Monoclonal antibody-tagged polyethylenimine (PEI)/poly(lactide) (PLA) nanoparticles for the enhanced delivery of doxorubicin in HER-positive breast cancers
Abstract
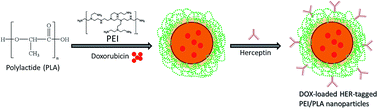
Poor therapeutic response and adverse side effects of chemotherapeutic agents are the major obstacles for effective chemotherapy against breast cancers. In this study, we reported a successful application of an Herceptin-tagged polyethylenimine (PEI) and poly (lactide) PEI/PLA nanoparticle system (hPPD) to deliver the chemotherapeutic drug doxorubicin (DOX) to HER2-positive breast cancer cells for improved efficacy of chemotherapy. The prepared nanoparticles were nanosized and exhibited a typical controlled drug release pattern suggesting its suitability for biomedical applications. The presence of Herceptin on the nanoparticle surface enhanced the accumulation of carrier on the cellular cytoplasm as is evident from the CLSM image. Our data showed that hPPD could significantly inhibit the proliferation of breast cancer cells compared to that with free DOX. Furthermore, Herceptin-based nanocarrier-mediated delivery of DOX could not only enhance the therapeutic outcome of using the anticancer drug, but also reduced the side effects of DOX in an effective manner in a xenograft tumor model. Overall, the Herceptin-tagged nanosystem demonstrates great potential as a novel therapeutic strategy in the anticancer treatment of HER2-positive breast cancers.

 Please wait while we load your content...
Please wait while we load your content...