Fabrication of recyclable superhydrophobic materials with self-cleaning and mechanically durable properties on various substrates by quartz sand and polyvinylchloride
Mengnan Qu *,
Shanshan Liu,
Jinmei He,
Juan Feng,
Yali Yao,
Lingang Hou,
Xuerui Ma and
Xiangrong Liu
*,
Shanshan Liu,
Jinmei He,
Juan Feng,
Yali Yao,
Lingang Hou,
Xuerui Ma and
Xiangrong Liu
College of Chemistry and Chemical Engineering, Xi'an University of Science and Technology, Xi'an 710054, China. E-mail: mnanqu@gmail.com
First published on 16th August 2016
Abstract
The recyclable superhydrophobic materials are successfully prepared by employing surface-functionalized quartz sand particles embedded into polyvinylchloride. The as-prepared superhydrophobic materials not only exhibited normal superhydrophobicity but also can retain excellent chemical stability and fascinating mechanical durability after many rigorous tests. These materials can pass the 5H pencil hardness test and maintain good superhydrophobicity even after 500 cm abrasion by a mechanical reciprocating abrasion test loaded of 500 g. Importantly, the debris that is scraped from the superhydrophobic materials can be recycled and easily reused to prepare the superhydrophobic materials. This method is suitable for large-scale production because it uses inexpensive and environmentally friendly materials and gets rids of sophisticated equipment, special atmospheres and harsh operation conditions. It's meaningful to a wide range of future applications in industry and real life.
Introduction
When the wettability of a solid surface displays water contact angles (WCA) above 150° and slide angle (SA) less than 10°, this is referred to as a superhydrophobic surface. Superhydrophobic surfaces inspired by the lotus,1–3 salvinia,4,5 water-strider legs,6,7 rose petals,8,9 and gecko's feet10 have attracted tremendous scientific interest due to their excellent properties of anti-icing,11 self-cleaning,12–14 anti-fogging,15 corrosion resistance16,17 and so on. The key element to construct a superhydrophobic surface is the combination of suitable micro- and nano-structures and low-surface-energy materials.18–22 Up to now, many researchers have successfully constructed superhydrophobic surfaces by chemical vapor deposition,23 chemical etching,24 sol–gel technique,25 solution-immersion method,26 spray coating27 and laser fabrication.28 The phenomenon of superhydrophobicity has been explained by two renowned theories proposed by Wenzel29 and Cassie30 independently.Most superhydrophobic surfaces exhibit excellent performance and promise a wide range of potential applications. However, the micro/nano hierarchical structure is vulnerable to damage when the superhydrophobic material is squeezed and it is difficult to maintain a good superhydrophobicity, which will greatly restrict the practical application of superhydrophobic materials. In recent years, many scholars have found that methods to achieve wear-resisting superhydrophobicity can be classified into several categories: (a) building a micro/nano hierarchical rough structure,31 (b) using a mechanical durability material,32–34 (c) choosing a lubricating material of wear-resistance.35 Lyons et al.36 fabricated superhydrophobic polymer surfaces with excellent abrasion resistance by a simple lamination templating method. Surfaces remained superhydrophobic after more than 5500 abrasion cycles at a pressure of 32.0 kPa. Xue et al.37 fabricated the washable and wear-resistant superhydrophobic poly(ethylene terephthalate) (PET) textile surfaces with a self-cleaning property by treating the microscale fibres with alkali followed by coating with polydimethylsiloxane (PDMS). Importantly, the textiles maintained superhydrophobicity even when the textiles are ruptured by severe abrasion. Zhou et al.38 reported that a cross-linked elastomeric thin film possessing a nanocomposite structure with a rough and low free-energy surface can endow fabrics with highly durable superhydrophobicity.
Among polymers, polyvinylchloride (PVC) is one of the most versatile materials and is widely used in different applications because of its good mechanical property and high resistance against corrosive chemicals.39 The intrinsic PVC can be declared to be hydrophobic at most. To the best of our knowledge, no studies have been reported on how to fabricate mechanically durable and stable superhydrophobic materials with PVC on various substrates using a facile and low-cost method.
In our study, by employing diethoxydimethylsilane (DDS) surface-functionalized quartz sand nanoparticles embedded into a polymer of PVC, we are able to construct solid superhydrophobic surfaces on various substrates (including glass slides, cardboards, or plastic pieces) without any additional hydrophobic capping layer. The obtained composite materials can pass the 5H pencil hardness test and exhibit good superhydrophobicity, excellent chemical stability and fascinating mechanical durability after many rigorous tests. Additionally, the fabricated materials possess great self-cleaning and recycling properties, which is meaningful to a wider range of future applications in industry and real life.
Results and discussion
PVC is a promising candidate for the preparation of durable superhydrophobic materials due to its excellent solvent and acid/alkali resistance, outstanding stability and mechanical durability. Scheme 1 schematically shows the fabrication process of the superhydrophobic materials.40–42 Firstly, DDS hydrolysed and the SiOC2H5 group transformed into Si–OH. Then the surface modified quartz sand particles were obtained after the Si–OH groups reacted with the hydroxyl groups of quartz sand particles. After these modified quartz sand particles were embedded into PVC, a composite material with proper hierarchical roughness was prepared. The inner structures would expose and the new rough structures were regenerated after the surface suffered an abrasion. The surfaces of the as-prepared material on various substrates have the static water contact angles of 156 ± 1°, as is shown in Fig. 1a–c. The water droplets already cannot stay on as-prepared material surface and easily roll down the sample surface when there is no apparent tilt of the surfaces. The water droplets exhibited a perfect spherical shape on the surface and were able to easily roll off the surface when the as-prepared material surface was rolled up (Fig. 1c). The result demonstrates that the as-prepared superhydrophobic material has not only good water repellency but also good flexibility. Fig. 1d is a free-standing superhydrophobic material which was prepared by pouring the final mixture into a mold and removal of the mold. It demonstrates that the superhydrophobic materials with different shape can be easily obtained or varied by this method. The as-prepared material can be regarded as a real superhydrophobic “material”, not just the usual superhydrophobic “surface”. Also it should be noted that superhydrophobic surfaces were fabricated in this work just by applying DDS and PVC to the quartz sand particles without using any fluorochemicals, which was different from the previous report.38 | ||
| Scheme 1 Schematic illustration of the preparation of the durable superhydrophobic materials by employing surface-functionalized quartz sand particles embedded into polyvinylchloride. | ||
To further investigate the mechanical durability of the as-prepared surface, an abrasion test which is similar to Lu et al.43 conducted was carried out. The process of abrasion test was illustrated in Fig. 2. The as-prepared material, bearing a total weight of 500 g, was driven to move on 600 # sandpaper with a speed of 3 cm s−1 and had sufficient contact with the abrasion surface. The contact area was 20 mm × 20 mm. After abrasion, the material surface was cleaned by water and blown with air. The variation in the values of both the WCA and SA on the as-prepared surface after an increasing length of abrasion is shown in Fig. 3a, where it can be seen, after 500 cm of abrasion, the WCA of the as-prepared surface decreases to 155° and the WCAs and SAs value undergoes a slight change with the number of abrasion length. While 500 cm abrasion length made the sample worn out, the surfaces still remained superhydrophobicity, as was shown in Fig. 3b, indicating excellent durability against abrasion.
 | ||
| Fig. 2 Schematic illustration of the abrasion test for the prepared surface at 500 g loading on sandpaper (600 #). | ||
As mentioned previously, the vulnerability of the micro/nano structure is the key factor which greatly hindered the real application of superhydrophobic surface. Thus the samples were further characterized by pencil hardness and water contact angle. Typical nanoparticle derived films44 exhibit at contact angles above 150° however exhibit very low pencil hardness, typically 8B. Fig. 4a shows the changes in apparent hardness and water contact angle as a function of PVC content in 10 mL THF from 0.0 g to 0.6 g in the as-prepared superhydrophobic materials. With the increasing of PVC content, the hardness of the as-prepared superhydrophobic materials gradually enhanced and WCAs remained above 155°. While the PVC content was more than 0.5 g in 10 mL THF, the hardness of the as-prepared superhydrophobic materials was upto 5H and no changed, but WCAs dropped to 148°. Fig. 4b showed the monotonic tensile stress–strain testing data of the as-prepared material.45,46 The mechanical test was performed with 3 specimens in order to obtain highly convincing statistical results. As shown in Fig. 4b, the yielding stress, ultimate tensile stress and elongation at break of the as-prepared material were 11.27 MPa, 3.2 MPa and 6.2%, respectively. At very low strains, the stress increases linearly with the strain. This is followed by an anelastic regime, then yielding, and finally a decrease in the stress (strain softening). The experiment shows the expected qualitative features which are greater mechanical strength and tenacity.
 | ||
| Fig. 4 (a) Variation in hardness and water contact angle of the as-prepared material surface with content of PVC in 10 mL THF. (b) Strain–stress curve of the as-prepared material. | ||
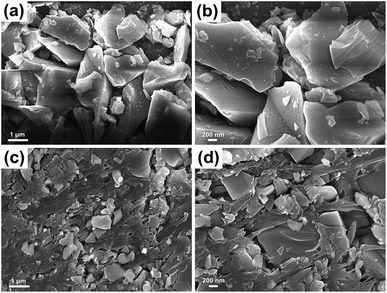
In contrast to most superhydrophobic surfaces, the liquid repellency in this study exhibits high tolerance against mechanical damage, i.e., damage does not deteriorate the superhydrophobicity. It suggests that this is caused by the self-similar micro/nano framework structure that essentially preserves the multivalued topography even upon abrasion. SEM micrographs show that the as-prepared materials surface contains micrometer scale roughness features which have an additional nanoscale roughness from the DDS nanoparticles (Fig. 5). Fig. 5a and b show the quartz sand particles and DDS uniformly dispersed throughout the surface layer forming a binary complex micro/nano structure, which is necessary for superhydrophobicity. Fig. 5c and d are the images of the quartz sand particles added PVC. Compared with Fig. 5a and b, the main difference is that there are remarkable highly porous networks. Fig. 5c and d showed that PVC is cross-linked into a network structure and pore size of the mesh structure is very uneven, since PVC is a polymer containing a small amount of amorphous crystalline structure. Different sizes and shapes of sheet-like quartz sand particles are dispersedly embedded in network of PVC. Even the accumulation of sheet-like quartz sand particles are observed in some larger holes. With the inevitable presence of van der Waals force between molecules and the embedded structure, micro/nanoparticles of quartz sand and silane are connected together closely to form a stable porous mesh structure by PVC polymer. The resulting nano/microscale hierarchical roughness and porous networks are well known to enhance hydrophobicity and durability.
Further evidence of the roughness is provided by TEM images of the quartz sand particles modified by DDS and added PVC (Fig. 6). Fig. 6a reveals there are the quartz sand particles which have the diameter of about several micrometers. Fig. 6a also shows there are some irregular nanoscale particles attached to this larger quartz sand particle. Fig. 6b reveals that there are also quartz sand particles have the diameters of about several hundred nanometers. Both the different particle sizes and the rough surface contribute to the construction of hierarchical structures, potentially enhancing hydrophobicity of the durable superhydrophobic materials.
 | ||
| Fig. 6 TEM images at different magnifications of the quartz sand particles modified by DDS and added PVC. The scale bars represent: (a) 200 nm and (b) 100 nm. | ||
FTIR spectrum of the unmodified quartz sand particles (Fig. 7a), the quartz sand particles modified by DDS (Fig. 7b) and the quartz sand particles modified by DDS and added PVC (Fig. 7c) were shown in Fig. 7. Several characteristic absorption peaks were observed in the range 400–4000 cm−1. For the quartz sand particles modified by DDS and added PVC, peaks at 2920 cm−1 and 2853 cm−1 were attributed to the asymmetric and symmetric CH2 stretching vibrations, respectively.47 The peak at 1430 cm−1 was assigned to the CH2 deformation vibration and the peak at ∼1100 cm−1 was assigned to the Si–O–Si stretching vibration. The peaks observed at 617 cm−1 are due to the C–Cl bond. These results indicated that DDS and PVC have been successfully grafted onto the surface of quartz sand particles.
 | ||
| Fig. 7 FTIR spectrum of the unmodified quartz sand particles (a), the quartz sand particles modified by DDS (b) and the quartz sand particles modified by DDS and added PVC (c). | ||
Fig. 8 shows the XPS spectra of the pure quartz sand particles (Fig. 8a), the quartz sand particles modified by DDS (Fig. 8b) and the as-prepared superhydrophobic material (Fig. 8c). Fig. 8a shows only the elements of C, O and Si were observed in this case. After the particles modified by DDS (Fig. 8b), there is an increase in the oxygen, silicon and carbon peaks than that in Fig. 8a, which belonged to characteristic components of DDS. Compared with the quartz sand particles without PVC treatment, Fig. 8c reveals that two obvious peaks of Cl 2s, Cl 2p appeared after added PVC. This is further affirmed that the DDS and PVC are well bound to the quartz sand particles.
 | ||
| Fig. 8 XPS spectra of the pure quartz sand particles (a), the quartz sand particles modified by DDS (b) and the as-prepared superhydrophobic material (c). | ||
The durable superhydrophobic surfaces may contact to various environments and for a long time in daily practical uses. As is shown in Fig. 9a, variations of the contact angles and slide angles for the superhydrophobic surface as functions of pH value of water droplet. The pH value of the water droplet was adjusted by aqueous HCl and aqueous NaOH. It is clear that the as-prepared surface retains superhydrophobicity in pH values ranging from 1.0 to 13.0 because of their contact angles greater than 153° and slide angles lower than 10°. It suggests that the current superhydrophobic material is durable to the attack of strong acid and strong alkali environments. To assess the robustness against a pressure-induced wetting, an immersion test was conducted. The durable superhydrophobic surface acts like a sliver mirror when viewed at a glancing angle, owing to the total light reflection caused by the trapped air pockets in the rough interstices. This trapped air can effectively prevent a wetting on the durable superhydrophobic surface underwater.30 Fig. 9b displays the change of the water contact angles and slide angles of the durable superhydrophobic surface as a function of the immersion time in water. The surface was almost dry to the touch, but the WCA was slightly decreased to 150° from 156° and SA was 6° increased to 8° as the superhydrophobic materials was immersed in water for 20 days. After continuous immersion in water more than 20 days, the surface lost superhydrophobicity and water droplets on the material surface didn't roll down while the surface was vertical. After removal from the water, the surface was completely wetting; conversely, after drying at 60 °C for 1 h and rubbing with the 600 # sandpaper for 20 cm, the superhydrophobicity was restored and the WCA was increased greatly to 156°.
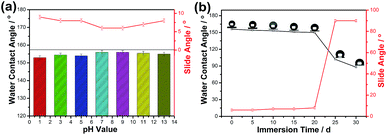 | ||
| Fig. 9 (a) Influence of pH values of water droplets and (b) influence of immersion time in water, respectively, on water contact angles and slide angles of the superhydrophobic surfaces. | ||
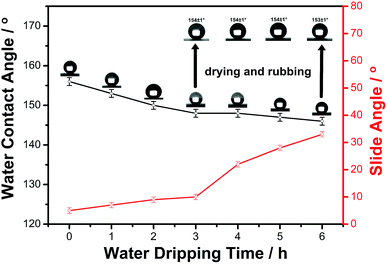
The water-impacting test was conducted under the reported procedure.48 Fig. 10 showed the change in water contact angles and slide angles of the as-prepared surface during the water dripping test. As can be seen, the surface remained superhydrophobic after 1 h of water dripping (contact angle ∼153°), and after 2 h (∼7200 water droplets) of impacting, the contact angle of the surface decreases slightly below the superhydrophobic region and the slide angle increased to 33° after 6 h of impacting. However, after drying at 60 °C for 1 h and rubbing with the 600 # sandpaper for 20 cm, the superhydrophobicity of the as-prepared surface was recovered and the WCA was increased greatly to 154°, which further proved the outstanding mechanical stability of as-prepared superhydrophobic material.
In many applications, surfaces were exposed to extreme temperatures, and thus the stability and durability of the superhydrophobic materials were assessed. It was observed that the contact angle and slide angle of the as-prepared surface can be measured by test temperature. As can be seen in Table 1, the sample was investigated at various temperatures ranging from −10 to 200 °C for 2 h periods, and after recovering to the room temperature for 20 min, it exhibited no discernible change in its hydrophobicity behavior. Upon increasing the annealing temperature to 250 °C, the as-prepared material converted from superhydrophobic to superhydrophilic. These results prove that it is possible to tune hydrophobicity of the prepared materials by applying appropriate temperature and duration, and the high level of stability and durability.
| Temperature (°C) | −10 | 0 | 100 | 150 | 200 | 250 |
| Water contact angle (°) | 153 ± 1 | 155 ± 1 | 156 ± 1 | 154 ± 1 | 153 ± 1 | 0 |
| Slide angle (°) | 8 ± 1 | 8 ± 1 | 6 ± 1 | 7 ± 1 | 9 ± 1 | — |
The superhydrophobic property of the present surface is comparable to traditional superhydrophobic surfaces, even if the prepared material could provide a new route to a self-cleaning ability. As is shown in Fig. 11, the self-cleaning effect of the as-prepared surface is also investigated by applying hydrophilic methylene blue powder as contaminants to the surface. A hanging water drop of nearly 10 μL was produced from a syringe needle and slowly dragged on the dust-accumulated area. The water drops effectively to collect the dust particles and the dusted surface became clean while rolling on the superhydrophobic surface. Therefore, it can be concluded that the superhydrophobic surface can protect materials from pollution in practical applications.
 | ||
| Fig. 11 Self-cleaning ability of the surfaces simply investigated. The self-cleaning performance of the as-prepared surface was tested by hydrophilic dust (methylene blue powders). | ||
Environmental concerns and making full use of raw materials are attracting more and more attentions. A recyclable superhydrophobic material would sure reduce or even overcome certain potential problems caused by the normal low surface energy materials.49–51 Therefore we applied a method that was conducted in the previous reports52 to investigate the recyclability of the as-prepared superhydrophobic materials that have been coated on substrates. Fig. 12 demonstrates the recycling process. The debris that scraped from the coating by a chisel was collected and put into the solvent of THF; the resultant suspension was stirred at ambient temperature for 1 h and then returned to the initial liquid-formation of coating. The coating suspension was then drop-coating onto a substrate (including glass slides, cardboards, or plastic pieces) and dried at 80 °C for 2 h, to remake the superhydrophobic material. Then the superhydrophobic material was reconstructed, the water droplet stayed spherical (contact angle ∼ 156°) on recycled materials surface and rolled off easily, indicating that the low energy materials could be recycled and reused.
Conclusion
In summary, we successfully developed a versatile strategy to generate robust superhydrophobic and simultaneously durable materials with great self-cleaning and recycling properties on various substrates. By employing DDS surface-functionalized quartz sand nanoparticles embedded into a polymer of PVC, we were able to construct solid superhydrophobic materials having both appropriate roughness and low surface energy but without any additional hydrophobic capping layer. The materials maintained superhydrophobic property after more than 500 cm length by a standard mechanical reciprocating abrasion loaded of 500 g. Additionally, we demonstrated the materials can endure the 5H pencil hardness test. Further systematic analyses on their functions and applications were conducted involving the wettability, high hardness, corrosion resistance, droplet impact, self-cleaning and recycling, etc. It is found that the materials can retain excellent chemical stability and fascinating mechanical durability after many rigorous tests. These materials are of interest to academic research but may also have important industrial applications.Experimental
Materials
Quartz sand particles were purchased from a local store. And before used, they were dried at 120 °C for 5 h to remove water. The DDS was purchased from Sigma-Aldrich. The PVC was purchased from Shaanxi North Yuan Chemical Group Co., Ltd. Ethanol and tetrahydrofuran (THF) were analytical and used as received without any purification. And deionized water was used in all process.Fabrication of the durable superhydrophobic materials
5.0 g quartz sand particles were ultrasonically dispersed in the mixture solution of 8.0 mL deionized water and 2.0 mL DDS for 20 min. The as-prepared mixture was then reacted at 60 °C of water bath for 2 h under magnetic stirring. The particles were collected by suction filtration using a Buchner funnel to get the modified quartz sand particles. After that, they were dried in an oven at 120 °C for 2 h.0.5 g PVC was dissolved in 10 mL of THF at room temperature, and the mixture was marked as PVC-THF. 0.5 g the modified quartz sand particles were subsequently ultrasonically dispersed in 3 mL PVC-THF for 20 min. Then the mixture was heated in a water bath at 40 °C with magnetic stirring reaction for 6 h. When the temperature of the reaction system returned to room temperature, the mixture was carefully dispensed onto substrates, including glass slides, cardboards, or plastic pieces. After the addition, the samples were covered with a suitably sized, so that the coating to dry slowly at ambient temperature, to prevent excessive evaporation of the solvent. Finally, the samples were cured at 80 °C for 2 h. After the samples cooling to room temperature, the surfaces were rubbed with 600 grit sandpaper. In the subsequent analysis and testing, glass slides were selected as substrates since it is simple and versatile.
Characterization
The chemical compositions of the materials were examined by Fourier-transform infrared spectrophotometer (FTIR, Perkin Elmer FTIR System 2000) and X-ray photoelectron spectrometer (XPS, K-Alpha of Thermo Electron Corporation). The microstructures of the materials were observed by scanning electron microscopy (SEM, JEOL JSM-6460LV) operated at 15 kV. Before the SEM investigations, all the samples were coated with gold cluster. Nanostructure of the materials was visualized by bright-field images with a transmission electron microscope (TEM, JEOL JEM-3010). The WCAs and SAs of the surfaces were determined using a contact-angle goniometer (JC2000DM, China) at ambient temperature. The volume of the water droplets was approximately 5 μL delivered by using a micropipet for static WCAs and SAs. The WCA and SA values were the averages of four measurements made on different areas of the surfaces. The mechanical stability of the obtained samples was evaluated by friction test and pencil hardness test. The friction test was carried out by investigating the relationship between WCA and SA changes of the obtained surface and abrasion lengths. 600 # sandpaper was used as an abrasion surface, on which as-prepared surface was moved back and forth with a total distance, while a load of 500 g was applied to the as-prepared surface. The pencil hardness tests for the coatings were carried out according to the standard of Wolff-Wilbom. It uses pencil leads of different hardness grades (6B–6H). Furthermore, variations of the WCAs and SAs for the superhydrophobic surface were as functions of pH values of water droplet, immersion time in water and the water dripping test. The stress-strain curve was obtained by a universal tester (Instron 3365, UK). Samples to be tested were 40.00 mm long, 6.31 mm wide and 2.31 mm thickness.Acknowledgements
The authors thank the National Natural Science Foundation of China (Grant No. 21473132, 21373158), the Shannxi Science and Technology Department (Grant No. 2014JM2047, 2013KJXX-41) for continuing financial support.References
- W. Barthlott and C. Neinhuis, Planta, 1997, 202, 1–8 CrossRef CAS
.
- B. Bhushan, Y. C. Jung, A. Niemietz and K. Koch, Langmuir, 2009, 25, 1659–1666 CrossRef CAS PubMed
.
- L. Feng, S. Li, Y. Li, H. Li, L. Zhang, J. Zhai, Y. Song, B. Liu, L. Jiang and D. Zhu, Adv. Mater., 2002, 14, 1857–1860 CrossRef CAS
.
- W. Barthlott, T. Schimmel, S. Wiersch, K. Koch, M. Brede, M. Barczewski, S. Walheim, A. Weis, A. Kaltenmaier and A. Leder, Adv. Mater., 2010, 22, 2325–2328 CrossRef CAS PubMed
.
- C.-Y. Yang, Y.-L. Tsai, C.-Y. Yang, C.-K. Sung, P. Yu and H.-C. Kuo, Appl. Phys. Express, 2014, 7, 087001 CrossRef
.
- D. L. Hu, B. Chan and J. W. Bush, Nature, 2003, 424, 663–666 CrossRef CAS PubMed
.
- X. Gao and L. Jiang, Nature, 2004, 432, 36 CrossRef CAS PubMed
.
- L. Feng, Y. Zhang, J. Xi, Y. Zhu, N. Wang, F. Xia and L. Jiang, Langmuir, 2008, 24, 4114–4119 CrossRef CAS PubMed
.
- J. Xi and L. Jiang, Ind. Eng. Chem. Res., 2008, 47, 6354–6357 CrossRef CAS
.
- B. Bhushan, Nanotribology and Nanomechanics II, Springer, New York, 2011 Search PubMed
.
- L. Cao, A. K. Jones, V. K. Sikka, J. Wu and D. Gao, Langmuir, 2009, 25, 12444–12448 CrossRef CAS PubMed
.
- A. Solga, Z. Cerman, B. F. Striffler, M. Spaeth and W. Barthlott, Bioinspiration Biomimetics, 2007, 2, 126–134 CrossRef PubMed
.
- B. Bhushan, Philos. Trans. R. Soc., A, 2009, 367, 1445–1486 CrossRef CAS PubMed
.
- C.-H. Xue, S.-T. Jia, J. Zhang and J.-Z. Ma, Sci. Technol. Adv. Mater., 2010, 11, 1–15 CrossRef
.
- X. Gao, X. Yan, X. Yao, L. Xu, K. Zhang, J. Zhang, B. Yang and L. Jiang, Adv. Mater., 2007, 19, 2213–2217 CrossRef CAS
.
- K.-C. Chang, H.-I. Lu, C.-W. Peng, M.-C. Lai, S.-C. Hsu, M.-H. Hsu, Y.-K. Tsai, C.-H. Chang, W.-I. Hung, Y. Wei and J.-M. Yeh, ACS Appl. Mater. Interfaces, 2013, 5, 1460–1467 CAS
.
- J. Ou, W. Hu, M. Xue, F. Wang and W. Li, ACS Appl. Mater. Interfaces, 2013, 5, 3101–3107 CAS
.
- H. Kim, K. Noh, C. Choi, J. Khamwannah, D. Villwock and S. Jin, Langmuir, 2011, 27, 10191–10196 CrossRef CAS PubMed
.
- L. Xu, R. G. Karunakaran, J. Guo and S. Yang, ACS Appl. Mater. Interfaces, 2012, 4, 1118–1125 CAS
.
- Z. Chen, L. Hao, A. Chen, Q. Song and C. Chen, Electrochim. Acta, 2012, 59, 168–171 CrossRef CAS
.
- X. Yao, L. Xu and L. Jiang, Adv. Funct. Mater., 2010, 20, 3343–3349 CrossRef CAS
.
- X. Zhu, Z. Zhang, X. Xu, X. Men, J. Yang, X. Zhou and Q. Xue, J. Colloid Interface Sci., 2012, 367, 443–449 CrossRef CAS PubMed
.
- Z. She, Q. Li, Z. Wang, L. Li, F. Chen and J. Zhou, ACS Appl. Mater. Interfaces, 2012, 4, 4348–4356 CAS
.
- J. Yu, L. Qin, Y. Hao, S. Kuang, X. Bai, Y.-M. Chong, W. Zhang and E. Wang, ACS Nano, 2010, 4, 414–422 CrossRef CAS PubMed
.
- B. Qian and Z. Shen, Langmuir, 2005, 21, 9007–9009 CrossRef CAS PubMed
.
- A. V. Rao, S. S. Latthe, S. A. Mahadik and C. Kappenstein, Appl. Surf. Sci., 2011, 257, 5772–5776 CrossRef CAS
.
- K. J. Lin, D. Y. Zang, X. G. Lia and X. G. Geng, RSC Adv., 2016, 6, 47096–47100 RSC
.
- C. Dong, Y. Gu, M. Zhong, L. Li, K. Sezer, M. Ma and W. Liu, J. Mater. Process. Technol., 2011, 211, 1234–1240 CrossRef CAS
.
- R. N. Wenzel, Ind. Eng. Chem. Res., 1936, 28, 988–994 CrossRef CAS
.
- A. B. D. Cassie and S. Baxter, Trans. Faraday Soc., 1944, 40, 546–551 RSC
.
- S. Luo, Q. B. Zheng, X. H. Jie and L. Y. Zhang, RSC Adv., 2016, 6, 47588–47594 RSC
.
- C. Su, Y. Xu, F. Gong, F. Wang and C. Li, Soft Matter, 2010, 6, 6068–6071 RSC
.
- Z.-X. Zhang, T. Zhang, X. Zhang, Z. X. Xin, X. Deng and K. Prakashan, RSC Adv., 2016, 6, 12530–12536 RSC
.
- X. Tian, T. Verho and R. H. A. Ras, Science, 2016, 352, 142–143 CrossRef CAS PubMed
.
- H. Y. Wang, J. Y. Zhao, Y. Z. Zhu, Y. Meng and Y. J. Zhu, J. Colloid Interface Sci., 2013, 402, 253–258 CrossRef CAS PubMed
.
- Q. F. Xu, B. Mondal and A. M. Lyons, ACS Appl. Mater. Interfaces, 2011, 3, 3508–3514 CAS
.
- C. H. Xue, Y. R. Li, P. Zhang, J. Z. Ma and S. T. Jia, ACS Appl. Mater. Interfaces, 2014, 6, 10153–10161 CAS
.
- H. Zhou, H. Wang, H. Niu, A. Gestos, X. Wang and T. Lin, Adv. Mater., 2012, 24, 2409–2412 CrossRef CAS PubMed
.
- T. C. Chen, H. T. Liu, H. F. Yang, W. Yan, W. Zhu and H. Liu, RSC Adv., 2016, 6, 43937–43949 RSC
.
- B. G. Wei, Q. Chang, C. X. Bao, L. Dai, G. Z. Zhang and F. P. Wu, Colloids Surf., A, 2013, 434, 276–280 CrossRef CAS
.
- H. Y. Wang, X. G. Zhang, Z. J. Liu, Y. X. Zhu, S. Q. Wu and Y. J. Zhu, RSC Adv., 2016, 6, 10930–10937 RSC
.
- H. Y. Li, R. G. Wang, H. L. Hu and W. B. Liu, Appl. Surf. Sci., 2008, 255, 1894–1900 CrossRef CAS
.
- Y. Lu, W. J. Xu, J. L. Song, X. Liu, Y. J. Xing and J. Sun, Appl. Surf. Sci., 2012, 263, 297–301 CrossRef CAS
.
- K. L. Cho, I. I. Liaw, A. H.-F. Wu and R. N. Lamb, J. Phys. Chem. C, 2010, 114, 11228–11233 CAS
.
- Z. He, Y. Liang, W. Yang, H. Uchino, J. Yu, W.-H. Sun and C. C. Han, Polymer, 2015, 56, 119–122 CrossRef CAS
.
- B. Bending, K. Christison, J. Ricci and M. D. Ediger, Macromolecules, 2014, 47, 800–806 CrossRef CAS
.
- X. Hong, J. Tyson, J. Middlecoff and D. Collard, Macromolecules, 1999, 32, 4232 CrossRef CAS
.
- A. Yildirim, T. Khudiyev, B. Daglar, H. Budunoglu, A. K. Okyay and M. Bayindir, ACS Appl. Mater. Interfaces, 2013, 5, 853–860 CAS
.
- C. Douvris and O. V. Ozerov, Science, 2008, 321, 1188–1190 CrossRef CAS PubMed
.
- M. K. Whittlesey and E. Peris, ACS Catal., 2014, 4, 3152–3159 CrossRef CAS
.
- T. L. Gianetti, R. G. Bergman and J. Arnold, Chem. Sci., 2014, 5, 2517–2524 RSC
.
- N. Wang, Y. Lu, D. Xiong, C. J. Carmaltb and I. P. Parkinb, J. Mater. Chem. A, 2016, 4, 4107 CAS
.
| This journal is © The Royal Society of Chemistry 2016 |