad peaks and a lower crystallinity compared with non-biogenic HAP, which was probably due to the carbonate incorporation in bHAP.7
 |
| | Fig. 1 The XRD patterns of prepared biogenic hydroxyapatite (bHAP), humic acid-impregnated bHAP (HA-bHAP), non-biogenic HAP and the reference pattern of pure HAP (JCPDS no. 09-0432). | |
SEM images provide the direct information about the size and typical shape of the as-synthesized samples. The morphology of bHAP and HA-bHAP was investigated by SEM and the results suggested that the synthetic bHAP (Fig. 2a) was made up of nearly spheroidal particles of narrow size distribution having an average particle size of 300 nm, and the nanometric primary particles agglomerated tightly into micrometric aggregates of various shape and size. According to the literature,17,18 the synthesis conditions such as dispersant species, solvent systems, drying methods and solution pH had great effects on the nucleation and crystal growth of HAP. It was considered that dispersant species had small effect on the shape of the HAP particles, and well dispersed HAP nanoparticles could be synthesized in water solvent.17 As shown in Fig. 2a, the aggregation of bHAP took place resulting in larger crystals was probably due to the atmospheric drying without any protect measures. Furthermore, previous study indicated that the growth unit of bHAP on the c axis was the coordination anions Ca–P6O24, but on both a and b axes were OH–Ca6.19 Under the present experimental conditions, the initial value of the pH of reaction solvent was about 10.0, which directly affected the crystal growth. Hence the positively charged OH–Ca6 formed quickly, which could be easily adsorbed to a and b axes, because the surface of bHAP nucleus had a negative charge.20 Therefore, the growth velocity on a, b and c axes would be at a same speed, forming spherical bHAP particles. However, the HA-bHAP sample (Fig. 2b) exhibited more agglomeration after HA impregnation and consisted of irregular particles varying in size from nanoparticles to μm particles that appeared to be agglomerates of nanoparticles.
 |
| | Fig. 2 The SEM micrographs of the bare bHAP (a) and HA-bHAP (b). | |
The binding of HA on surface of bHAP was confirmed by FTIR spectroscopy. Fig. 3 illustrated the FTIR spectra of unimpregnated bHAP and HA-bHAP, in the 400–4000 cm−1 wave number range. In both spectra of bHAP and HA-bHAP, there were some uniform peaks of groups. The FTIR spectra of bHAP showcased characteristic peaks for PO43− (473, 565, 603, 962, 1043 and 1092 cm−1), OH− (629 and 3567 cm−1), and adsorbed water (1637 and 3420 cm−1), which indicated that the synthetic sample was pure HAP.21 While bands at 876 cm−1 and around 1420 and 1458 cm−1 were that of CO32− ions which suggested incorporation of carbonate ion in synthesized bHAP. Their appearance could be caused by a high activity of the initial component CaO and the presence of CO2 in the atmosphere during the synthesis process.22 These carbonate derived bands have been mentioned in other literature where bHAP was prepared from eggshell waste,23 and the bands were different from the single band of carbonate, which indicated that CO32− groups existed in bHAP. FTIR spectra of HA-bHAP (Fig. 3) showed the characteristic absorption of the HA at 1602 and 1701 cm−1, which were attributed to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond stretching vibration in carboxylic salt and free carboxylic acid, respectively. Spectroscopic analysis indicated the successful impregnation of bHAP with HA. It was generally believed the binding of HA to bHAP surface was mainly through surface complexation between the oxygen atom of functional groups of HA and calcium ions of bHAP.15
O bond stretching vibration in carboxylic salt and free carboxylic acid, respectively. Spectroscopic analysis indicated the successful impregnation of bHAP with HA. It was generally believed the binding of HA to bHAP surface was mainly through surface complexation between the oxygen atom of functional groups of HA and calcium ions of bHAP.15
 |
| | Fig. 3 The FT-IR spectra of the bare bHAP and HA-bHAP. | |
3.2 Comparison of MB adsorption onto bHA and HA-bHAP
Comparison of the MB adsorption onto bHAP and HA-bHAP (Fig. 4) demonstrated that the HA-bHAP exhibited much higher adsorption capacity for MB than bare bHAP adsorbent. In our previous work,6 it was found that the maximum MB adsorption capacity of synthetic HAP was only 14.27 mg g−1, which was comparable to bHAP. However, the bHAP exhibited a significant advantage over HAP that the needless of the utilization of expensive calcium reagent. In contrast, HA-bHAP exhibited a much higher adsorption ability towards MB than both HAP and bHAP. The difference of the adsorption capacity between the bare bHAP and HA-bHAP illustrated that the HA impregnated on the surface of HA-bHAP and provided the main activated sites for MB adsorption. Similar findings have been reported by Zhang et al.,11 Anirudhan et al.,14 Vinod and Anirudhan,16 and Chen et al.,24 who reported the effective adsorption of MB by HA-coated Fe3O4 nanoparticles, HA immobilized polymer/bentonite composite, HA immobilized pillared clay, and HA-coated magnetic nanoparticles, respectively. Therefore, it was considered that the binding of negatively charged HA on the HA-bHAP surface might be the main reason why the observed adsorption capacity of MB onto HA-bHAP was much higher than that of bare bHAP. For further experiments, the HA impregnated bHAP was selected as adsorbent.
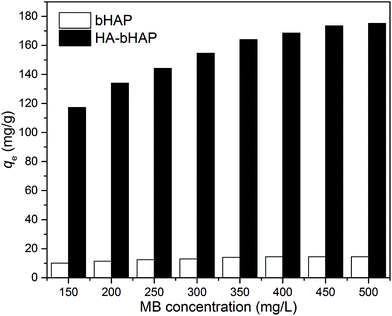 |
| | Fig. 4 Adsorbed amount of HA-bHAP compared with bare bHAP (adsorption conditions: adsorbent dosage = 1.0 g L−1, initial pH 7.0, contact time = 4 h, and temperature = 298 K). | |
3.3 Effect of pH on MB adsorption onto HA-bHAP
The pH of the solution has a significant influence on the adsorption of dyes, since it determines the surface charge of the adsorbent, the degree of ionization and speciation of the adsorbate. In order to establish the effect of pH on the adsorption of MB, the batch equilibrium studies at different pH values were carried out over a pH range of 3.0–10.0. It could be seen from Fig. 5 that when the pH of MB solution was increased from 3.0 to 10.0, the equilibrium adsorption capacity increased from 74.0 to 93.2 mg g−1. A similar result of pH effect was also reported for the adsorption of MB onto HA immobilized pillared clay16 and HA immobilized polymer/bentonite composite.14 The weaker MB adsorption under lower solution pH might be explained by a protonation of the acidic functional groups like carboxylate of HA and competition adsorption between the MB and hydrogen ions.11 It is generally accepted that MB is a very weak base and reacts only in the solutions of strong acids to yield low amounts of protonated cations,25 and it is safe to assume that MB is a positively charged, unprotonated cation, throughout the pH range investigated. At higher solution pH, the HA-bHAP became negatively charged, which enhanced the adsorption of positively charged dye cations through electrostatic forces of attraction. However, a considerable amount of MB was adsorbed by HA-bHAP at lower pH values, which suggested that a pure electrostatic interaction between the negatively charged HA-bHAP and the positively charged MB cannot be the only mechanism of adsorption. A different type of interaction such as hydrophobic interaction and complex adsorption which have been suggested by different authors might account for the adsorption process.11,26 It was worthy to note that the adsorption of MB onto HA-bHAP decreased the final pH (Fig. 5). According to the literature,27 this decrease in pH was mainly due to ion exchange contribution in the adsorption process. After HA impregnation, a large number of oxygen containing functional groups such as carboxylic, carboxylic in lactone-like binding structure, phenolic hydroxyl and carboxylic groups were introduced onto bHAP surface and played an important role in the cationic dye adsorption. It was thus considered that the adsorption of cationic dye released hydrogen ions from the surface functional groups of HA-bHAP by an ion exchange mechanism and resulting in a decrease in solution pH at equilibrium. A similar behaviour was observed for MB adsorption on poorly crystalline HAP,6 HA-immobilized amine modified polyacrylamide/bentonite composite,27 and pine cone biomass of Pinus radiata.28
 |
| | Fig. 5 Influence of initial pH on MB (methylene blue) adsorption onto HA-bHAP (dark line) and relationships between initial pH and final pH values obtained after equilibration of HA-bHAP with MB solution (blue line) (adsorption conditions: adsorbent dosage = 1.0 g L−1, contact time = 4 h, and temperature = 298 K). | |
3.4 Effect of contact time on MB adsorption and adsorption kinetics
The adsorption capacity of HA-bHAP to MB versus contact time was illustrated in Fig. 6 at the initial concentrations of 100, 200, and 500 mg L−1 at 298 K. Results showed that adsorption occurred rapidly in the beginning and slowly reached equilibrium at about 4 h. Thus a 4 h contact time was recommended in all experiments. In order to elucidate the adsorption process and adsorption mechanism, three kinetic models including pseudo-first-order, pseudo-second-order and intra-particle diffusion models were selected to fit the adsorption kinetic experimental data. The kinetics model fitted with the experimental data were presented in Fig. S1 in ESI† and the parameters were summarized in Table 1. The correlation coefficients (R2) of pseudo-second-order model were greater than the pseudo-first-order and intra-particle diffusion models, and the calculated qe agreed much better with the experimental data than that of pseudo-first-order model, which suggested that MB adsorption onto HA-bHAP could be better fitted by the pseudo-second-order kinetic model based on the assumption that the rate determining step might be chemisorption between adsorbent and adsorbate. Pseudo-second-order kinetics of dye adsorption was also observed for several other adsorbents.11,29,30
 |
| | Fig. 6 Effect of contact time and initial dye concentration on adsorption capacity of MB on HA-bHAP (adsorption conditions: adsorbent dosage = 1.0 g L−1, initial pH 7.0, and temperature = 298 K). | |
Table 1 Parameters for different adsorption kinetics models
| Fitting model |
Parameter |
Initial MB concentration (mg L−1) |
| 100 |
200 |
500 |
| Pseudo-first-order model |
k1 (min−1) |
1.85 × 10−2 |
2.19 × 10−2 |
2.83 × 10−2 |
| qe,cal |
36.09 |
80.56 |
90.65 |
| R2 |
0.9460 |
0.9596 |
0.9019 |
| Pseudo-second-order model |
k2 (g (mg min)−1) |
0.1513 |
0.0984 |
0.1750 |
| qe,cal |
87.49 |
135.14 |
181.82 |
| R2 |
0.9984 |
0.9953 |
0.9994 |
| Intra-particle diffusion model |
kp1 (mg (g min1/2)−1) |
6.39 |
12.32 |
12.22 |
| C1 (mg g−1) |
39.13 |
42.63 |
78.07 |
| R2 |
0.8410 |
0.9539 |
0.9803 |
| kp2 (mg (g min1/2)−1) |
2.89 |
4.90 |
6.98 |
| C2 (mg g−1) |
52.25 |
67.68 |
106.52 |
| R2 |
0.9343 |
0.9757 |
0.8686 |
| kp3 (mg (g min1/2)−1) |
0.80 |
1.37 |
0.88 |
| C3 (mg g−1) |
73.82 |
110.63 |
164.38 |
| R2 |
0.9630 |
0.9718 |
0.9155 |
| qe,exp (mg g−1) |
86.65 |
131.72 |
177.80 |
3.5 Effect of ionic strength on MB adsorption
The wastewater containing dyes has commonly higher salt concentration and leads to high ionic strength, which might affect the adsorption of dyes onto adsorbent.31 In order to investigate the effect of inorganic salts on dye adsorption process, the experiments were carried out using 200 mg L−1 initial MB solution containing various NaCl or CaCl2 concentrations ranging from 0 to 0.2 mol L−1. It was observed (Fig. 7) that the adsorption capacity increased slightly from 136.6 to 143.7 mg g−1, as the NaCl concentration increased from 0 to 0.1 mol L−1. Similar results have been reported for the adsorption of MB and other cationic dyes by sepiolite,32 activated carbon,33 hazelnut shell,34 and alkali-activated multiwalled carbon nanotubes.35 The results in Fig. 7 also indicated that a further increase in NaCl concentration from 0.15 to 0.2 mol L−1 resulted in a decrease of adsorption capacity from 139.6 to 119.8 mg g−1. This result was different from those reported by Mak and Chen9 and Yang et al.,36 who found that the adsorption characteristics of MB were insensitive to changes in ionic strength (KCl or NaCl) of solution. But in their investigation, the highest concentration of salts is 0.05 and 0.1 mol L−1, which is quite different from the present experiment. In addition, it could be seen from Fig. 7 that the adsorption capacities decreased with increasing CaCl2 concentration (0–0.2 mol L−1) for the adsorbent, and CaCl2 had significantly more adverse effects than NaCl. The result was consistent with that of Maurya et al.37 and Han et al.,38 who reported that the effect of divalent ions was more prominent than that of monovalent ions for MB adsorption. While the monotonous declining trend of MB adsorption with the increase of ionic strength have also been reported for several adsorbents such as Ashoka leaf powder,31 and hydrogel composite.39
 |
| | Fig. 7 Effects of ionic strength on the adsorption of MB by HA-bHAP (adsorption conditions: adsorbent dosage = 1.0 g L−1, initial pH 7.0, contact time = 4 h, and temperature = 298 K). | |
It was considered that the presence of salt in the solution might have two opposite effects on dyes adsorption. On the one hand, since the salt screened the electrostatic interaction of opposite changes of the adsorbent surface and the dye molecules, the adsorbed amount should decrease with increase of ionic strength.32 The negative effect of CaCl2 on MB adsorption was stronger than NaCl (>0.1 mol L−1) because unit mol divalent Ca2+ contributed more positive charge than unit mol monovalent Na+.38 On the other hand, the salt caused an increase in the degree of dissociation of the dye molecules by facilitating the protonation.40 Thus the adsorption capacity would increase as the dissociated free dye ions for electrostatically binding onto oppositely charged solid surface increased.
3.6 Adsorption isotherms and thermodynamic analyses
Adsorption isotherms can provide some physicochemical information on how the adsorption proceeds and how adsorbate interacts with adsorbent surface. The adsorption isotherms of MB on HA-bHAP at temperatures of 283, 298, and 313 K were shown in Fig. 8. The adsorption of MB on HA-bHAP increased from 124.8 mg g−1 to 149.5 mg g−1 when temperature was increased from 283 to 313 K at an initial concentration of 200 mg L−1. The increase in the adsorption capacity of MB with temperature illustrated that MB adsorption on HA-bHAP favored a high temperature. Adsorption equilibrium data have been tested by using Langmuir (Fig. 8a), Freundlich (Fig. 8b) and Sips (also known as Langmuir–Freundlich, Fig. 8c) isotherms at various temperatures.
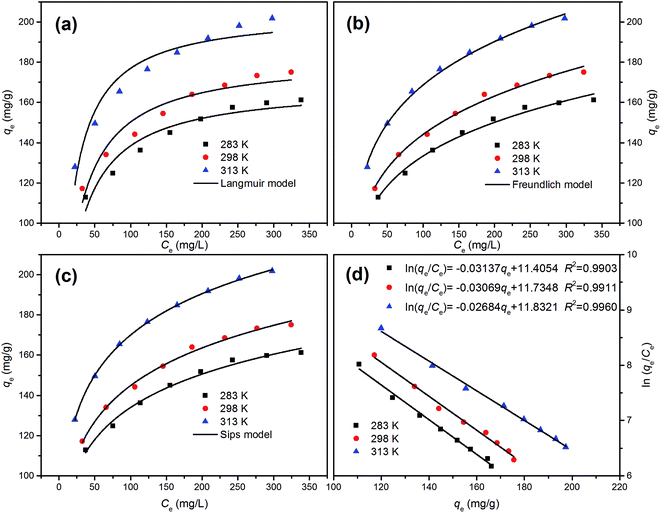 |
| | Fig. 8 Nonlinear Langmuir (a), Freundlich (b) and Sips (c) isotherm models at different temperatures for the adsorption of MB onto HA-bHAP; (d) plots of ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe/Ce vs. qe for the MB adsorption on HA-bHAP at different temperatures (qe: mg g−1, Ce: mg mL−1). qe/Ce vs. qe for the MB adsorption on HA-bHAP at different temperatures (qe: mg g−1, Ce: mg mL−1). | |
Nonlinear Langmuir, Freundlich, and Sips isotherms at different temperatures for the adsorption of MB by HA-bHAP were shown in Fig. 8 and the calculated parameters were summarized in Table 2. According to the results, the experimental data were better fitted by the Freundlich and Sips models (0.9868 < R2 < 0.9995) than the Langmuir isotherm model. The Sips isotherm is a composite of the Langmuir and Freundlich isotherms and can reduce to either at its limits. KS values for Sips isotherm were between 0.00207 and 0.00407, which was very small. In this case, Sips isotherm equation followed the Freundlich isotherm model, indicating a heterogeneous multilayer adsorption process.41 The comparison of the three adsorption models (Fig. 8a–c) also showed the Sips isotherm to be the best with a high similarity with the Freundlich isotherm. The Freundlich model revealed a thermodynamically favorable adsorption of MB onto HA-bHAP as the Freundlich constants 1/n were less than 1. In the present study, adsorption capacity of MB was compared to other adsorbents in terms of MB adsorption. From Table 3, it could be seen that the HA-bHAP used in this study possessed a higher MB adsorption capacity than many other adsorbents reported in the literature. Furthermore, the feasibility of HA-bHAP adsorbent for the adsorption of other cationic dyes such as malachite green and crystal violet, results in Fig. S2 (see ESI†) indicated that HA-bHAP also exhibited excellent performance for these cationic dyes.
Table 2 Parameters of adsorption isotherms of MB on HA-bHAP at different temperatures
| Fitting model |
Parameter |
Temperature (K) |
| 283 |
298 |
313 |
| Langmuir |
qm (mg g−1) |
168.81 |
182.54 |
205.22 |
| KL (L mg−1) |
0.04558 |
0.04661 |
0.06274 |
| R2 |
0.9259 |
0.9322 |
0.9274 |
| Freundlich |
KF (mg g−1 (L mg−1)1/n) |
60.69 |
63.04 |
75.69 |
| 1/n |
0.1709 |
0.1795 |
0.1740 |
| R2 |
0.9868 |
0.9920 |
0.9968 |
| Sips |
qm (mg g−1) |
335.61 |
376.33 |
393.47 |
| KS (L mg−1) |
0.00247 |
0.00207 |
0.00407 |
| 1/n |
0.2926 |
0.2973 |
0.3043 |
| R2 |
0.9875 |
0.9926 |
0.9995 |
Table 3 The comparison of maximum MB adsorption capacities of various adsorbents on the basis of Langmuir isotherm modela
| Adsorbent |
qm (mg g−1) |
Isotherm model |
Equilibrium time (h) |
Adsorbent dosage (g L−1) |
pH |
Temperature (K) |
References |
| * not available, ** obtained from pseudo-second-order model, *** values were converted from μmol g−1. |
| HAP |
0 |
* |
0.5 |
0.2 |
* |
298 |
48 |
| Clay |
6.3 |
Langmuir |
1.0 |
1.0 |
* |
293 |
49 |
| Natural phosphate |
7.232 |
Langmuir |
1.0 |
1.0 |
* |
298 |
50 |
| Activated carbon |
9.813 |
Langmuir |
1.0 |
20 |
7.5 |
303 |
51 |
| Poorly crystalline HAP |
14.27 |
Langmuir |
2 min |
1.0 |
9.0 |
283 |
6 |
| HA based biopolymeric membrane |
20.83 |
Langmuir |
2.0 |
0.3 |
9.0 |
308 |
30 |
| Halloysite nanotubes |
40.82** |
** |
72 |
1.0 |
* |
298 |
52 |
| CMCD-MNP(C) |
77.5 |
Langmuir |
20 min |
0.024–0.026 |
8.0 |
298 |
45 |
| HA-coated Fe3O4 nanoparticles |
93.1 |
Langmuir |
2.0 |
2.0 |
7.0 |
293 |
11 |
| Citric acid modified kenaf core fibres |
131.6 |
Langmuir |
4.0 |
2.0 |
7.0 |
333 |
29 |
| HAP/meso-silica |
134.0 |
Langmuir |
0.5 |
0.2 |
* |
298 |
48 |
| CMCD-MNP(P) |
138.9 |
Langmuir |
50 min |
0.024–0.026 |
8.0 |
298 |
45 |
| HA immobilized PILC |
194.6*** |
Langmuir |
3.0 |
2.0 |
6.0 |
303 |
16 |
| Polyacrylic acid-bound MNP |
199.0 |
Langmuir |
1.0 |
1.0 |
* |
298 |
9 |
| HA coated Fe3O4 nanoparticles |
200.0 |
Langmuir |
24 |
1.0 |
7.0 |
298 |
24 |
| HA immobilized-polymer/bentonite |
207.4*** |
Langmuir |
2.0 |
2.0 |
6.0 |
303 |
14 |
| Fe3O4-embedded GO |
246 |
Langmuir |
5 min |
2.0 |
12 |
293 |
53 |
| HA modified biogenic HAP |
205.22 |
Langmuir |
4.0 |
1.0 |
7.0 |
313 |
This work |
| HA modified biogenic HAP |
393.47**** |
Sips |
4.0 |
1.0 |
7.0 |
313 |
This work |
Furthermore, the thermodynamic parameters were applied to evaluate the orientation and feasibility of MB adsorption by HA-bHAP. The thermodynamic parameters (ΔG0, ΔH0 and ΔS0) of the adsorption of MB were calculated using equations as below:
| |
ΔG0 = −RT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K0 K0
| (3) |
| |
 | (4) |
where Δ
G0 is the standard free energy of adsorption (kJ mol
−1);
R is the universal gas constant (8.314 J (mol K)
−1);
T is the temperature in kelvin;
K0 is the thermodynamic equilibrium constant, which was determined by plotting ln
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe
qe/
Ce vs. qe and extrapolating to zero
qe using a graphic method described by Khan and Singh.
42 Regression straight lines were fitted through the data points by the least-squares method, and their intersections with the vertical axis gave the value of
K0 (
Fig. 8d). Δ
S0 is the standard entropy change (J (mol K)
−1) and Δ
H0 is the standard enthalpy change (kJ mol
−1). The values of Δ
H0 and Δ
S0 could be obtained from the slope and intercept of a plot of ln
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K0
K0 against 1/
T.
All the thermodynamic parameters were listed in Table 4. The positive value of ΔH0 suggested that the adsorption process is endothermic, and the negative ΔG0 values implied the feasibility and spontaneous nature of the adsorption process. Furthermore, it was clearly that the ΔG0 was decreased when the temperature increased from 283 K to 313 K, which suggested the increase in feasibility of adsorption at higher temperatures. Additionally, the positive ΔS0 value indicated an increase in disorder and randomness at the solid/solution interface during the adsorption. Spontaneous, endothermic and entropy-increasing processes have also been reported for the adsorption of MB on citric acid modified kenaf core fibres,29 HA based biopolymeric membrane,30 and magnetic amine/Fe3O4 functionalized biopolymer resin.43
Table 4 Thermodynamic parameters of the MB adsorption on HA-bHAP at different temperatures
| Temperature (K) |
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K0 K0 |
ΔG0 (kJ mol−1) |
ΔH0 (kJ mol−1) |
ΔS0 (J (mol K)−1) |
| 283 |
11.41 |
−26.82 |
10.56 |
132.42 |
| 298 |
11.73 |
−29.06 |
| 313 |
11.83 |
−30.78 |
3.7 Adsorption mechanisms
For understanding of the interaction of HA-bHAP with MB, FTIR data were introduced to gain insight into the adsorption mechanism. Fig. 9 showed the spectra of MB and MB loaded HA-bHAP. The vibration bands of MB matched well with the published data.44 The sharp peak corresponding to the stretching vibration of aromatic ring at 1600 cm−1 was very prominent; two medium sharp peaks at 1490 and 1396 cm−1 could be assigned to C–N stretching vibrations of the dye heterocycle; the characteristic symmetric bending vibrations of CH3 in dimethylamino groups of MB appeared at 1338–1355 cm−1.45 Several weak peaks between 1250, 886 and 669 cm−1 were ascribed to the C–H in plane and out of plane bending vibrations in heterocycle. After MB adsorption, most of these characteristic peaks of MB loaded HA-bHAP remained the same as those of HA-bHAP. However, a shift of the 1602 cm−1 peak (now at 1600 cm−1) in the spectrum for HA-bHAP was observed, and the peak sharpen and showed a significant decrease in intensity, which indicated that the carboxyl groups might be involved into interaction with MB. Two new weak adsorption bands around 1338 and 1396 cm−1 could also be observed and should be assigned to C–N stretching vibrations and the CH3 group vibrations, respectively, reflecting the evidence for the strong interaction between MB and HA-bHAP. MB is a type of cationic dye which could be adsorbed easily by electrostatic forces on negatively charged surfaces. Therefore, the change in carboxyl vibration bands after adsorption might be ascribed to the electrostatic attraction between HA-bHAP and MB.35,46 The strong binding of HA on bHAP surface provided more negatively charged groups responsible for enhanced adsorption of MB.
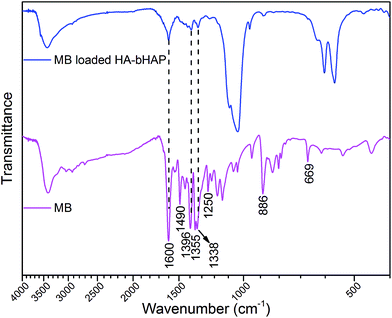 |
| | Fig. 9 FTIR spectra of MB and MB loaded HA-bHAP. | |
3.8 Desorption studies
Regeneration of adsorbent and recovery of adsorbate are important aspects to minimize the waste and reuse of the adsorbent. Desorption results can also be used to investigate the mechanism of an adsorption process. If the dye adsorbed onto the adsorbent can be desorbed by water, it can be concluded that the attachment of the dye onto the adsorbent is by weak bonds. If the strong acids, such as HNO3 and HCl, or strong bases, such as NaOH can desorb the dye, it can be concluded that the attachment of the dye onto the adsorbent is by ion exchange or electrostatic attraction. If organic acids, for example, such as CH3COOH, can desorb the dye, it can be said that the adsorption of the dye onto the adsorbent is by chemisorption.47 In the present work, the low MB adsorption capability for HA-bHAP at low solution pH value implied that MB adsorbed onto HA-bHAP might be desorbed in acidic solution, however, the acidic agents could not be used for desorption of MB from HA-bHAP since solubility of bHAP in aqueous media increased with lowering pH value. In this study, a mixture of 0.5 mol L−1 KCl and 0.5 mol L−1 KH2PO4 was used as desorption agent. Results showed that the desorption efficiency of MB from MB-loaded adsorbent was found to be above 91.8% at the first cycle. The regenerated HA-bHAP sample was reused for four consecutive cycles. Fig. 10 demonstrated the recycling of HA-bHAP in the removal of MB. Only a slight loss in adsorption capacity was observed after four cycles of consecutive adsorption–desorption, indicating that HA-bHAP was highly efficient recyclable adsorbent for the removal of MB.
 |
| | Fig. 10 MB adsorption on virgin and regenerated HA-bHAP adsorbent with four adsorption–regeneration cycles (initial HA concentration = 100 mg L−1, temperature = 298 K). | |
4. Conclusions
The present study provides new insights into the potential for using HA, a common component of dissolved organic matter, to enhance MB adsorption by biogenic HAP derived from eggshell waste. The higher adsorption capacity towards MB of HA-bHAP than bare bHAP was attributed to the electrostatic attraction between cationic dye and negatively charged adsorbent. The adsorption reaction was best described by Sips isotherm and pseudo-second-order kinetic models. The HA-bHAP showed a high capacity for recycling through a simple regeneration procedure. Results indicated that HA impregnation to biogenic HAP might offer an efficient and low-cost approach for MB removal from wastewater.
Acknowledgements
This work is supported by the National Natural Science Foundation of China (No. 41303081 and 41373111), the Natural Science Foundation of Jiangsu Province (No. SBK2016020293), the Research Fund for the Doctoral Program of Higher Education (No. 20113207110014 and 20133207120019), the Foundation for Talent Recommendation Program of Nanjing Normal University (No. 2009103XGQ0063 and 2011105XGQ0247), and PAPD (a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions, No. 164320H116).
References
- J. M. Herrmann, M. Vautier and C. Guillard, J. Catal., 2001, 201, 46–59 CrossRef.
- M. A. Brown and S. C. de Vito, Environ. Sci. Technol., 1993, 23, 249–324 CrossRef CAS.
- P. Pandey, R. P. Singh, K. N. Singh and P. Manisankar, Environ. Sci. Pollut. Res., 2013, 20, 238–249 CrossRef CAS PubMed.
- H. Zhou and J. Lee, Acta Biomater., 2011, 7, 2769–2781 CrossRef CAS PubMed.
- V. C. Nguyen and Q. H. Pho, Sci. World J., 2014, 273082 Search PubMed.
- W. Wei, L. Yang, W. H. Zhong, S. Y. Li, J. Cui and Z. G. Wei, Dig. J. Nanomater. Bios., 2015, 10, 1343–1363 Search PubMed.
- D. S. R. Krishna, A. Siddharthan, S. K. Seshadri and T. S. Kumar, J. Mater. Sci.: Mater. Med., 2007, 18, 1735–1743 CrossRef CAS PubMed.
- S. Meski, S. Ziani and H. Khireddine, J. Chem. Eng. Data, 2010, 55, 3923–3928 CrossRef CAS.
- S. Y. Mak and D. H. Chen, Dyes Pigm., 2004, 61, 93–98 CrossRef CAS.
- L. Peng, P. Qin, M. Lei, Q. Zeng, H. Song, J. Yang, J. Shao, B. Liao and J. Gu, J. Hazard. Mater., 2012, 209, 193–198 CrossRef PubMed.
- X. Zhang, P. Zhang, Z. Wu, L. Zhang, G. Zeng and C. Zhou, Colloids Surf., A, 2013, 435, 85–90 CrossRef CAS.
- L. Yang, Z. Wei, W. Zhong, J. Cui and W. Wei, Colloids Surf., A, 2016, 490, 9–21 CrossRef CAS.
- A. A. El-Zahhar and N. S. Awwad, J. Environ. Chem. Eng., 2016, 4, 633–638 CrossRef CAS.
- T. S. Anirudhan, P. S. Suchithra and P. G. Radhakrishnan, Appl. Clay Sci., 2009, 43, 336–342 CrossRef CAS.
- W. Wei, L. Yang, W. Zhong, J. Cui and Z. Wei, Dig. J. Nanomater. Bios., 2015, 10, 663–680 Search PubMed.
- V. P. Vinod and T. S. Anirudhan, Water, Air, Soil Pollut., 2003, 150, 193–217 CrossRef CAS.
- P. Wang, C. Li, H. Gong, X. Jiang, H. Wang and K. Li, Powder Technol., 2010, 203, 315–321 CrossRef CAS.
- I. Smičiklas, A. Onjia and S. Raičević, Sep. Purif. Technol., 2005, 44, 97–102 CrossRef.
- J. Zhang, D. Jiang, J. Zhang, Q. Lin and Z. Huang, Ceram. Int., 2010, 36, 2441–2447 CrossRef CAS.
- Y. Y. Zhang and Y. S. Dong, Adv. Mater. Res., 2014, 912, 119–122 CrossRef.
- G. Y. Liu, J. Hu, Z. K. Ding and C. Wang, Mater. Chem. Phys., 2011, 130, 1118–1124 CrossRef CAS.
- D. L. Goloshchapov, V. M. Kashkarov, N. A. Rumyantseva, P. V. Seredin, A. S. Lenshin, B. L. Agapov and E. P. Domashevskaya, Ceram. Int., 2013, 39, 4539–4549 CrossRef CAS.
- D. Liao, W. Zheng, X. Li, Q. Yang, X. Yue, L. Guo and G. Zeng, J. Hazard. Mater., 2010, 177, 126–130 CrossRef CAS PubMed.
- R. P. Chen, Y. L. Zhang, X. Y. Wang, C. Y. Zhu, A. J. Ma and W. M. Jiang, Desalin. Water Treat., 2015, 55, 539–548 CrossRef CAS.
- A. Czímerová, L. Jankovič and J. Bujdák, J. Colloid Interface Sci., 2004, 274, 126–132 CrossRef PubMed.
- G. F. Malash and M. I. El-Khaiary, J. Colloid Interface Sci., 2010, 348, 537–545 CrossRef CAS PubMed.
- T. S. Anirudhan and P. S. Suchithra, J. Environ. Sci., 2009, 21, 884–891 CrossRef CAS.
- T. K. Sen, S. Afroze and H. M. Ang, Water, Air, Soil Pollut., 2011, 218, 499–515 CrossRef CAS.
- M. S. Sajab, C. H. Chia, S. Zakaria, S. M. Jani, M. K. Ayob, K. L. Chee, P. S. Khiew and W. S. Chiu, Bioresour. Technol., 2011, 102, 7237–7243 CrossRef CAS PubMed.
- S. S. Shenvi, A. M. Isloor, A. F. Ismail, S. J. Shilton and A. Al Ahmed, Ind. Eng. Chem. Res., 2015, 54, 4965–4975 CrossRef CAS.
- N. Gupta, A. K. Kushwaha and M. C. Chattopadhyaya, J. Taiwan Inst. Chem. Eng., 2012, 43, 604–613 CrossRef CAS.
- M. Doğan, Y. Özdemir and M. Alkan, Dyes Pigm., 2007, 75, 701–713 CrossRef.
- Y. S. Al-Degs, M. I. El-Barghouthi, A. H. El-Sheikh and G. M. Walker, Dyes Pigm., 2008, 77, 16–23 CrossRef CAS.
- M. Doğan, H. Abak and M. Alkan, J. Hazard. Mater., 2009, 164, 172–181 CrossRef PubMed.
- J. Ma, F. Yu, L. Zhou, L. Jin, M. Yang, J. Luan, Y. Tang, H. Fan, Z. Yuan and J. Chen, ACS Appl. Mater. Interfaces, 2012, 4, 5749–5760 CAS.
- S. T. Yang, S. Chen, Y. Chang, A. Cao, Y. Liu and H. Wang, J. Colloid Interface Sci., 2011, 359, 24–29 CrossRef CAS PubMed.
- N. S. Maurya, A. K. Mittal, P. Cornel and E. Rother, Bioresour. Technol., 2006, 97, 512–521 CrossRef CAS PubMed.
- X. Han, W. Wang and X. Ma, Chem. Eng. J., 2011, 171, 1–8 CrossRef CAS.
- Y. Liu, Y. Zheng and A. Wang, J. Environ. Sci., 2010, 22, 486–493 CrossRef CAS.
- F. Blockhaus, J. M. Séquaris, H. D. Narres and M. J. Schwuger, J. Colloid Interface Sci., 1997, 186, 234–247 CrossRef CAS PubMed.
- L. Jin, Q. Sun, Q. Xu and Y. Xu, Bioresour. Technol., 2015, 197, 348–355 CrossRef CAS PubMed.
- A. A. Khan and R. P. Singh, Colloids Surf., 1987, 24, 33–42 CrossRef CAS.
- W. Song, B. Gao, X. Xu, L. Xing, S. Han, P. Duan, W. C. Song and R. Jia, Bioresour. Technol., 2016, 210, 123–130 CrossRef CAS PubMed.
- E. S. Dragan and D. F. A. Loghin, Chem. Eng. J., 2013, 234, 211–222 CrossRef CAS.
- A. Z. M. Badruddoza, G. S. S. Hazel, K. Hidajat and M. S. Uddin, Colloids Surf., A, 2010, 367, 85–95 CrossRef CAS.
- L. Ai, C. Zhang, F. Liao, Y. Wang, M. Li, L. Meng and J. Jiang, J. Hazard. Mater., 2011, 198, 282–290 CrossRef CAS PubMed.
- I. D. Mall, V. C. Srivastava, G. V. A. Kumar and I. M. Mishra, Colloids Surf., A, 2006, 278, 175–187 CrossRef CAS.
- C. Li, X. Ge, S. Liu and F. Liu, Adv. Mater. Res., 2012, 463, 543–547 Search PubMed.
- A. Gürses, S. Karaca, C. Doğar, R. Bayrak, M. Açıkyıldız and M. Yalçın, J. Colloid Interface Sci., 2004, 269, 310–314 CrossRef.
- N. Barka, A. Assabbane, A. Nounah, L. Laanab and Y. A. Ichou, Desalination, 2009, 235, 264–275 CrossRef CAS.
- V. B. Rao and S. R. M. Rao, Chem. Eng. J., 2006, 116, 77–84 CrossRef CAS.
- M. Zhao and P. Liu, Microporous Mesoporous Mater., 2008, 112, 419–424 CrossRef CAS.
- C. J. Zhou, W. J. Zhang, H. X. Wang, H. Y. Li, J. Zhou, S. H. Wang, J. Y. Liu, J. Luo, B. S. Zou and J. D. Zhou, Arabian J. Sci. Eng., 2014, 39, 6679–6685 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra08715a |
|
| This journal is © The Royal Society of Chemistry 2016 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 × g for 30 min. Then the product was washed twice with distilled water and twice with ethanol and dried at 80 °C with a vacuum dryer for 24 h to obtain fine white powder which was subsequently sintered at 100 °C in an oven for 6 h to finally produce bHAP material. This powdered sample was then used for the preparation of HA impregnated bHAP (HA-bHAP). HA was impregnated onto bHAP at pH 5.0 using the batch adsorption techniques reported by our previous work.15 Briefly, the initial pH of HA solution (pH 5.0, 2000 mg L−1) was adjusted and 2.0 g of bHAP was added in the HA solution and shaken at 200 rpm for 24 h. After attaining equilibrium, the suspension was centrifuged and the residue was washed with 0.01 mol L−1 NaClO4 solution followed by deionized water. The obtained HA-bHAP sample was then freeze-dried, ground, and stored for future experiments. The amount of unadsorbed HA was determined using UV-visible spectrophotometer (UV-2450, Shimadzu, Japan) at a wavelength of 254 nm. The amount of HA loaded onto bHAP was calculated and found to be 118.7 mg g−1.
000 × g for 30 min. Then the product was washed twice with distilled water and twice with ethanol and dried at 80 °C with a vacuum dryer for 24 h to obtain fine white powder which was subsequently sintered at 100 °C in an oven for 6 h to finally produce bHAP material. This powdered sample was then used for the preparation of HA impregnated bHAP (HA-bHAP). HA was impregnated onto bHAP at pH 5.0 using the batch adsorption techniques reported by our previous work.15 Briefly, the initial pH of HA solution (pH 5.0, 2000 mg L−1) was adjusted and 2.0 g of bHAP was added in the HA solution and shaken at 200 rpm for 24 h. After attaining equilibrium, the suspension was centrifuged and the residue was washed with 0.01 mol L−1 NaClO4 solution followed by deionized water. The obtained HA-bHAP sample was then freeze-dried, ground, and stored for future experiments. The amount of unadsorbed HA was determined using UV-visible spectrophotometer (UV-2450, Shimadzu, Japan) at a wavelength of 254 nm. The amount of HA loaded onto bHAP was calculated and found to be 118.7 mg g−1.



![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond stretching vibration in carboxylic salt and free carboxylic acid, respectively. Spectroscopic analysis indicated the successful impregnation of bHAP with HA. It was generally believed the binding of HA to bHAP surface was mainly through surface complexation between the oxygen atom of functional groups of HA and calcium ions of bHAP.15
O bond stretching vibration in carboxylic salt and free carboxylic acid, respectively. Spectroscopic analysis indicated the successful impregnation of bHAP with HA. It was generally believed the binding of HA to bHAP surface was mainly through surface complexation between the oxygen atom of functional groups of HA and calcium ions of bHAP.15


![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K0
K0

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe/Ce vs. qe and extrapolating to zero qe using a graphic method described by Khan and Singh.42 Regression straight lines were fitted through the data points by the least-squares method, and their intersections with the vertical axis gave the value of K0 (Fig. 8d). ΔS0 is the standard entropy change (J (mol K)−1) and ΔH0 is the standard enthalpy change (kJ mol−1). The values of ΔH0 and ΔS0 could be obtained from the slope and intercept of a plot of ln
qe/Ce vs. qe and extrapolating to zero qe using a graphic method described by Khan and Singh.42 Regression straight lines were fitted through the data points by the least-squares method, and their intersections with the vertical axis gave the value of K0 (Fig. 8d). ΔS0 is the standard entropy change (J (mol K)−1) and ΔH0 is the standard enthalpy change (kJ mol−1). The values of ΔH0 and ΔS0 could be obtained from the slope and intercept of a plot of ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K0 against 1/T.
K0 against 1/T.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K0
K0





