Long-term influence of aeration on arsenic trapping in a ZVI/sand bed reactor†
Abstract
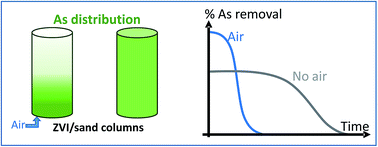
Arsenic toxicity and occurrence in the environment necessitate the development of easy-to-handle and cheap water treatment processes. Zero Valent Iron (ZVI) supports meet these requirements. However many ZVI by-products might be generated in the unit reactor process, mainly influenced by physico-chemical conditions. This work deals with the influence of aeration onto arsenic trapping in ZVI/sand columns. The monitoring of a pilot unit that consisted of four reactors (two aerated and two non-aerated), at different durations (three days, three weeks and three months), enables the characterization of arsenic sorption capacity. In an aerated system, the highest arsenic removal levels were observed at the beginning of the experiment, and the performances greatly decreased with time. In the non-aerated systems, the highest arsenic trapping capacities were obtained (220 mgAs per gFe in the three months of running the system) but the arsenic removal percentage remained under 60%. Support analyses after dismantling were performed to characterize the arsenic sorption and the ZVI by-products generated. They confirmed the influence of aeration on the arsenic distribution along the reactor: a homogeneous repartition in non aerated conditions whereas it was highly heterogeneous under aerated conditions, with far higher concentrations at the inlet. This long-term study highlights that aeration modifies the oxidation of ZVI, resulting in by-products with high arsenic sorption capacities but for a limited duration whereas the absence of aeration limits the oxidation of ZVI into sorbant by-products but it significantly increases the duration of arsenic trapping and consequently the lifetime of the bed reactor.

 Please wait while we load your content...
Please wait while we load your content...