A pseudo-kinetics approach for time-series metabolomics investigations: more reliable and sensitive biomarkers revealed in vincristine-induced paralytic ileus rats†
Abstract
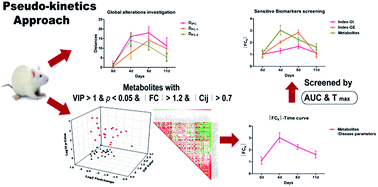
Time-series metabolomics studies can provide snapshots of the metabolic status during the progress of disease and drug treatment. In order to reveal the dynamic development of a disease, a pseudo-kinetics approach based on calibration and integration of absolute fold changes (FCk) along the time points was proposed. A distance based discriminative trajectory was applied to assess global metabolic alterations during ileus progress upon principle component analysis (PCA). The area under the curve (AUC) and peak time (Tmax) of the absolute FCk–time curve were integrated as complementary thresholds to screen sensitive biomarkers compared with the typical ileus parameters of gastric emptying (GE) and gastrointestinal transit (GI). As a result, docosahexaenoic acid, glycocholic acid, glutamine and acetyl-carnitine were identified as sensitive biomarkers for early prediction of paralytic ileus, which were related to intestinal inflammation and mucosal injury. Furthermore, the discrimination abilities of these four biomarkers were validated using receiver-operating characteristic (ROC) analysis. Collectively, this study demonstrated that our proposed pseudo-kinetics approach is effective to screen more reliable and sensitive biomarkers along a pathologic progress.

 Please wait while we load your content...
Please wait while we load your content...