Hybrid bicelles as a pH-sensitive nanocarrier for hydrophobic drug delivery†
Li Lin‡
a,
Xiaoyou Wang‡b,
Yanyu Guoa,
Kuan Rena,
Xiaoda Lia,
Lijia Jinga,
Xiuli Yue*a,
Qiang Zhang*b and
Zhifei Dai*b
aSchool of Life Science and Technology, School of Municipal and Environmental Engineering, Harbin Institute of Technology, Harbin 150001, China. E-mail: xiulidx@163.com
bBeijing Key Laboratory of Molecular Pharmaceutics and New Drug Delivery System, College of Engineering, School of Pharmaceutical Sciences, Peking University, Beijing 100191, China. E-mail: zqdodo@bjmu.edu.cn; zhifei.dai@pku.edu.cn
First published on 17th August 2016
Abstract
Hydrophobic doxorubicin was successfully loaded into hybrid discoid bicelles generated from proamphiphilic organoalkoxysilane and dihexanoyl phosphatidylcholine at the ratio of 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 by conventional Bangham method in combination with sol–gel reaction and self-assembly process. The drug-loaded hybrid bicelles with about 60 nm diameter and 6 nm thickness were found to exhibit pH-sensitive release behavior, good biocompatibility and remarkably high stability towards surfactant solubilization, long-term storage, and many factors susceptible to destabilize conventional phospholipid bicelles. The hybrid bicelles were proved to have higher cellular uptake via endocytosis and adhesion than spherical cerasomes. The endocytosis of hybrid bicelles was related to clathrin, macropinocytosis and was energy-dependent. Both in vitro and in vivo results showed that the drug loaded bicelles can effectively inhibit tumor growth. In other words, such hybrid bicelles can be employed as a novel promising nanocarrier for hydrophobic drugs.
2 by conventional Bangham method in combination with sol–gel reaction and self-assembly process. The drug-loaded hybrid bicelles with about 60 nm diameter and 6 nm thickness were found to exhibit pH-sensitive release behavior, good biocompatibility and remarkably high stability towards surfactant solubilization, long-term storage, and many factors susceptible to destabilize conventional phospholipid bicelles. The hybrid bicelles were proved to have higher cellular uptake via endocytosis and adhesion than spherical cerasomes. The endocytosis of hybrid bicelles was related to clathrin, macropinocytosis and was energy-dependent. Both in vitro and in vivo results showed that the drug loaded bicelles can effectively inhibit tumor growth. In other words, such hybrid bicelles can be employed as a novel promising nanocarrier for hydrophobic drugs.
Introduction
Currently, chemotherapy remains one of the most important means of malignant tumor treatment.1 Most cancer chemotherapeutics are hydrophobic small molecules. Due to their small size and poor solubility, many of these cancer drugs show poor bioavailability via oral or parenteral delivery, resulting in limited accumulation in tumors, and hence poor therapeutic efficacy. In order to unlock the full therapeutic potential of hydrophobic small-molecule cancer drugs, a variety of innovative water-soluble nanoscale vehicles (10–100 nm diameter), such as liposomes,2 micelles3–7 or nanoemulsions, have been developed to deliver a class of structurally diverse hydrophobic drugs. Especially, bicelles, or bilayered micelles, have recently received increasing interest as a new type of nanocarrier for hydrophobic drugs that allow controlled drug release, and some of these have entered clinical trials.8,9Bicelles are comprised of a mixture of short-chain amphiphiles, typically 1,2-dihexanoyl-sn-glycero-3-phosphocholine (DHPC, segregated to edge regions of high curvature), and long-chain amphiphiles, typically 1,2-dimyristoyl-sn-glycero-3-phosphocholine (DMPC, self-assembled into planar bilayers).10 As a model of biological membrane system, bicelles combine the advantages of lipid vesicles and the classical mixed micelles.11,12 Bicelles do not have inner aqueous compartment like liposomal vesicles, but keep a bilayered domain which maintains many key dynamic and conformational properties of liquid crystalline phase bilayers.13 Bicelles with biomembrane-mimetic properties have opened a new field for the studies of drug partitioning,14,15 structure of membrane proteins16–18 and function of membrane associated proteins.19,20 As their dimension is in the range of 10–50 nm and thickness is about 4–6 nm, bicelles have the ability to penetrate through the narrow intercellular spaces of the stratum corneum of the skin.21 Moreover, bicelles can modify skin biophysical parameters and change the skin's barrier function to enhance drug penetration.22,23 The nanodisk structures were also used as effective carriers for antimicrobial peptides or anti-tumor drugs with extended drug release.23–25 Besides, evidences indicate that nanoparticles in disk-like shape display higher circulation times,26,27 higher cellular uptake ability21–29 and higher microvascular adhesion30,31 than in spherical shape. All of these results proved that the bicelle is very promising in drug delivery.
More importantly, as a lipid bilayer, the intrinsic versatility of bicelles permits formulations with a variety of hydrophobic drugs by entrapping physically into their hydrophobic domains.32,33 In addition, the ability to engineer the protein component of bicelles creates additional opportunities for targeted delivery of bioactive agents to tissues and cells, holding promise for enhancing therapeutic efficacy. Nevertheless, bicelles have problems with drug-loading stability, which is strongly influenced by the in vivo environment.34–38
The bicelles are subject to electrostatic, hydrophobic, and van der Waals interactions with plasma proteins, which may lead to destabilization of the bicelles and drug leakage during circulation often before reaching their tumor sites.39 It is reported that the nanodiscs spontaneously transform into rod-like micelles or vesicles because of the elevation of temperature.40 Most of anti-cancer drugs are toxic not only to tumor cells but also to all tissues they contact. The instability limited the application of bicelles.26–28 Therefore, we have pressing need to develop more stable bicelles to encapsulate and deliver hydrophobic anticancer drugs to tumors and the near vicinity of tumor cells.
The organic–inorganic hybrid composites have been shown to increase the stability of supermolecular assemblies.41–48 Recently, Kikuchi et al. have successfully constructed a new type of organic–inorganic hybrid bicelles (HBicelles) to overcome general problems associated with current phospholipid bicelles.41,42 Such hybrid bicelle has attracted wide attention since its polyorganosiloxane surface offers significantly higher stability than conventional phospholipid bicelles.49–54 Moreover, nontoxic polyorganosiloxane surface may protect the inner lipid bilayer and is facile for conjugation with various ligands for targeting delivery via silane-coupler chemistry. Importantly, many researchers report that silicon materials hold satisfactory degradation behavior, and could degrade into nontoxic orthosilicic acid.55–58 Therefore, such hybrid bicelles are essential due to their potential applications for the encapsulation of a range of hydrophobic guest species.
The current study demonstrates for the first time that the hybrid bicelles can be employed as a novel promising nanocarrier for hydrophobic drugs. An anticancer drug of hydrophobic doxorubicin (HDOX) was selected as a model drug. The HDOX loaded hybrid bicelles were generated from cerasome-forming lipid (CFL) and dihexanoyl phosphatidylcholine (DHPC) at the ratio of 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 by conventional Bangham method in combination with sol–gel reaction and self-assembly process (Fig. 1). We investigated the morphology, stability, encapsulation efficiency and drug release behavior in vitro. To further exploit the potential use of the HBicelles as hydrophobic drugs delivery systems, the biosafety and endocytosis mechanism were studied. In addition, the anti-tumor efficacy of HDOX-loaded HBicelles (HDOX@HBicelles) in vitro and in vivo was also evaluated.
2 by conventional Bangham method in combination with sol–gel reaction and self-assembly process (Fig. 1). We investigated the morphology, stability, encapsulation efficiency and drug release behavior in vitro. To further exploit the potential use of the HBicelles as hydrophobic drugs delivery systems, the biosafety and endocytosis mechanism were studied. In addition, the anti-tumor efficacy of HDOX-loaded HBicelles (HDOX@HBicelles) in vitro and in vivo was also evaluated.
Results and discussion
Characterization of blank HBicelles and HDOX@HBicelles
The HBicelles were fabricated from proamphiphilic organoalkoxysilane and DHPC at the molar ratio of 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
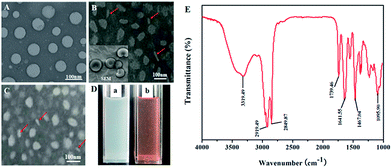
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 by conventional thin film hydration method.41 The lipid bilayer membrane self-assembled by in situ sol–gel processes (Si–OCH2CH3 + H2O → Si–OH + CH3CH2OH followed by 2Si–OH → Si–O–Si + H2O) on the surface (Fig. 1). The structure and morphology of the blank HBicelles were observed with transmission electron microscopy (TEM) and scanning electron microscopy (SEM) (Fig. 2B and C). As shown in Fig. 2B, the particles with ellipsoidal and rod-like shape represented face-on bicelles and edge-on bicelles, respectively. The inserted SEM image further proved that the prepared bicelles were disc-like. The TEM and SEM images also demonstrated that the diameter of HBicelles was about 60 nm and thickness was about 6 nm. The sizes of HBicelles were consistent with the dynamic light scattering (DLS) measurements. For comparative study, we prepared hybrid liposomal cerasomes with the spherical shape and the diameter of about 60 nm (Fig. 2A). The similar diameter of cerasome and HBicelle may eliminate the effect of particle size on the experimental results.
2 by conventional thin film hydration method.41 The lipid bilayer membrane self-assembled by in situ sol–gel processes (Si–OCH2CH3 + H2O → Si–OH + CH3CH2OH followed by 2Si–OH → Si–O–Si + H2O) on the surface (Fig. 1). The structure and morphology of the blank HBicelles were observed with transmission electron microscopy (TEM) and scanning electron microscopy (SEM) (Fig. 2B and C). As shown in Fig. 2B, the particles with ellipsoidal and rod-like shape represented face-on bicelles and edge-on bicelles, respectively. The inserted SEM image further proved that the prepared bicelles were disc-like. The TEM and SEM images also demonstrated that the diameter of HBicelles was about 60 nm and thickness was about 6 nm. The sizes of HBicelles were consistent with the dynamic light scattering (DLS) measurements. For comparative study, we prepared hybrid liposomal cerasomes with the spherical shape and the diameter of about 60 nm (Fig. 2A). The similar diameter of cerasome and HBicelle may eliminate the effect of particle size on the experimental results.
Hydrophobic DOX (HDOX) could be incorporated into the lipid bilayer membrane of HBicelles via hydrophobic interaction. The TEM images showed that the HDOX@HBicelles remained disc-like structure and the size was about 60 nm (Fig. 2C). Besides, Fig. 2D shows the HBicelles with or without HDOX loading were well dispersed and very stable in water at room temperature even after storage over a week. This may be attributed to the crosslinked siloxane network formed on the surface of the HBicelles, which was proved by Fourier transform infrared (FT-IR) spectroscopy (Fig. 2E). The prepared HBicelle exhibited significant peaks at 1111.82 cm−1 and 3430.95 cm−1 corresponding to stretching vibration of Si–O–Si and O–H, respectively.
To further demonstrate the preferable stability of HBicelles, traditional phospholipid bicelles (PBicelles) were prepared for comparative study. Triton X-100 is a kind of nonionic surfactant which can insert into the lipid membrane, form voids and aggravate the existing holes, inducing leakage and fracture of lipid membrane. HBicelles or PBicelles were incubated with Triton X-100 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, w/w), and the diameters were measured at 0.5 h and 24 h respectively. As shown in Fig. S1A,† HBicelles and PBicelles were both stable in water without Triton X-100 for 24 h, yet the incorporation of Triton X-100 caused a sharp drop on particle number of PBicelles at 0.5 h, and no particles were detected after 24 h. And the zeta potential data showed similar results (Fig. S1B†). HBicelles, however, were barely influenced by Triton X-100, probably due to the protection by the crosslinked siloxane network on their surface, giving rise to better stability than PBicelles.
1, w/w), and the diameters were measured at 0.5 h and 24 h respectively. As shown in Fig. S1A,† HBicelles and PBicelles were both stable in water without Triton X-100 for 24 h, yet the incorporation of Triton X-100 caused a sharp drop on particle number of PBicelles at 0.5 h, and no particles were detected after 24 h. And the zeta potential data showed similar results (Fig. S1B†). HBicelles, however, were barely influenced by Triton X-100, probably due to the protection by the crosslinked siloxane network on their surface, giving rise to better stability than PBicelles.
The shape of nanoparticles has been identified as a key factor affecting cell internalization which was important to the cancer therapeutic efficacy.59–62 The internalization of cerasomes and HBicelles was evaluated by flow cytometry and confocal laser scanning microscopy (CLSM). The rhodamine B labeled HBicelles and cerasomes were incubated with MDA-MB-231 cells for 3 h at 37 °C and 4 °C to test their cellular uptake and adhesion respectively, and the fluorescence intensity was quantified by flow cytometry. As shown in Fig. 3A, the mean fluorescence intensity (MFI) of HBicelles was about 1.8 times and 3.2 times stronger than that of cerasomes at 37 °C and 4 °C, respectively. Also, the HBicelles group exhibited higher intracellular fluorescent spot count than cerasome group (Fig. S2†), indicating more HBicelle was internalized than cerasome. Then, further insight into the cellular internalization was gained by CLSM, where HBicelles showed more intracellular red fluorescence at 37 °C, and more adhesion signal around the cell at 4 °C (Fig. 3B). Similar phenomenon was also observed in the other cell lines including MCF-7, HUVEC and LX-2 cells (Fig. S3–S5†). To get a more complete understanding of particle internalization, the uptake rates of HBicelles and cerasomes by MDA-MB-231 cells were investigated by determining the MFI in cells at continuous time points using real-time CLSM (Fig. 3C). According to the quantitative analysis on real-time CLSM images, HBicelle showed higher MFI in cells than cerasomes at all inspection time points. Also, HBicelle were internalized faster than cerasome, and its MFI reached maximum plateau in a shorter time. It showed that the MFI of cells reached to a maximum (250) after the HBicelles incubated with the cells for 900 s, while the MFI of cells incubated with cerasomes only reached to 200 after 1600 s. Thus, the result indicated that HBicelles had accelerated and boosted uptake by MDA-MB-231 cells compared with cerasomes. These results supported that different shape of nanocarriers could lead to altered cellular interaction behavior, and the discoid HBicelles exhibited much easier cellular adhesion (4 °C) and internalization (37 °C) than spherical cerasomes.
To more clearly delineate the role of specific endocytosis pathways involved in HBicelles and cerasome cellular internalization, MDA-MB-231 cells were pre-incubated with biomedical inhibitors of clathrin-mediated endocytosis63 (chlorpromazine (Chlo), sucrose (Sucr)), caveolin-mediated endocytosis (methylated-β-cyclodextrin (MβCD)), macropinocytosis (amiloride hydrochloride (AmiH)) and energy-dependent process (4 °C incubation). Cells incubated with HBicelles and cerasomes only were served as control. As shown in Fig. 3D, Chlo, Sucr and 4 °C inhibited the cellular uptake of HBicelles down to 68.25%, 47.22% and 2.07%, respectively, while the three inhibitors inhibited 18.64%, 71.22% and 98.47% of cellular uptake of cerasomes. No change was observed in AmiH and MβCD group for either HBicelle or cerasome. It indicated that clathrin-mediated and energy-dependent endocytosis were the prominent endocytic pathway for cellular entry of HBicelles and cerasomes.
Lysosome is an important organelle in the intracellular transport of nanocarriers, and can affect drug bioavailability and activity.64 To investigate the colocalization of HBicelles and cerasomes with lysosome, we incubated MDA-MB-231 cells with nanocarriers and labeled lysosomes with Lysotracker. Lysosomes were dyed red and nanocarriers were visualized as green fluorescence. As shown in Fig. 3E, the green fluorescence of HBicelles was much higher than that of cerasome, which was consistent with the cellular uptake result. Strong yellow fluorescence in the cytoplasm merged of red lysosome and green nanocarrier could be observed, which was more obvious for HBicelles group than cerasome group. For quantitative analysis, the Pearson's overlap coefficients of cerasomes and HBicelles with lysosomes were 0.068 ± 0.160 and 0.450 ± 0.261 respectively (n = 9, p < 0.01). These results indicated that significantly more HBicelles were transported into lysosomes than cerasomes at the time point of observation (Fig. 3E).
As is known to all, low toxicity is a prerequisite for the nanoparticles as drug carriers. The toxicity of nanocarriers on MDA-MB-231 cells was evaluated by reactive oxygen species (ROS), lactate dehydrogenase (LDH) and fluorescent recovery after photobleaching (FRAP). The production of ROS induced by nanocarriers can damage external or internal cells and can also contribute to inflammation.65 To study the ROS in cells treated with HBicelles and cerasomes for 3 h, the fluorescence (green) of 2′,7′-dichlorodihydrofluorescein (DCF) oxidized by intracellular reactive oxygen species from non-fluorescent dichlorodihydrofluorescein diacetate (DCFH) was measured. No significant difference of ROS was observed between cerasomes and HBicelles (Fig. 4A). Besides, a fluorescence assay based on the intracellular lactate dehydrogenase (LDH) release was used as a marker of cell membrane integrity, for LDH release in supernatant indicates the disruption of plasma membrane.66 The result in Fig. 4B showed that the LDH release of HBicelles and cerasomes was 8.45% and 7.09% (lower than 10%). There was no significant effect on cytotoxicity for HBicelles and cerasomes after 24 h. To assess whether or not the presence of nanocarriers affected the lipid membrane fluidity, the FRAP technique was employed to detect the rate of recovery. MDA-MB-231 cell membrane were labeled with 1,1-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI) and incubated with HBicelles or cerasomes for 1.5 h. Then, a small membrane area was irreversibly photobleached and the time duration of half fluorescent recovery (T1/2) was recorded to evaluate the fluidity of cell membrane. As shown in Fig. 4C, compared to control group (T1/2 = 5.4 s), the mean T1/2 of HBicelle and cerasome treated group was 9.3 s and 7.7 s, respectively. There was no significant difference observed between cerasome and HBicelle groups. In a word, HBicelles and cerasomes have good biocompatibility and there was no significant difference in cytotoxicity.
Drug release behavior in vitro of HDOX@HBicelles
In order to determine the best drug-loading content (DLC) and encapsulation efficiency (EE), different ratios of drug to lipids were used during the preparation of HDOX@HBicelles (Table S1†). The results showed that the DLC and EE were changed with the ratio of drug to lipid. At the ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30, the DLC and EE were evaluated to be 2.26 ± 0.06% and 67.82 ± 1.73%. In addition to high DLC and EE, the drug release behavior also plays an important role in the application of nanocarrier. The drug release test from HDOX@HBicelles was performed in buffer solution at different pH values with free HDOX used as control. The cumulative release profiles are shown in Fig. 5. From the in vitro cumulative release studies, free HDOX was seen to release rapidly and almost completed within 30 h at pH 7.4. In comparison with free HDOX, HDOX@HBicelles displayed a biphasic release pattern characterized by an initial faster release followed by a sustained-release phase. About 30% HDOX was released from HBicelles in the first 10 hours, and about 50% HDOX was released after 90 h. It has been reported that pH values in blood circulation, early endosomes, late endosomes and lysosomes are about 7.4, 6.5, 5.5 and 4.5, respectively.24 In order to investigate the drug release behavior of the HDOX@HBicelles in the different organelles after cellular uptake, different pH conditions were simulated, and the drug release behavior were detected. Interestingly, Fig. 5 shows that the HDOX@HBicelles possessed pH-sensitivity. As pH values decreased, the drug release rates from HBicelles increased. After 84 h, the cumulative release percentage reached 86% at pH 4.5, while the percentage at pH 7.4 was only 51%. The HBicelle edge regions of DHPC may become unstable in acidic environment, leading to leakage of HBicelle content. The above results indicate that HBicelles is a promising pH-sensitive drug controlled-release system, which can be used sensitive drug controlled-release system, which can be used as an effective drug nanocarrier with high stability during blood circulation and high cumulative release rate after being selectively taken up by tumor tissue due to the acidic microenvironments in the extracellular tissues of the tumors and intracellular lysosomes and endosomes. Therefore, the HBicelles as a drug nanocarrier was very suitable for cancer chemotherapy. This may lead to a better chemotherapeutic efficacy and an improved patient acceptance and compliance.
30, the DLC and EE were evaluated to be 2.26 ± 0.06% and 67.82 ± 1.73%. In addition to high DLC and EE, the drug release behavior also plays an important role in the application of nanocarrier. The drug release test from HDOX@HBicelles was performed in buffer solution at different pH values with free HDOX used as control. The cumulative release profiles are shown in Fig. 5. From the in vitro cumulative release studies, free HDOX was seen to release rapidly and almost completed within 30 h at pH 7.4. In comparison with free HDOX, HDOX@HBicelles displayed a biphasic release pattern characterized by an initial faster release followed by a sustained-release phase. About 30% HDOX was released from HBicelles in the first 10 hours, and about 50% HDOX was released after 90 h. It has been reported that pH values in blood circulation, early endosomes, late endosomes and lysosomes are about 7.4, 6.5, 5.5 and 4.5, respectively.24 In order to investigate the drug release behavior of the HDOX@HBicelles in the different organelles after cellular uptake, different pH conditions were simulated, and the drug release behavior were detected. Interestingly, Fig. 5 shows that the HDOX@HBicelles possessed pH-sensitivity. As pH values decreased, the drug release rates from HBicelles increased. After 84 h, the cumulative release percentage reached 86% at pH 4.5, while the percentage at pH 7.4 was only 51%. The HBicelle edge regions of DHPC may become unstable in acidic environment, leading to leakage of HBicelle content. The above results indicate that HBicelles is a promising pH-sensitive drug controlled-release system, which can be used sensitive drug controlled-release system, which can be used as an effective drug nanocarrier with high stability during blood circulation and high cumulative release rate after being selectively taken up by tumor tissue due to the acidic microenvironments in the extracellular tissues of the tumors and intracellular lysosomes and endosomes. Therefore, the HBicelles as a drug nanocarrier was very suitable for cancer chemotherapy. This may lead to a better chemotherapeutic efficacy and an improved patient acceptance and compliance.
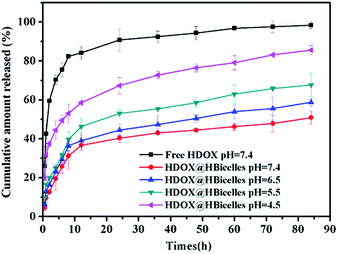 | ||
| Fig. 5 Cumulative release of HDOX from HDOX@HBicelles as a function of time at different pH conditions. Data were presented as mean ± SD (n = 3). | ||
Biocompatibility evaluation of HBicelles
The cytotoxicity of HBicelles was evaluated on three kinds of cells, including human normal epithelial cell line (HUVECs), T cells and bone marrow dendritic cells (BMDCs). As shown in Fig. 6A, no obvious deduction in HUVEC cell viability was observed at concentrations ranging from 62.5 to 2000 mg L−1, and the cell viability was still 84.20 ± 1.68% at a concentration up to 2000 mg L−1 after 72 h incubation with HBicelles. The hemolysis assay was carried out to test the membrane-damage-induced hemoglobin release from erythrocyte after incubation with HBicelles. Fig. 6B showed that the hemolysis rate was below 10% after treatment with free HDOX, blank HBicelles and HDOX@HBicelles. Blank HBicelles had minor influence on erythrocyte membrane, and encapsulation of HDOX into HBicelles reduced the toxicity towards the erythrocyte.Immune cells from Sprague-Dawley (SD) rats were further used to evaluate the immune toxicity of HBicelles. It was found that HBicelles exhibited minor cytotoxicity to both BMDCs and T cells. After incubation with 1000 mg L−1 HBicelles for 24 h, the cell viability of T cells and BMDC cells were 98.35 ± 1.19% (Fig. S5B†) and 97.69 ± 0.95% (Fig. S5C†), respectively. Fluorescent cell staining was carried out for assessment of cell viability with calcein-AM and propidium iodide (PI). As shown in Fig. S5A,† no dead cell was observed at concentration up to 1000 mg L−1, which was well consistent with the MTT results. The data provided an additional evidence that HBicelles owned good biocompatibility.
Anti-tumor efficacy of HDOX@HBicelles in vitro and in vivo
To evaluate the in vitro anti-tumor activity of HDOX@HBicelles, HeLa cells were incubated with HDOX@HBicelles at different concentrations for 24 h, 48 h and 72 h, respectively, and cell viability was tested by MTT assay with the group of free HDOX treatment as control. It was found that cancer cell viability was dependent on the incubation time (Fig. 7). The half maximal inhibitory concentration (IC50) of HDOX@HBicelles was 4.21 mg L−1, which was much higher than free HDOX (0.99 mg L−1) after 48 h incubation. After 72 h incubation, HDOX@HBicelles showed improved cytotoxicity relative to 24 h and 48 h incubation. Cancer cell viability was also observed to be dependent on the HDOX concentration. The higher the drug concentrations, the stronger cell-killing effects. At the drug concentration of 10 mg L−1, the HDOX@HBicelles were found to be as active as the free drug. It is consistent with the result of the release study. The siloxane framework on the HDOX@HBicelles surface enhanced the stability of the lipid bilayer and reduced the HDOX release rate from the bicelles. As a result, only a fraction of the loaded HDOX was released over the experimental period when the bicelles come into contact with medium and cells.The morphology of cell nuclei was observed by optical microscopy after various treatments for 24 h (Fig. 7D). No change of the cell nuclei morphology was observed after treatment with blank HBicelles. In contrast, the number of cells incubated with free HDOX and HDOX@HBicelles was significantly reduced.
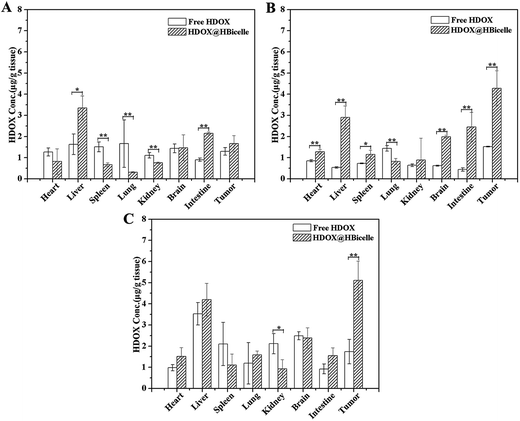
In vivo distribution is one of the most important pharmacokinetic properties of a drug carrier. And it is a major determinant of a half-life and dosing frequency of a drug agent. So, the HDOX concentrations in different organs after administration were measured to evaluate the in vivo biodistribution of HDOX@HBicelles (Fig. 8). The distribution of HDOX in heart, spleen, and lung was reduced by encapsulating into the HBicelles at 1 h after intravenous injection. The HDOX@HBicelles were mainly trapped in reticuloendothelial system (RES) of liver, spleen, lung and intestine at 4 h after administration. Comparing the HDOX contents of organs at 4 h and 24 h postinjection, it was found that the HDOX content of these organs were decreased over time. It indicated that HDOX@HBicelles could be cleared or degraded from the RES organs.67 It was found that the accumulation of HDOX in the tumor increased with time for the HDOX@HBicelles group. Compared with free HDOX, the tumor distribution of HDOX@HBicelles increased by 1.04-, 1.88-, and 2.11-fold after intravenous injection for 1 h, 4 h, and 24 h, respectively. This demonstrated that the HDOX@HBicelles could passively accumulate in the tumor.
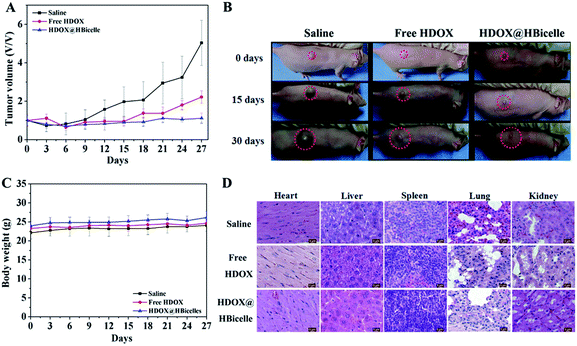
The anti-tumor efficiency of the HDOX@HBicelles in vivo was investigated on MDA-MBA-231-bearing nude mice. Saline, free HDOX or HDOX@HBicelles were administrated by intravenous injection (dose: 5 mg HDOX per kg) when tumor volume reached approximately 50 mm3. The efficiency of tumor inhibition was evaluated by tumor sizes, and representative mice photographs were recorded at certain days (Fig. 9A and B). In saline treated group, tumor volume increased rapidly, from 50 mm3 to approximately 700 mm3 over 27 days. On the contrary, different tumor growth inhibitions were displayed in other two groups. The tumor volume in mice treated only with free HDOX increased from 50 mm3 to 200 mm3 over 27 days, suggesting the dosage of free HDOX in our study could not effectively inhibit the tumor due to the short half-life and low accumulation in tumor sites. Excitingly, in the HDOX@HBicelles treated group, the tumor volume was still about 50 mm3 over 27 days. It is in agreement with the photographs of representative mice shown in Fig. 9B. Consequently, the HBicelles were stable enough to retain HDOX inside and to be delivered to the tumor by enhanced permeability and retention (EPR) during blood circulation. The results demonstrated that the HDOX@HBicelles could successfully inhibit tumor growth and hold the promise of improving therapeutic efficacy.
The in vivo biocompatibility of HDOX@HBicelles was also studied. The body weights of mice in three groups (saline, free HDOX, HDOX@HBicelles) were recorded every three days. As shown in Fig. 9C, no apparent difference was observed in the body weights of mice among three groups within 27 days observation, suggesting low in vivo toxicity of HDOX@HBicelles. The toxicity of HDOX@HBicelles on healthy tissues in vivo was assessed. The representative organs (heart, liver, spleen, lung, kidney and tumor) of the three groups were collected at 27 days after treatment and observed by H&E staining. H&E stained images showed that neither visible organ damage nor inflammation could be observed in any organs of the treated mice (Fig. 9D). The excellent anti-tumor activity and good biocompatibility suggested that the HDOX@HBicelles could be used as an effective hydrophobic drug carrier for in vivo cancer treatment.
Conclusion
The most important finding of this research is that the hybrid discoid bicelles with high loading content of hydrophobic drugs were successfully constructed by the Bangham method in combination of sol–gel reaction and self-assembly process. The polyorganosiloxane network on the lipid bilayer surface acted as a protection towards surfactant solubilization, long-term storage, and many factors susceptible to destabilize conventional bare phospholipid bicelles. In addition, the bicelles showed excellent biocompatibility, superior cellular adhesions and uptake. Most importantly, such hybrid bicelle can be used as a promising pH-sensitive drug controlled-release system with high stability during blood circulation and high cumulative release rate after being selectively taken up by tumor tissue due to the acidic microenvironments in the extracellular tissues of the tumors and intracellular lysosomes and endosomes. The doxorubicin loaded bicelles exhibited effective inhibition on tumor cells in vitro and in vivo. In general, compared to other silicon-lipid hybrid nanocarriers,68,69 traditional spherical cerasome70,71 and lipid bicelles,25 hybrid bicelles show simple preparation, improved characterization (i.e. cellular uptake, pH-sensitive release, higher stability etc.), and more efficiently improved toxicity and tumor accumulation. We therefore anticipate that the hybrid bicelles will be a promising candidate as nanocarrier for hydrophobic drugs with excellent chemotherapeutic efficacy, improved patient acceptance and compliance.Experimental section
Materials
Aminopropyltriethoxysilane (APTES, 99.9%), methylthiazolyl tetrazolium (MTT) and amiloride hydrochloride were purchased from Sigma-Aldrich. 1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC·HCl) was purchased from Shanghai Medpep Co., Ltd. Hexadecylamine was obtained from ABCR GmbH Company. Doxorubicin hydrochloride (DOX·HCl) was obtained from Beijing Huafeng United Technology. Succinic acid anhydride was procured from Sinopharm Chemical Reagent Co., Ltd. LysoTracker@Red DND-99 was purchased from Invitrogen. 1,2-Dihexanoyl-sn-glycero-3-phosphochocholine (DHPC) and Lissamine rhodamine B was purchased from Avanti Polar Lipids, Inc. Fluorescent probe 1,1-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI), 1,1-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine-4-chlorobenzenesulfonate salt (DiD) and lactate dehydrogenase (LDH) assay kit were obtained from Beyotime Institute of Biotechnology. Reactive Oxygen Species (ROS) assay kit was purchased from Nanjing KeyGEN Biotech. Co., Ltd. All chemicals and reagents were commercially obtained and used directly without further purification.Preparation of HDOX@HBicelles and rhodamine B labeled HBicelles
Synthesis of cerasome-forming lipid (CFL) was referred to previous literature.48 Appropriate amounts of CFL and DHPC (molar ratio was 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) were dissolved in chloroform. The solvent was evaporated in a vacuum rotary evaporator; the thin lipid film formed on the wall of the flask was dried overnight in vacuum. Then, ultrapure water was added into the flask and hydrated for 30 min to 1 h at 55–65 °C. After water bath ultrasonication for 10 min, the ultrasonic waves of probe-type sonicator were applied to the resultant dispersion for 5 min at the output amplitude of 30. Prior to the observation, the samples were incubated overnight at room temperature.
2) were dissolved in chloroform. The solvent was evaporated in a vacuum rotary evaporator; the thin lipid film formed on the wall of the flask was dried overnight in vacuum. Then, ultrapure water was added into the flask and hydrated for 30 min to 1 h at 55–65 °C. After water bath ultrasonication for 10 min, the ultrasonic waves of probe-type sonicator were applied to the resultant dispersion for 5 min at the output amplitude of 30. Prior to the observation, the samples were incubated overnight at room temperature.
HDOX-loaded and rhodamine B labeled HBicelles were also prepared as described above. HDOX was obtained by treating DOX·HCl with triethylamine according to the report methods.72 For HDOX loading or fluorescent labeling, different amounts of HDOX or fluorescent probes (L-α-phosphatidylethanolamine-N-(lissamine rhodamine B sulfonyl)) in chloroform were added into the solution of CFL/DHPC (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) in chloroform. The un-encapsulated HDOX was removed by centrifuging at 1500 rpm for 10 min.
2) in chloroform. The un-encapsulated HDOX was removed by centrifuging at 1500 rpm for 10 min.
For comparison, traditional phospholipid bicelles (PBicelles) were prepared with 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC) and DHPC following the same procedure. Characterization of blank HBicelles and HDOX@HBicelles: the as-prepared bicelles were examined by scanning electron microscopy (SEM, Hitachi S-4800, Tokyo, Japan) and transmission electron microscopy (TEM, Hitachi, H-7650). The TEM specimens were prepared by depositing the bicelle solutions onto a carbon-coated copper grid and stained with 4% (w/v) uranyl acetate for 5 min. Then, the specimens were washed several times with distilled water and air-dried prior to imaging. The SEM specimens were prepared by dropping an aliquot of bicelles solution onto copper foil. The specimens were imaged after sputtered with platinum for 2 min.
The hydrodynamic diameter and zeta potential of bicelles were analyzed with a 90Plus/BI-MAS DLS analyzer (Brookhaven Zeta PALS instruments). All the samples were carried out in triplicate. Fourier transform infrared (FTIR) spectrum of blank HBicelles was recorded using a Varian Resolution FTIR spectrophotometer (Varian FTS 3100, USA). And lyophilized samples were subjected to prepare the KBr disk as a specimen.
Cell interaction of HBicelles and cerasomes
FRAP (Fluorescent Recovery After Photobleaching) assay was employed to test the membrane fluidity. MDA-MB-231 cell membrane was labeled with DiI for 0.5 h before 1.5 h incubation with blank HBicelles. A small membrane area was irreversibly photobleached, and fluorescent recovery was measured by CLSM (confocal laser scanning microscope). The time duration it took for the area to reach half recovery (T1/2) was recorded to assess the fluidity of cell membrane after incubation with bicelles. The cells were imaged and analyzed using a Leica TCS SP5 laser scanning confocal microscope (Heidelberg, Germany).LDH (lactate dehydrogenase) assay was carried out to test the membrane integrity after the cells were incubated with blank HBicelles. MDA-MB-231 cells were seeded into 96-well plates and incubated with blank HBicelles for 24 h at 37 °C. The LDH in the culture medium was measured using the LDH analysis kit.
ROS (Reactive Oxygen Species) assay was conducted to assess the nanomaterial-caused injury. MDA-MB-231 cells were cultured in glass-bottomed dishes and co-incubated with blank HBicelles for 3 h. ROS was detected with the ROS assay kit by CLSM.
Cellular uptake and mechanisms
Flow cytometry and CLSM were performed to examine the cellular uptake of rhodamine B labeled HBicelles. Cells were cultured in 6-well plates or glass-bottomed dishes. The cells were washed thrice and incubated with 2.0 mL formulation at 37 °C for 3 h for uptake examination or at 4 °C for adhesion detection. Colocalization test was carried out to analyze the colocalization of HBicelles with lysosomes after internalization. In colocalization test, cells were incubated with LysoTracker for 45 min at 37 °C before 1 h incubation of HBicelles.To study the potential uptake mechanism of HBicelles, MDA-MB-231 cells were pretreated with following endocytosis inhibitors individually for 30 min at 37 °C: 10 μg mL−1 of chlorpromazine, 5 mM of methyl-β-cyclodextrin, 3 mM of amiloride hydrochloride or 0.4 M of sucrose. To study the effect of temperature on the cellular uptake, the cells were cultured at 37 °C and 4 °C, respectively. Following pre-incubation for 30 min, cells were incubated with rhodamine B labeled HBicelles for another 2 h. Then cells were washed carefully with PBS three times, trypsinized and resuspended in 0.4 mL PBS. The fluorescent intensity of cells was measured by a flow cytometer (Cytomics FC 500, Beckman coulter).
Evaluation of encapsulation efficiency and drug loading content
The amount of HDOX loaded in the HBicelles was measured spectrophotometrically.59,73 Firstly, fresh prepared HDOX@HBicelles (0.1 mL) were suspended in 0.9 mL HCl solution (1 M). After vigorous stirring overnight, the fluorescence intensity of the DOX solutions was measured at an excitation wavelength of 480 nm and an emission wavelength of 590 nm. The amount of HDOX was determined using fluorescence spectrophotometer (Varian Cary Eclipse) and the corresponding standard calibration curve. The drug loading content (DLC%) and encapsulation efficiency (EE%) were evaluated by following equations:Drug release behavior in vitro
Briefly, the release of HDOX from HBicelles was determined by dialysis tube method in phosphate buffer saline at different pH values. 2 mL dispersion of HDOX@HBicelles was added to 50 mL PBS preheated to 37 °C. 2 mL samples were taken at predetermined time intervals and replaced with the same amount of PBS. The release experiment lasted for 90 h. Then the amount of HDOX released from HBicelles was determined by a fluorescence spectrophotometer (Cary Eclipse, Varian, Palo Alto, CA) and corresponding standard curve. The excitation and emission wavelengths were 480 nm and 590 nm. All experiments were carried out in triplicate.Biocompatibility evaluation and cytotoxicity assay
Human umbilical vein endothelial cells (HUVECs), bone marrow dendritic cells (BMDCs), T cells and MDA-MB-231 cells were selected to evaluate the biocompatibility of blank HBicelles in vitro. BMDCs were generated by culturing bone marrow stem cells from SD rats in complete Iscove's Modified Dulbecco's Medium (cIMDM) with 20 ng mL−1 recombinant granulocyte macrophage colony stimulating for 6 days at 37 °C. And T cells obtained from spleen of SD rats were also cultured in cIMDM at 37 °C. The biocompatibility and cytotoxicity were evaluated by MTT method.Briefly, HUVECs were seeded in a 96-well plate at a density of 1 × 105 cells per well and incubated for 24 h. Then, different concentrations of blank HBicelles were added in cells and incubated for another 24 h, 48 h, and 72 h. Every concentration was added to five wells as parallel control and wells without bicelles were negative control. The biocompatibility of HBicelles on HUVECs was assessed using the standard MTT method. BMDCs and T cells were also used for biocompatibility evaluation according to the same procedure as described above. After incubation with bicelles for 24 h, cell viability was determined by double staining with calcein-AM and PI, and observed using fluorescence microscope.
Hemolysis assay was carried out by incubating mice blood samples with HBicelles. Blood samples were harvested from mice heart into heparinized test tubes, centrifuged at 3500 rpm for 15 min, washed and diluted with saline. The red blood cells obtained were incubated with 1 mg mL−1 HBicelles at 37 °C for 1 h with gentle shaking. Then the samples were centrifuged at 3000 rpm for 5 min, and the supernatant was collected and measured by UV-vis spectrophotometry at 576 nm. Negative and positive control groups were incubated with saline (0%) and water (100%) respectively.
To investigate the cytotoxicity of HDOX@HBicelles, 1 × 105 HeLa cells were seeded in 96-well plate and incubated for 24 h. Following attachment, the cells were exposed to different concentrations of free HDOX or HDOX@HBicelles for 24 h, 48 h and 72 h. Cells without any bicelles was used as control. The viability of cells was detected by the MTT method as previously described. The morphology of cell nuclei (stained with DAPI) was observed by optical microscopy after different treatments.
Tissue distribution and anti-tumor efficacy of HDOX@HBicelles in vivo
Female nude mice (BALB/c, 5–6 weeks old) were inoculated subcutaneously with MDA-MB-231 breast tumor cells. When the tumor volume reached ∼50 mm3, the tumor-bearing mice were randomly divided into three groups (n = 8) and were treated by intravenous injection with 150 μL (dose: 5 mg HDOX per kg) saline, free HDOX and HDOX@HBicelles every three days for three times, respectively. The tumor sizes were measured by Vernier caliper every three days, tumor volume was calculated as tumor volume = (tumor length) × (tumor width)2/2. The weight of mice was also detected every three days. In addition, mice were sacrificed after 27 days and representative organs including heart, liver, lung, spleen, kidney and tumor were collected, fixed in formaldehyde solution, embedded in paraffin, followed by section, stained with hematoxylin and eosin (H&E) and observed by optical microscope.All animal procedures were approved by the Institutional Animal Care and Use Committee at the Institute of Biophysics of Peking University and conducted in compliance with the Guideline on Administration of Lab Animals in China approved by State Council.
Statistical analysis
Results are presented as mean ± SD. Statistical analysis of the data were performed using the Student's t-test. Values of p < 0.05 considered to be statistically significant.Acknowledgements
This work was financially supported by State Key Program of National Natural Science of China (Grant No. 81230036), National High Technology Research and Development Program of China (no. 2013AA032201), National Natural Science Foundation of China (No. 21273014 and No. 81371580), National Natural Science Foundation for Distinguished Young Scholars (No. 81225011) and the Foundation for Innovative Research Groups of the National Natural Science Foundation of China (Grant No. 81421004).Notes and references
- C. Wang, Sci. Bull., 2015, 60, 403–404 CrossRef.
- W. J. Mulder, G. J. Strijkers, G. A. van Tilborg, D. P. Cormode, Z. A. Fayad and K. Nicolay, Acc. Chem. Res., 2009, 42, 904 CrossRef CAS PubMed.
- Y. M. Zhao, F. Fay, S. Hak, J. Manuel Perez-Aguilar, B. L. Sanchez-Gaytan, B. Goode, R. Duivenvoorden, C. de Lange Davies, A. Bjørkøy, H. Weinstein, Z. A. Fayad, C. Pérez-Medina and W. J. Mulder, Nat. Commun., 2016, 7, 11221 CrossRef CAS PubMed.
- N. Kamaly, Z. Xiao, P. M. Valencia, A. F. Radovic-Moreno and O. C. Farokhzad, Chem. Soc. Rev., 2012, 41, 2971 RSC.
- H. Cabral, Y. Matsumoto, K. Mizuno, Q. Chen, M. Murakami, M. Kimura, Y. Terada, M. R. Kano, K. Miyazono, M. Uesaka, N. Nishiyama and K. Kataoka, Nat. Nanotechnol., 2011, 6, 815 CrossRef CAS PubMed.
- C. Y. Shi, D. D. Guo, K. Xiao, X. Wang, L. L. Wang and J. T. Luo, Nat. Commun., 2015, 6, 7449 CrossRef PubMed.
- J. A. MacKay, M. Chen, J. R. McDaniel, W. Liu, A. J. Simnick and A. Chilkoti, Nat. Mater., 2009, 8, 993 CrossRef PubMed.
- J. Hrkach, D. Von Hoff, M. Mukkaram Ali, E. Andrianova, J. Auer, T. Campbell, D. De Witt, M. Figa, M. Figueiredo, A. Horhota, S. Low, K. McDonnell, E. Peeke, B. Renarajan, A. Sabnis, E. Schnipper, J. J. Song, Y. H. Song, J. Summa, D. Tompsett, D. Trompsett, G. Troiano, T. Van Geen Hoven, J. Wright, P. LoRusso, P. W. Kantoff, N. H. Bander, C. Sweeney, O. C. Farokhzad, R. Langer and S. Zale, Sci. Transl. Med., 2012, 4, 128ra39 Search PubMed.
- T. Y. Kim, D. W. Kim, J. Y. Chung, S. G. Shin, S. C. Kim, D. S. Heo, N. K. Kim and Y. J. Bang, Clin. Cancer Res., 2004, 10, 3708 CrossRef CAS PubMed.
- C. R. Sanders 2nd and J. P. Schwonek, Biochemistry, 1992, 31, 8898 CrossRef CAS.
- C. R. Sanders and R. S. Prosser, Structure, 1998, 6, 1227 CrossRef CAS PubMed.
- B. B. Lucyanna, R. Gelen, C. Merce, R. Laia, L. I. Carmern, L. M. Alfons de and L. Olga, Pharmaceutics, 2011, 3, 636 CrossRef PubMed.
- X. L. Liang, X. L. Yue, Z. F. Dai and J. Kikuchi, Chem. Commun., 2011, 47, 4751 RSC.
- W. L. Klotz, M. R. Schure and J. P. Foley, J. Chromatogr. A., 2002, 962, 207 CrossRef CAS PubMed.
- T. R. Oliveira, C. R. Benatti and M. T. Lamy, Biochim. Biophys. Acta, 2011, 1808, 2629 CrossRef CAS PubMed.
- C. R. Sanders and K. Oxenoid, Biochim. Biophys. Acta, 2000, 1508, 129 CrossRef CAS.
- R. E. Minto, P. R. Adhikari and G. A. Lorigan, Chem. Phys. Lipids, 2004, 132, 55 CrossRef CAS PubMed.
- U. H. Dürr, M. Gildenberg and A. Ramamoorthy, Chem. Rev., 2012, 112, 6054 CrossRef PubMed.
- J. A. Whiles, R. Deems, R. R. Vold and E. A. Dennis, Bioorg. Chem., 2002, 30, 431 CrossRef CAS PubMed.
- T. Yamaguchi, T. Uno, Y. Uekusa, M. Yagi-Utsumi and K. Kato, Chem. Commun., 2013, 49, 1235 RSC.
- L. Barbosa-Barros, C. Barba, M. Cocera, L. Coderch, C. López-lqlesias, A. de la Maza and O. López, Int. J. Pharm., 2008, 352, 263 CrossRef CAS PubMed.
- G. Rodríguez, L. Barbosa-Barros, L. Rubio, M. Cócera, C. López-Iglesias, A. de la Maza and O. López, Colloids Surf., B, 2011, 84, 390 CrossRef PubMed.
- W. P. Zhang, J. Sun and Z. G. He, J. Pharm. Sci., 2013, 8, 143 Search PubMed.
- M. M. Zetterberg, K. Reijmar, M. Pränting, A. Engström, D. I. Andersson and K. Edwards, J. Controlled Release, 2011, 156, 323 CrossRef CAS PubMed.
- W. P. Zhang, J. Sun, Y. Liu, M. Y. Tao, X. Y. Ai, X. N. Su, C. F. Cai, Y. L. Tang, Z. Feng, X. D. Yan, G. L. Chen and Z. G. He, Mol. Pharm., 2014, 11, 3279 CrossRef CAS PubMed.
- S. Muro, C. Garnacho, J. A. Champion, J. Leferovich, C. Gajewski, E. H. Schuchman, S. Mitragotri and V. R. Muzykantov, Mol. Ther., 2008, 16, 1450 CrossRef CAS PubMed.
- N. P. Truong, M. R. Whittaker, C. W. Mak and T. P. Davis, Expert Opin. Drug Delivery, 2015, 12, 129 CrossRef CAS PubMed.
- S. Barua, J. W. Yoo, P. Kolhar, A. Wakankar, Y. R. Gokarn and S. Mitragotri, Proc. Natl. Acad. Sci. U. S. A., 2013, 110, 3270 CrossRef CAS PubMed.
- R. Agarwal, V. Singh, P. Jurney, L. Shi, S. V. Sreenivasan and K. Roy, Proc. Natl. Acad. Sci. U. S. A., 2013, 110, 17247 CrossRef CAS PubMed.
- N. Doshi, B. Prabhakarpandian, A. Rea-Ramsey, K. Pant, S. Sundaram and S. Mitragotri, J. Controlled Release, 2010, 146, 196 CrossRef CAS PubMed.
- G. Adriani, M. D. de Tullio, M. Ferrari, F. Hussain, G. Pascazio, X. Liu and P. Decuzzi, Biomaterials, 2012, 33, 5504 CrossRef CAS PubMed.
- S. May and A. Fahr, Drug Delivery Strategies for Poorly Water-Soluble Drugs, John Wiley & Sons Ltd., 2013 Search PubMed.
- N. Ahrmed, C. E. Mora-Hueratas, C. Jaafar-Maalej, H. Fessi and A. Elaissari, Drug Delivery Strategies for Poorly Water-Soluble Drugs, John Wiley & Sons Ltd., 2013 Search PubMed.
- S. Kim, Y. Shi, J. Y. Kim, K. Park and J. X. Cheng, Expert Opin. Drug Delivery, 2010, 7, 49 CrossRef CAS PubMed.
- J. Liu, F. Zeng and C. Allen, Eur. J. Pharm. Biopharm., 2007, 65, 309 CrossRef CAS PubMed.
- A. E. Nel, L. Mädler, D. Velegol, T. Xia, E. M. Hoek, P. Somasundaran, F. Klasessig, V. Castranova and M. Thompson, Nat. Mater., 2009, 8, 543 CrossRef CAS PubMed.
- C. J. Cheng, G. T. Tietjen, J. K. Saucier-Sawyer and W. M. Saltzman, Nat. Rev. Drug Discovery, 2015, 14, 239 CrossRef CAS PubMed.
- K. Letchford and H. M. Burt, Mol. Pharm., 2012, 9, 248 CrossRef CAS PubMed.
- H. T. Chen, S. Kim, W. He, H. F. Wang, P. S. Low, K. Park and J. X. Cheng, Langmuir, 2008, 24, 5213 CrossRef CAS PubMed.
- L. V. Dam, G. Karlsson and K. Edwards, Biochim. Biophys. Acta, 2004, 1664, 241 Search PubMed.
- K. Yasuhara, S. Miki, H. Nakazono, A. Ohta and J. Kikuchi, Chem. Commun., 2011, 47, 4691 RSC.
- K. Yasuhara, H. Hayashi and J. Kikuchi, Chem. Lett., 2012, 41, 1223 CrossRef CAS.
- S. Tekobo and E. Pinkhassik, Chem. Commun., 2009, 7, 1112 RSC.
- A. Shimojima and K. Kuroda, Chem. Rec., 2006, 6, 53 CrossRef CAS PubMed.
- S. Inagaki, S. Guan, Y. Fukushima, A. T. Ohsuna and O. Terasaki, J. Am. Chem. Soc., 1999, 121, 9611 CrossRef CAS.
- J. H. Jung, S. Shinkai and T. Shimizu, Chem. Rec., 2003, 3, 212 CrossRef CAS PubMed.
- T. Ogoshi and Y. Chujo, Compos. Interfaces, 2005, 11, 539 CrossRef CAS.
- S. Sreejith, T. T. M. Huong, P. Borah and Y. Zhao, Sci. Bull., 2015, 60, 665–678 CrossRef CAS.
- Z. F. Dai, W. J. Tian, X. L. Yue, Z. Z. Zheng, J. J. Qi, N. Tamai and J. Kikuchi, Chem. Commun., 2009, 21, 2032 RSC.
- K. Katagiri, M. Hashizume, K. Ariga, T. Terashima and J. Kikuchi, Chemistry, 2007, 13, 5272 CrossRef CAS PubMed.
- S. Z. Li, Y. Ma, X. L. Yue, Z. Cao, S. Q. Liu and Z. F. Dai, J. Dispersion Sci. Technol., 2010, 31, 1727 CrossRef CAS.
- K. Matsui, Y. Sasaki, T. Komatsu, M. Mukai, J. Kikuchi and Y. Aoyama, Bioorg. Med. Chem. Lett., 2007, 17, 3935 CrossRef CAS PubMed.
- Y. Sasaki, K. Matsui, Y. Aoyama and J. Kikuchi, Nat. Protoc., 2006, 1, 1227 CrossRef CAS PubMed.
- X. L. Liang, X. L. Yue, Z. F. Dai and J. Kikuchi, Chem. Commun., 2011, 47, 4751 RSC.
- A. Tzur-Balter, Z. Shatsberg, M. Beckerman and A. N. ESegal, Nat. Commun., 2015, 6, 6208 CrossRef CAS PubMed.
- E. Tolstik, L. A. Osminkina, C. Matthäus, M. Burkhardt, K. E. Tsurikov, U. A. Natashina, V. Y. Timoshenko, R. Heintzmann, J. Popp and V. Sivakov, Nanomedicine, 2016, 12, 1931–1940 CAS.
- L. T. Canham, Adv. Mater., 1995, 7, 1033–1037 CrossRef CAS.
- Y. Choi, J. E. Lee, J. H. Lee, J. H. Jeong and J. Kim, Langmuir, 2015, 31, 6457–6462 CrossRef CAS PubMed.
- J. A. Chamipon, Y. K. Katare and S. Mitragotri, J. Controlled Release, 2007, 121, 3 CrossRef PubMed.
- S. Venkataraman, J. L. Hedrick, Z. Y. Ong, C. Yang, P. L. R. Ee, P. T. Hammond and Y. Y. Yang, Adv. Drug Delivery Rev., 2011, 63, 1228 CrossRef CAS PubMed.
- F. Zhao, H. Meng, L. Yan, B. Wang and Y. L. Zhao, Sci. Bull., 2015, 60, 3–20 CrossRef CAS.
- W. Zhang and C. Gao, Sci. Bull., 2015, 60, 1973–1979 CrossRef CAS.
- C. Chen, Sci. Bull., 2015, 60, 1787–1788 CrossRef.
- T. Zong, L. Mei, H. Gao, P. Zhu, K. Shi, J. Chen, Y. Wang, F. Gao and Q. He, Mol. Pharm., 2014, 11, 2346 CrossRef CAS PubMed.
- G. Oberdörster, E. Oberdörster and J. Oberdörster, Environ. Health Perspect., 2005, 113, 823 CrossRef.
- J. Kasper, M. I. Hermanns, C. Bantz, M. Maskos, R. Stauber, C. Pohl, R. E. Unger and J. C. Kirkpatrick, Part. Fibre Toxicol., 2011, 8, 6 CrossRef CAS PubMed.
- X. L. Huang, L. L. Li, T. L. Liu, N. J. Hao, H. Y. Liu, D. Chen and F. Q. Tang, ACS Nano, 2011, 5, 5390 CrossRef CAS PubMed.
- R. Yasmin, A. Tan, K. E. Bremmell and C. A. Prestidge, J. Pharm. Sci., 2014, 9, 2950–2959 CrossRef PubMed.
- S. Rao, A. Tan, B. J. Boyd and C. A. Prestidge, Nanomedicine, 2014, 12, 2745–2759 CrossRef PubMed.
- Y. S. Jin, X. L. Yue, Q. Y. Zhang, X. Y. Wu, Z. Cao and Z. F. Dai, Acta Biomater., 2012, 9, 3372–3380 CrossRef PubMed.
- Z. Cao, X. L. Yue, Y. S. Jin, X. Y. Wu and Z. F. Dai, Colloids Surf., B, 2012, 10, 97–104 CrossRef PubMed.
- Y. Ma, X. L. Liang, S. Tong, G. Bao, Q. S. Ren and Z. F. Dai, Adv. Funct. Mater., 2013, 23, 815 CrossRef CAS.
- X. L. Liang, J. Gao, L. D. Jiang, J. W. Luo, L. J. Jing, X. D. Li, Y. S. Jin and Z. F. Dai, ACS Nano, 2015, 9, 1280 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Characterization, cellular uptake and adhesion, drug loading performance and biocompatibility. See DOI: 10.1039/c6ra18112k |
| ‡ The authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2016 |