Carbon nanotube-based stimuli-responsive nanocarriers for drug delivery
R. Bafkary and
S. Khoee*
Polymer Laboratory, Chemistry Department, School of Science, University of Tehran, Tehran, Iran. E-mail: khoee@khayam.ut.ac.ir; Fax: +98 66495291; Tel: +98 9127180849
First published on 12th August 2016
Abstract
In cancer therapy, smart nanocarriers are one of the most important nanoscale vectors of therapeutic agents. In this study, magnetic carbon nanotubes are functionalized with stimuli-responsive polymeric shells, which have thermal sensitivity and redox sensitivity due to the presence of PNIPAM-co-PHEMA and disulfide bonds, respectively. The chemical–physical properties of the nanovectors have been specified and used in a pinpointed DOX delivery system. At 25 °C, 12% of DOX was released from poly(NIPAM-HEMA-SS)/MN-MWCNTs in 12 h; as the temperature increased to 41 °C, the cumulative release amount of DOX in 12 h increased to 41%. Moreover, it was shown that in the presence of DTT, a more rapid release rate of DOX was observed. The in vitro hemolysis and in vivo biochemical analysis results revealed negligible toxicity of poly(NIPAM-HEMA-SS)/MN-MWCNTs in mice at a high dosage in the course of a 10 day experiment. The temperature responsive cytotoxicity of DOX–poly(NIPAM-HEMA-SS)/MN-MWCNTs was determined in vitro. In addition, with increasing temperature, the viability significantly decreased; the cell survival ratio was reduced from 59% ± 1% at 37 °C to 50% ± 2% at 41 °C at a concentration of 2 μg mL−1 because of the increased drug release under these conditions, likely as it was observed during in vitro drug release.
Introduction
Controlled drug delivery is one of the most important and novel fields of study in biomedical research. Its most potent advantages are high sensitivity and selectivity, prolonged duration, better bioavailability and minimized systemic side effects, which increase the efficiency of therapeutics.1,2Since the introduction of nanotubes by Long and Yang, many groups have researched this topic.3 In medical fields, CNTs have been studied extensively by different researchers as efficient drug carriers.3 Based on their number of graphene layers, CNTs can be categorized into single-wall CNTs (SWCNTs) and multi-wall CNTs (MWCNTs).
The remarkable material properties of CNTs, such as high electrical conductivity, thermal resistance, thermal stability, and mechanical strength, have made them promising candidates for different applications. Additionally, features such as their nanosized diameter, ultralight weight, hollow and layered structures, high specific surface area (i.e. surface area to volume ratio), and notable drug cargo sufficiency have made CNTs promising nanomachines for transferring many drugs, including doxorubicin (DOX),4,5 mitoxantrone,6 epirubicin,7 camptothecin,8 and methotrexate.9
However, despite all these desirable characteristics, CNTs must be modified with smart polymers prior to use in controllable drug delivery systems. Great efforts have been made to develop stimuli-sensitive drug delivery systems, where drugs can only be released in the presence of a special stimulus.10 Stimuli-mediated release of drugs is an appropriate method that can be used to reach this goal. In this trend, smart nanocarriers can respond to multiple stimuli, such as temperature,11 pH,12 magnetic field,13 ionic strength14 or enzymes,15 in target tissue.
Poly(N-isopropylacrylamide) (PNIPAM), a temperature sensitive polymer, was taken into consideration as a carrier for drug delivery systems due to its lower critical solution temperature (LCST) of around 32 °C; it has been eagerly investigated owing to its possible applications in drug delivery.16 The LCST of PNIPAM can be increased by copolymerization with other hydrophilic monomers.17 Hydroxyethyl methacrylate (HEMA) is a hydrophilic monomer which can be used to increase the LCST of PNIPAM.17
PNIPAM chains are not degraded under physiological conditions. However, when PNIPAM is crosslinked with degradable cross-link agents, the polymer can be degraded. In biological systems, disulfide bonds in polymer structures play an important role for both remedial and medicinal applications owing to their resistance to oxidation and their decomposition under reducing conditions.18 It is noted that disulfide crosslinked polymers can be degraded by separating the disulfide bond (–S–S–) into thiol functional groups (–SH HS–) with reducing agents such as dithiothreitol (DTT) and glutathione (GSH).19 The degraded polymers can easily release drugs due to their swelling in the cell culture medium. Different disulfide crosslinkers have been applied, although the solubility of the common S–S crosslinker in water is limited.20 Galaev et al. synthesized disulfide crosslinkers based on methacrylated cystine.21 The major benefit of this crosslinker is its high water solubility due to the carboxyl groups, which allows polymerization in aqueous solution.
Recently, targeted delivery of anticancer drugs has become a major research interest, and different targeting strategies have been proposed and applied to minimize the systemic toxicity of therapeutic agents. In cancer therapy, to maintain the effective dose of a drug in the blood, a high dose of the therapeutic agent should be maintained in the body for a long time, which leads to high-risk peripheral responses.22 Accordingly, in magnetic target approaches, like endowing magnetic feature to CNT, an external magnetic field can retain the carrier at target tissue and facilitate its extravascular uptake.
DOX is a popular anticancer drug in chemotherapy and has an acceptable effect against a large number of tumors. Nevertheless, DOX presents serious side effects, such as nephrotoxicity, cardiotoxicity, and hepotoxicity to healthy cells.23 Due to this disadvantage, it is necessary to deliver DOX via proper nanovectors to reduce the side effects of DOX. The main goal of this paper is the design and utilization of a multi stimuli-responsive nanovector by thermal sensitivity due to PNIPAM-co-PHEMA and redox sensitivity due to the presence of disulfide bonds for pinpointed DOX delivery into specific organs. The influences of temperature and enzyme reduction on the release of DOX from poly(NIPAM-HEMA-SS)/MN-MWCNTs were studied, and the structure, morphology, magnetic and LCST properties of the products were evaluated. Afterwards, the in vitro and in vivo biocompatibility of the resulting nanoparticles and their in vitro cytotoxicity against MCF7 cells were evaluated at different concentrations and temperatures by conducting 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assays.
Experimental
Material
N-Isopropylacrylamide (NIPAM), L-cystine, hydroxyethyl methacrylate, glycidyl methacrylate, ammonium iron(II) sulfate hexahydrate, methacryloyl chloride, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, and sodium dodecylbenzene sulfonate (SDBS) were obtained from Sigma-Aldrich. Potassium persulfate, hydrazine hydrate, ethylenediamine, N,N′-dicyclohexylcarbodiimide (DCC), potassium carbonate, tetrahydrofuran, chloroform, ethyl acetate and ethanol were provided by Merck Millipore. Carboxyl functionalized short MWCNTs were purchased from Neutrino Corporation (Neutrino Nanovation, Iran). DOX in the form of its hydrochloride salt was purchased from Korea United Pharm. INC (Chungnam, Korea). Human red blood cells (HRBCs) stabilized with EDTA were obtained from the Iran Blood Transfusion Institute. Deionized water used for all experiments was purified with a Milli-Q water system. All the chemicals were of analytical grade and were used without purification.Characterization
Transmission electron microscopy (Zeiss LEO 906) was used to define the morphologies and sizes of the magnetic MWCNTs and polymer-grafted MN-MWCNTs.The samples were dispersed in ethanol and sonicated thoroughly before drop casting. The structures and major functional groups of the compounds were determined using Fourier transform infrared (FTIR) spectroscopy (Bruker, Germany) via the KBr pellet method and nuclear magnetic resonance spectroscopy. The crosslinker was dissolved in D2O for the NMR measurements on a Bruker DRX-500 MHz spectrometer (USA). XPS analysis was carried out using an Al Kα (1486.6 eV) excitation source in a Thermo Electron Corporation spectrometer to verify the final structure. MN-MWCNTs and Poly(NIPAM-HEMA-SS)/MN-MWCNTs were also characterized by atomic force microscopy (AFM). In order to image the samples, prepared dispersions were diluted with water to obtain a concentration of 500 ng mL−1, and droplets were deposited onto a freshly cleaved mica substrate (1 cm2) and dried in air. AFM measurements were performed using Ntegra (NT-MDT, Russia) operated in contact mode at room temperature. Data analysis was carried out using “Gwyddion 2.9”, a free SPM data visualization and imaging tool released under the GNU General Public License. The CHNOS elemental analyses were performed using an Elementar Vario EL III Universal CHNOS Elemental Analyzer. Ultra-sensitive differential scanning calorimetry (US-DSC) measurements were performed from 15 °C to 70 °C (with a heating rate of 1 °C min−1) on a Micro Cal Inc. VP DSC under a nitrogen atmosphere with 0.1% (w/v) poly(NIPAM-HEMA-SS)/MN-MWCNT aqueous solution.
Preparation of magnetite MWCNT nanoparticles (MN-MWCNTs)
0.57 g of ammonium iron(II) sulfate hexahydrate was dissolved in 10 mL deionized water and, subsequently, 0.1 g of oxidized MWCNTs was added to this mixture, which was then sonicated for 15 min. Then, 1.25 mL hydrazine hydrate was added and ultrasonicated for 15 min (5 min on/3 min off). The pH of the resulting aqueous suspension was adjusted to 11 to 12 via NaOH, followed by reflux at boiling temperature for 2 h. The black MN-MWCNT hybrid was separated with an external magnetic field, washed with water and ethanol several times and dried under vacuum at 50 °C for 24 h; 0.23 g of product was obtained.Amine modification of MN-MWCNTs
The aminated MN-MWCNTs were prepared by functionalization of COOH-decorated magnetite MWCNTs with ethylenediamine.24 Briefly, 10 mg of the magnetite MWCNTs was ultrasonicated in 40 mL of THF. Next, 20 mL of a solution of 200 mg ethylenediamine in THF was added to the sonicated mixture. Afterward, 20 mL of THF solution containing 200 mg of DCC was poured dropwise into the above mixture and mechanically stirred in ambient temperature for 3 days under a purge of nitrogen. At the end, the aminated MN-MWCNTs were separated with a magnet, washed five times with THF and ethanol, and dried under vacuum at room temperature to obtain 10 mg of aminated MN-MWCNTs.Preparation of acrylated MN-MWCNTs
Amine-modified MN-MWCNTs were reacted with glycidyl methacrylate, and the reaction was carried out as follows: 10 mg aminated MN-MWCNTs were ultrasonicated for 10 min in chloroform (20 mL), and then 500 μL glycidyl methacrylate was added dropwise to the solution. The reaction mixture was heated to 50 °C during bath sonication for 12 h. The product was separated and washed with chloroform many times to remove the excess glycidyl methacrylate. Finally, the acrylated MN-MWCNTs were dried under vacuum overnight at 25 °C for 24 h; 10 mg of product was obtained.Synthesis of N,N′-bis(methacryloyl)-L-cystine (MAS-S)
MAS-S was synthesized according to the method described by Galaev et al.21 Briefly, L-cystine and NaOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 molar ratio) were dissolved in 30 mL of K2CO3 aqueous solution (5 vol%). After stabilization at 0 °C, 2 mmol of methacryloyl chloride were added slowly to this solution under a nitrogen purge, and the mixture was stirred magnetically at room temperature for 2 h in a closed vessel. Afterwards, the pH of the solution was tuned to 7, and then unreacted methacryloyl chloride was extracted with ethyl acetate. The aqueous phase was evaporated in a rotary evaporator. The residue was crystallized, first in ethanol and then in ethyl acetate, with 71% yield. The structure analysis of the synthesized disulfide crosslinker was completed using 1H-NMR spectroscopy.
2 molar ratio) were dissolved in 30 mL of K2CO3 aqueous solution (5 vol%). After stabilization at 0 °C, 2 mmol of methacryloyl chloride were added slowly to this solution under a nitrogen purge, and the mixture was stirred magnetically at room temperature for 2 h in a closed vessel. Afterwards, the pH of the solution was tuned to 7, and then unreacted methacryloyl chloride was extracted with ethyl acetate. The aqueous phase was evaporated in a rotary evaporator. The residue was crystallized, first in ethanol and then in ethyl acetate, with 71% yield. The structure analysis of the synthesized disulfide crosslinker was completed using 1H-NMR spectroscopy.
Preparation of poly(NIPAM-HEMA-SS)/MN-MWCNTs
10 mg of the acrylated MN-MWNTs in 60 mL of a 1.0 wt% SDBS aqueous solution was probe sonicated for 30 min (5 min on/3 min off). NIPAM (200 mg, 1.76 mmol), HEMA (10.7 μL, 0.09 mmol) and MAS-S (7.37 mg, 0.027 mmol) were dissolved in the dispersion, and the mixture was purged with N2 gas for 60 min to eliminate the remaining oxygen. Afterward, 14 mg potassium persulfate was added to the mixture. Polymerization was continued for 12 h at 60 °C and 600 rpm under nitrogen atmosphere. To recover the poly(NIPAM-HEMA-SS)/MN-MWCNTs and eliminate the SDBS molecules from their surfaces, the nanocarriers were dialyzed in a large amount of Milli-Q water (molecular weight cutoff of 12 kD) for 72 h and separated with a magnet; finally, 25 mg of product was obtained by freeze-drying.DOX-loading into and release from poly(NIPAM-HEMA-SS)/MN-MWCNTs
Encapsulation of DOX, a popular anticancer agent applied widely in cancer therapy, into the PNIPAM-HEMA/MN/MWCNTs was also studied. In brief, 1 mL of water solutions containing various concentrations of the drug (0.25, 0.5, 1, 1.25, 1.5 and 2 mg mL−1) were stirred with PNIPAM-HEMA/MN/MWCNTs (1 mg) in 2 mL PBS (pH = 7.4) for 24 h at room temperature in the dark. Then, the nanocarriers were separated by magnet and washed several times with PBS until the supernatant color disappeared. The content of free DOX in the solution was defined by measuring the absorbance at 490 nm using a calibration curve created under similar conditions. The drug encapsulation efficiency (%) is determined as (weight of DOX loaded in nanocarriers/weight of feeding DOX) × 100. The drug loading content (%) is determined as (weight of DOX loaded in nanocarriers/weight of nanocarriers) × 100.For the drug release studies, a suspension of DOX-loaded nanoparticles (2 mg) was dispersed in PBS (2 mL, 40 mM, pH = 7.4) solution and placed in a dialysis bag (molecular weight cutoff of 12 kDa) and dialyzed against the corresponding PBS (30 mL) at two different temperatures of 25 and/or 41 °C with a rotating speed of 90 rpm. Each experiment was repeated in triplicate. At pre-determined time intervals, PBS (5 mL) was removed and the concentration of released DOX was measured by UV-vis spectroscopy as explained above. The outer phase PBS was maintained at constant volume by replacing the removed volume with pre-warmed fresh release incubation medium (5 mL). In addition to the release described, DOX release was studied in the presence of DTT. For this purpose, suspensions of DOX-loaded nanocarriers were prepared in 20 mM DTT by a similar procedure to that described above.
Hemolysis assay
The in vitro hemolysis assays were carried out according to the method described by Yang et al.25 Briefly, the HRBCs were obtained via eliminating serum from the blood via centrifugation and were washed with sterile isotonic PBS solution five times.Next, the red blood cells were diluted with PBS solution, and 0.3 mL of the mixture was mixed with: (a) 1.2 mL of deionized water as a positive control; (b) 1.2 mL of PBS as a negative control and (c) 1.2 mL of poly(NIPAM-HEMA-SS)/MN-MWCNT suspensions at concentrations of 50, 100, 200 and 400 μg mL−1. The suspensions were shaken moderately and left to stand for 2 h at ambient temperature. After that, the suspensions were centrifuged, and the absorbance of the supernatants was scanned by UV-vis spectroscopy from 500 to 650 nm. The hemolysis values of the samples were calculated by dividing the absorbance difference between the sample and the negative control by the difference in absorbance at 541 nm between the negative and positive controls.
In vivo toxicity studies
Healthy male Balb/c mice (body weight about 20 g and 5 to 6 weeks) were purchased from the Pasteur Institute, Tehran, Iran. All procedures in this study, including the use of animals, were approved by the Research Council of Tehran University of Medical Sciences (Tehran, Iran), Ethics Committee on Animal Experiments, whose guidelines are in agreement with those of the National Institutes of Health for the use of live animals. Poly(NIPAM-HEMA-SS)/MN-MWCNTs dispersed in physiological saline (0.2 mL) at a final amount of 10 mg kg−1 were injected into Balb/c mice (n = 5 per group) via the tail vein. For the control group of Balb/c mice (n = 5), only physiological saline was injected.Serum biochemical analysis
The blood samples were taken from ophthalmic veins before sacrifice at 4 h and 10 days after injection. The serum was separated by centrifuge at 3500 rpm for 10 min. Two indexes for kidney functions (blood urea nitrogen (BUN) and creatinine) and two main hepatic indexes (alanine aminotransferase (ALT) and aspartate aminotransferase (AST)) were specified.In vitro cytotoxicity studies
The cytotoxicities of free DOX, pure poly(NIPAM-HEMA-SS)/MN-MWCNTs and DOX-loaded poly(NIPAM-HEMA-SS)/MN-MWCNTs against MCF-7 and MCF-10A cells were evaluated via MTT assay. The cells were cultivated in a 96-well plate at a density of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells per well and incubated at 37 °C in a humidified atmosphere including 5% CO2 for 24 h. After pre-incubation, the medium was removed and the cells were treated with 100 μL of fresh medium containing either free DOX, pure poly(NIPAM-HEMA-SS)/MN-MWCNT and DOX-loaded poly(NIPAM-HEMA-SS)/MN-MWCNT samples at various concentrations or temperatures with the same concentration of DOX. The incubation temperature was maintained at 37 and 41 °C for 12 h and was then adjusted to 37 °C for 36 h.
000 cells per well and incubated at 37 °C in a humidified atmosphere including 5% CO2 for 24 h. After pre-incubation, the medium was removed and the cells were treated with 100 μL of fresh medium containing either free DOX, pure poly(NIPAM-HEMA-SS)/MN-MWCNT and DOX-loaded poly(NIPAM-HEMA-SS)/MN-MWCNT samples at various concentrations or temperatures with the same concentration of DOX. The incubation temperature was maintained at 37 and 41 °C for 12 h and was then adjusted to 37 °C for 36 h.
After 48 h of incubation, 20 μL of 5.0 mg mL−1 MTT was added to each well, followed by incubation at 37 °C. After eliminating the supernatants, 150 μL DMSO was poured into each well, and the absorbance of the obtained solution was measured at 570 nm. Data are reported as mean ± SD.
Prussian blue staining
To study the cellular uptake behavior of poly(NIPAM-HEMA-SS)/MN-MWCNTs, DU145 cells were plated in 6-well plates with culture medium containing nanoparticles at two concentrations of 100 and 400 μg mL−1 and were incubated for 48 h. Then, the culture medium was removed and the cells were washed three times with PBS. Afterward, 4% formaldehyde solution was used to fix the cells for 30 min. The fixed cells were washed with PBS and stained using the Prussian blue method. Briefly, equivalent volume mixtures of 2% HCl solution and 2% potassium ferrocyanide(II) trihydrate were poured into each well, and the cells were left to incubate at room temperature. The contents of each well were washed with PBS three times. The Prussian blue staining was investigated using light microscopy.Results and discussion
The nanocarrier was prepared by free radical polymerization of NIPAM, HEMA and cystine-based disulfide crosslinker on the surface of well-dispersed acrylated magnetite MWCNTs in an aqueous solution of SDBS as surfactant. In order to graft the polymer on the surface of the MWCNTs, the unsaturated groups should be introduced on the MWCNTs in the following order: (i) preparation of the magnetite MWCNT nanoparticles, (ii) reaction between the carboxylic groups and an excess amount of ethylenediamine in the presence of DCC to produce free amine functional groups, and (iii) reaction of aminated MN-MWCNTs with glycidyl methacrylate to obtain acrylated MN-MWCNTs. Finally, free radical polymerization occurs in the presence of monomers, crosslinker and initiator in an aqueous solution of the SDBS under nitrogen purged conditions.SDBS was chosen as the surfactant because it enables better dispersion of MWCNTs in aqueous media. The MWCNTs are trapped inside micelles which form in the presence of SDBS, preventing accumulation of the CNTs. However, these steps present the advantage of reduced dimensions of nanocarriers for drug delivery. If appropriately engineered nano-sized delivery systems can be achieved, finer temporal control over drug release rates due to their large surface areas can be obtained. In this procedure, polymers were covalently attached to the CNTs. Covalent polymer systems have the advantage of greater polymer stability compared to non-covalent techniques. Moreover, the ability to direct and concentrate these vectors in a specific area of the body through the application of an external magnetic field gives an added advantage to such delivery systems. A schematic is shown in Scheme 1 that demonstrates the general concept of this study.
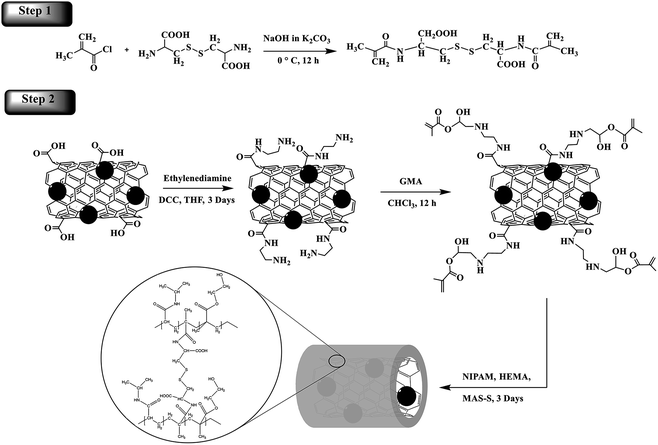 | ||
| Scheme 1 A schematic of the synthesis of the disulfide MAS-S crosslinker and the poly(NIPAM-HEMA-SS)/MN-MWCNTs. | ||
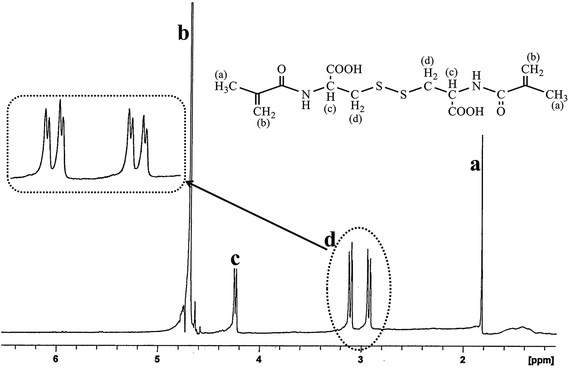
MAS-S was synthesized according to the method described by Galaev.21 MAS-S was prepared by the reaction of methacryloyl chloride with L-cystine in an extremely basic medium (Scheme 1). MAS-S is a hydrophilic crosslinker and has good solubility in water, which permits the polymerization/cross-linking of monomers in the emulsion system. The structure of MAS-S was determined using 1H-NMR and elemental analysis. The 1H-NMR (500 MHz, D2O) peak assignments are proved by the corresponding alphabetic proton labeling on the spectrum (Fig. 1) and in the molecular structure scheme. A peak at 1.82 ppm (3H, s) belongs to the CH3 (a) protons. The protons of the –CH2– (d) group appear at 2.91 ppm (2H, dd, J1 = 12.9 Hz, J2 = 3.0) and 3.11 ppm (2H, dd, J1 = 12.9 Hz, J2 = 3.0 Hz). Peaks at 4.24 ppm (1H, dd, J1 = 10 Hz, J2 = 3.0 Hz) and 4.641 ppm (2H, s) are related to the –CH (c) and ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 (b) groups, respectively. Formula: C14H20N2O6S2, elemental analysis: C, 44.62%; H, 3.34%; N, 7.42%; O, 25.48%; S, 17.01%.
CH2 (b) groups, respectively. Formula: C14H20N2O6S2, elemental analysis: C, 44.62%; H, 3.34%; N, 7.42%; O, 25.48%; S, 17.01%.
In order to investigate each step of the MWCNT functionalization, FT-IR spectroscopy was applied (Fig. 2). The FT-IR spectrum of the oxidized MWCNTs showed a peak at 1751 cm−1, corresponding to the carboxyl group stretching vibration26 (Fig. 2a). Broad peaks around 3100 to 3500 cm−1 are associated with the –OH stretching band in carboxyl and hydroxyl groups. The C–C stretching band that appeared at 1623 cm−1 is associated with the predicted phonon modes of the nanotubes.27 Two weakly sharp peaks at 2920 and 2853 cm−1 are related to the C–H stretching bands in the aliphatic –CH2 and CH3 groups.27
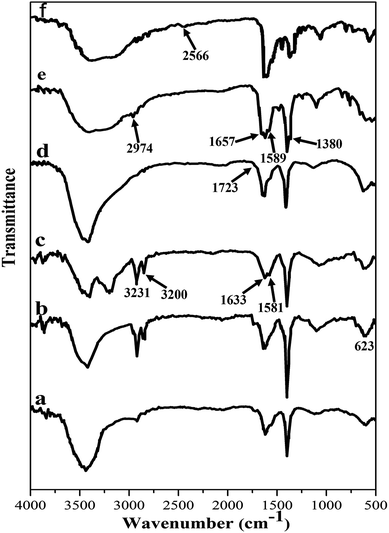 | ||
| Fig. 2 FTIR spectra of (a) MWCNTs, (b) MN-MWCNTs, (c) aminated MWCNTs, (d) acrylated MWCNTs, (e) poly(NIPAM-HEMA-SS)/MN-MWCNTs, and (f) poly(NIPAM-HEMA-SS)/MN-MWCNTs after DTT treatment. | ||
Magnetite MWCNTs (MN-MWCNTs) were synthesized using ammonium iron(II) sulfate hexahydrate. The presence of Fe3O4 nanoparticles in the MWCNT structure can be identified by the strong absorption band at 623 cm−1, which corresponds to the Fe–O band (Fig. 2b).27 In the infrared spectrum of aminated MN-MWCNTs, the broad peaks around 3400 cm−1 correspond to the absorption of –NH stretching. The N–H bending vibration of the amide group can be observed at 1581 cm−1. Two peaks at 3200 and 3231 cm−1 correspond to the stretching bands of the amine group. The absorption at 1633 cm−1 is attributed to the stretching of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O band of amide (Fig. 2c).24 In the acrylated MN-MWCNT, the peaks related to amine groups disappeared, and a carbonyl group peak is observed at 1723 cm−1 (Fig. 2d).
O band of amide (Fig. 2c).24 In the acrylated MN-MWCNT, the peaks related to amine groups disappeared, and a carbonyl group peak is observed at 1723 cm−1 (Fig. 2d).
By comparing the FTIR spectrum of poly(NIPAM-HEMA-SS)/MN-MWCNTs with the spectrum of acrylated MN-MWCNTs, significant differences at several wavelengths can be observed (Fig. 2e). The characteristic stretching vibration of the amide group at 1657 cm−1 is attributed to the amide I band belonging to PNIPAM, 1589 cm−1 is the amide II band assigned to N–H in-plane bending vibrations and 1492 cm−1 is the amide III band which is related to the C–N stretching peak.28,29 Additionally, wavelengths of about 1380 and 2974 cm−1 are attributed to the –CH(CH3)2 and –CH3 respectively.30
In the presence of DTT, the disulfide bonds of poly(NIPAM-HEMA-SS) will disintegrate. The decomposition of poly(NIPAM-HEMA-SS) in the presence of DTT was confirmed to be related to the reduction of S–S bonds (Scheme 2). The FT-IR spectrum of poly(NIPAM-HEMA-SS)/MN/MWCNTs after adding DTT shows the S–H stretching band at about 2566 cm−1 as the verification of the S–S bond dissociation of the disulfide crosslinker, while there is no noticeable peak before the addition of DTT (Fig. 2f).21
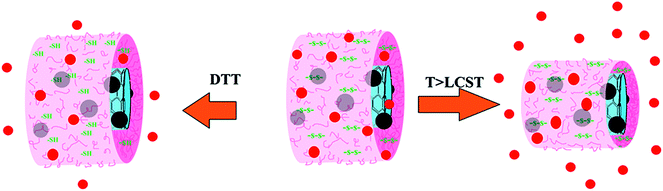 | ||
| Scheme 2 A schematic of DOX release from the poly(NIPAM-HEMA-SS)/MN-MWCNTs by increasing the temperature to above LCST and reducing the disulfide bonds with DTT enzyme. | ||
In order to verify the presence of the thermal and reduction-sensitive shell around the MN-MWCNTs, XPS analysis was performed.
The major XPS peaks at 54.8, 285.5, 533.23, 399.12 and 713.6 eV were assigned to Fe3p, C1s, O1s, N1s and Fe2p, respectively, and the peak at 163 eV was attributed to S2p of the MAS-S crosslinker (Fig. 3a).
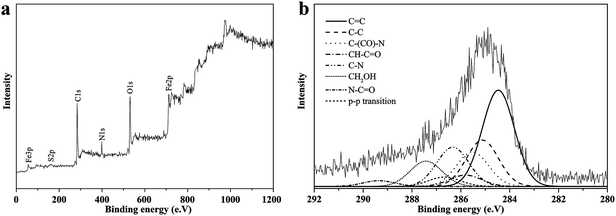 | ||
| Fig. 3 Wide-scan XPS survey (a) and high-resolution XPS survey of the C1s region (b) for poly(NIPAM-HEMA-SS)/MN-MWCNTs. | ||
The C1s core-level spectrum can be curve-fitted into several peaks, which indicates that there are different types of carbon in the structure: the graphite-like structure (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) at 284.5 eV; (C–C/C–H) of MWCNT at 285.1 eV; the π–π transition at 289.4 eV; the carbon attached to the amide group of PNIPAM (C–C
C) at 284.5 eV; (C–C/C–H) of MWCNT at 285.1 eV; the π–π transition at 289.4 eV; the carbon attached to the amide group of PNIPAM (C–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O: 285.5 eV); the carbon adjacent to the nitrogen of PNIPAM (C–N: 286.4 eV), the carbonyl group of PNIPAM (N–C
O: 285.5 eV); the carbon adjacent to the nitrogen of PNIPAM (C–N: 286.4 eV), the carbonyl group of PNIPAM (N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O: 287.5 eV); the carbon attached to the carbonyl group of HEMA (–CH–C
O: 287.5 eV); the carbon attached to the carbonyl group of HEMA (–CH–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O: 285.8 eV); and the alpha-methylene to the hydroxyl group of HEMA (–CH2–OH: 286.6 eV) (Fig. 3b).31,32
O: 285.8 eV); and the alpha-methylene to the hydroxyl group of HEMA (–CH2–OH: 286.6 eV) (Fig. 3b).31,32
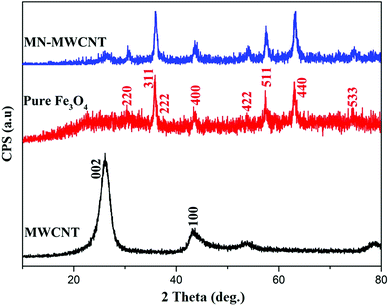
Fig. 4 represents the XRD peaks of the carboxylic acid functionalized MWCNTs, magnetic MWCNTs and pure Fe3O4 nanoparticles, respectively. The obtained hkl indices ((3 1 1), (2 2 0), (4 0 0), (2 2 2), (5 1 1), (4 2 2), and (4 4 0)) affirmed the cubic structure of the Fe3O4 nanoparticles.33 The secondary specified peaks can be demonstrated as the characteristic (1 0 1) and (0 0 2) indices related to graphite; also, the widening of the peak in the pattern demonstrates that the as-synthesized Fe3O4 crystallites are subminiature.34 The average crystallite dimension of Fe3O4 nanoparticles coated on MWCNTs was estimated on the basis of the maximum intensity peak at 311, utilizing the Debye–Scherrer formula, which is about 10 nm.
TEM was utilized to study the distribution and morphology of Fe3O4 nanoparticles on the MWCNTs and also the grafting of the biodegradable polymer on the MN-MWCNTs. The TEM images (Fig. 5a and b) demonstrate that the Fe3O4 nanoparticles have a mean size of 9.8 nm and are uniformly coated on the exterior walls of the MWCNTs. Some of these nanoparticles have been loaded inside the MWCNTs due to the penetration of ferric solution into the CNTs because of capillary forces during the saturation process.33 Most of the Fe3O4 nanoparticles were attached on the surface of the nanotubes; therefore, this phenomenon could be due to the closed tips of the nanotubes. The core/shell structures of the polymeric nanocarriers can be distinguished in the TEM images (Fig. 5c and d). The MWNT margin has become slightly dark due to the grafting of poly(NIPAM-HEMA-SS) on the surface of the MN-MWCNTs, and the width of the grafted stimuli-responsive polymer layer is about 3 to 5 nm. Also, as can be seen, the lengths of the CNTs in final nanoparticles are very similar to the lengths of the initial CNTs in the MN-MWCNTs, and they have maintained their tubular structures.
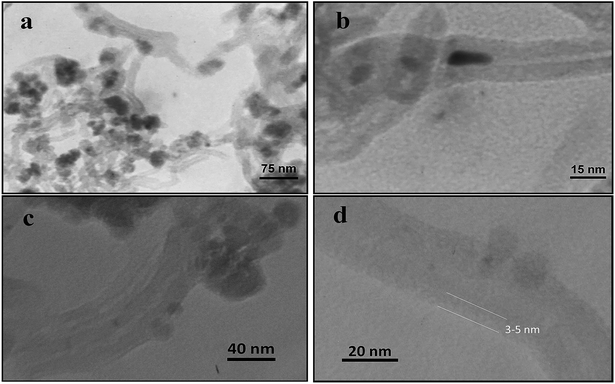 | ||
| Fig. 5 TEM micrographs of Fe3O4-filled pretreated MWCNTs (a, b) and poly(NIPAM-HEMA-SS)/MN/MWCNTs (c, d). | ||
It is remarkable that the high resolution TEM electron beam may destroy the coated polymer chains and may cause the loss of the polymer shell width, which emerges in the image.31
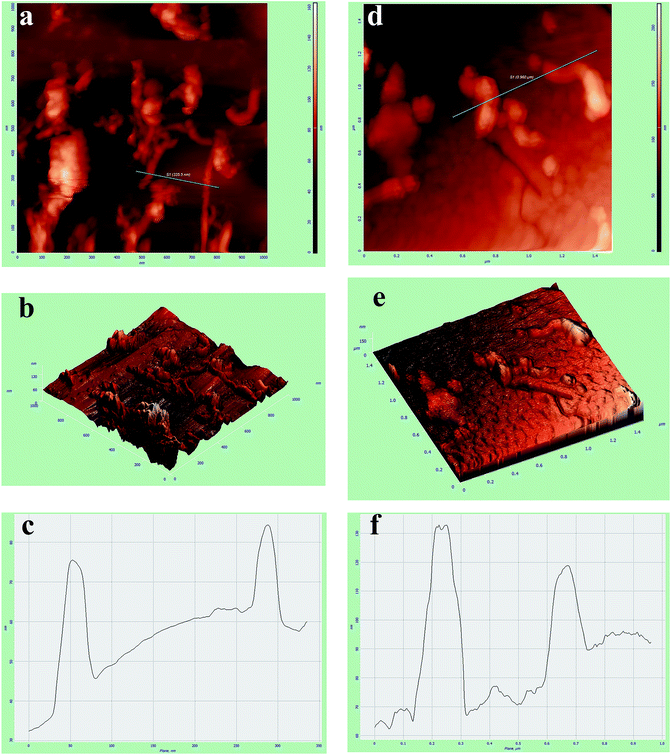
AFM was also applied to study the surface topography properties, such as roughness and thickness, of the MN-MWCNTs and poly(NIPAM-HEMA-SS)/MN-MWCNTs. Fig. 8 illustrates the AFM surface topology of the MN-MWCNTs using the height image 2D (Fig. 6a), 3D (Fig. 6b), and cross height (Fig. 6c) images. The 2D image of the MN-MWCNTs surface shows uniformly small particles of Fe3O4 on the whole surface of MWCNTs as spherical particles. The presence of magnetic nanoparticles with sizes of about 10 nm on the MN-MWCNTs surface is obviously displayed in the 2D AFM image.
The poly(NIPAM-HEMA-SS)/MN-MWCNT surface can be clearly distinguished from the MN-MWCNTs due to the polymeric shell grafting that evenly coats the surface of the MN-MWCNT particles. Based on the AFM images, it can be seen that the diameters of the MN-MWCNT particles increased significantly after modifying their surfaces. The desired polymer shell is obviously recognizable on the MN-MWCNTs in the height image (Fig. 6d), 3D (Fig. 6e), and cross height image (Fig. 6f).
All of the aforesaid characterizations are in agreement with each other, verifying the successful coating of poly(NIPAM-HEMA-SS) onto the surface of the MN-MWCNTs.
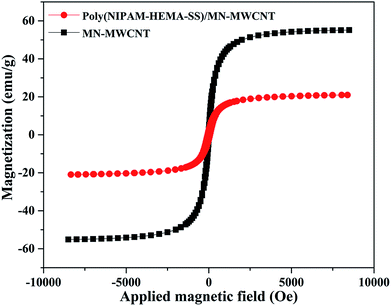
Fig. 7 shows the magnetization variation of the MWCNTs, the contained Fe3O4, and the poly(NIPAM-HEMA-SS)/MN-MWCNTs. The study of the variation of magnetization with an applied magnetic field gives useful information about the magnetic properties of magnetic polymeric particles. The magnetic hysteresis loop describes the characteristics of these particles. A vibrating sample magnetometer (VSM) was applied to investigate the magnetization of the prepared hybrid polymer. The outcomes of VSM analysis show the repercussions of magnetic CNTs on external magnetic fields, and the characteristic parameters are: saturation coercive force (Hc), magnetization (Ms), and remanence magnetization (Mr). The saturation magnetization quantity of the pure magnetic MWCNTs is 55 emu g−1 at 25 °C, with very low remanence and coercivity content, which demonstrates that the MN/MWCNTs are superparamagnetic, presumably because of the small size of the Fe3O4 nanoparticles.35 The saturation magnetization value of the polymer-coated MWCNTs was 19 emu g−1, which is acceptable for biomedical applications.35
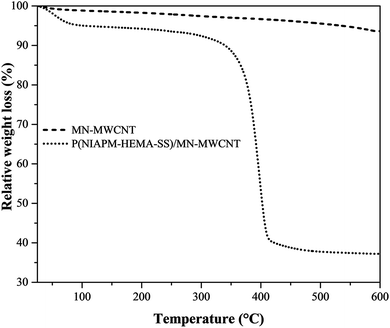
TGA of the MN-MWCNTs and poly(NIPAM-HEMA-SS)/MN-MWCNTs was carried out to evaluate the amount of polymer grafted on the surface of the MN-MWCNTs. Samples were heated from room temperature to 600 °C under purging N2 gas. The weight loss temperatures of the MN-MWCNTs that occur below 100 °C are possibly related to the evaporation of water molecules, trapped on the surface of the sample.36 This process was applied for the polymeric sample; the main mass loss of poly(NIPAM-HEMA-SS)/MN-MWCNTs occurs near 380 °C, which is related to the decomposition of the polymer shell. According to Fig. 8, the weight losses of the MN-MWCNTs and the poly(NIPAM-HEMA-SS)/MN-MWCNTs are 6.4% and 62.8%, respectively. Hence, the actual amount of stimuli-responsive shell on the MN-MWCNTs was calculated to be 56.4%, which can be considered as potent proof for the successful coating of the polymer onto the surface of the MN-MWCNTs.
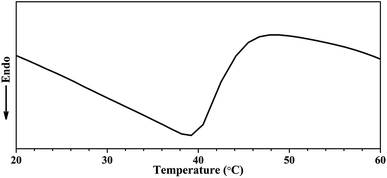
To study the transition behavior of the poly(NIPAM-HEMA-SS)/MN-MWCNTs, we performed ultrasensitive calorimetric measurements (micro-DSC). Micro-DSC can measure energy variations as small as 0.02 μcal s−1 included in a dynamic process of polymer chains in a dilute solution. Fig. 9 shows the typical micro-DSC curve of the poly(NIPAM-HEMA-SS)/MN-MWCNTs; it is obvious that the phase transition temperature is about 39 °C, which is higher than the LCST of neat PNIPAM.37 An increase in phase transition temperature is dependent on the presence of smaller ratios of hydrophilic monomers such as HEMA.28
Due to the hollow space inside the MN-MWCNTs and the three-dimensional net structure of stimuli-responsive polymer covering its surface, the poly(NIPAM-HEMA-SS)/MN-MWCNT carrier can be utilized as a nanoreservoir to store drugs. Stimuli-responsive systems take advantage of the significant variations in temperature and reducing enzymes to stimulate localized drug delivery to different regions of the body. To study the drug loading capacity, the temperature dependency of drug discharge and the effect of the presence of DTT on the drug release of the polymeric nanoparticles, DOX was used as a drug model. The UV spectroscopy analysis was used to characterize both the loading capacity and the encapsulation efficiency of the poly(NIPAM-HEMA-SS)/MN-MWCNTs. Table 1 presents the average encapsulation efficiencies (EE) and the loading capacities (LC) for different concentrations of DOX. The encapsulation efficiency was observed to be 71% and the loading capacity was about 41.5% when equal amounts of nanovector and drug (1 mg) were used. Nevertheless, the encapsulation efficiency decreases with increasing concentration of DOX; however, a corresponding growth in loading capacity is observed. The poly(NIPAM-HEMA-SS)/MN-MWCNTs were found to have a superior loading capacity of 49.5% when the ratio of drug to vector was about 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and the encapsulation efficiency was observed to be 49%, which proves that the poly(NIPAM-HEMA-SS)/MN-MWCNTs have good drug loading capacity and have the potential for use in drug delivery.
1, and the encapsulation efficiency was observed to be 49%, which proves that the poly(NIPAM-HEMA-SS)/MN-MWCNTs have good drug loading capacity and have the potential for use in drug delivery.
DOX![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) polymer ratio polymer ratio |
Encapsulation efficiency (%) | Loading capacity (%) |
|---|---|---|
0.25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
74 | 15.6 |
0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
74 | 27.0 |
0.75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
73 | 35.3 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
71 | 41.5 |
1.25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
68 | 45.9 |
1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
62 | 48.1 |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
49 | 49.5 |
In order to attain quantitative and qualitative information on DOX release from poly(NIPAM-HEMA-SS)/MN-MWCNTs nanoparticles, the release profile was determined using an in vitro dialysis technique in PBS buffer solution (pH = 7.4). The content of released DOX was specified using the UV absorbance of the solution removed from the dialysis tube. The DOX loaded poly(NIPAM-HEMA-SS)/MN-MWCNTs show considerable release behavior. Herein, due to their thermo-responsive shell, the release of the DOX particles can be controlled. Scheme 2 illustrates the mechanism of DOX release from the poly(NIPAM-HEMA-SS)/MN-MWCNTs at above LCST and in the presence of DTT as a reducing agent.
As shown in Fig. 10, the DOX release profile of the poly(NIPAM-HEMA-SS)/MN-MWCNTs displayed a significant shift due to temperature variation. For example, at 25 °C, 12% of the DOX was released from the poly(NIPAM-HEMA-SS)/MN-MWCNTs in 12 h; as the temperature increased to 41 °C, the cumulative amount of DOX released from the poly(NIPAM-HEMA-SS)/MN-MWCNTs in 12 h increased to 41%. Comparing the two release curves at any distinct time, the amount of drug released from the nanovectors at 41 °C is noticeably greater than the amount released at 25 °C.
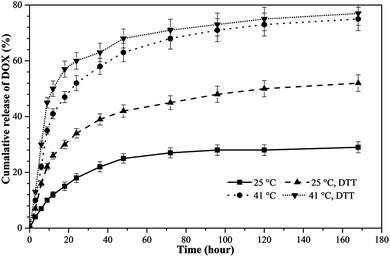 | ||
| Fig. 10 In vitro cumulative release of DOX from poly(NIPAM-HEMA-SS)/MN-MWCNTs at 25 °C and 41 °C in the absence and the presence of 20 mM DTT. | ||
The difference in DOX release below and above LCST could be related to the swelling–deswelling of the PNIPAM fraction on the surface polymeric layer of poly(NIPAM-HEMA-SS)/MN-MWCNTs. Above LCST, the faster release is due to the contraction of PNIPAM, which ejects the DOX from the polymeric shells to enable DOX delivery from the nanocarrier.
The reduction-triggered DOX release from the poly(NIPAM-HEMA-SS)/MN-MWCNTs was achieved using DTT as the reducing agent at various temperatures at pH = 7.4. It is obvious that in the presence of 20 mM DTT, more rapid release rates of DOX at 25 and 41 °C were observed, and the percent cumulative releases were 26% and 50% in 12 h, respectively; these values are meaningfully higher than those in the absence of DTT. These observations prove that the polymeric shell plays a fantabulous and important stimulating role in the release mechanism because of its sensitivity to changes in the temperature and the presence of reducing enzymes. Consequently, the poly(NIPAM-HEMA-SS)/MN-MWCNTs can actually be used as a drug delivery agent in the physiological environment.
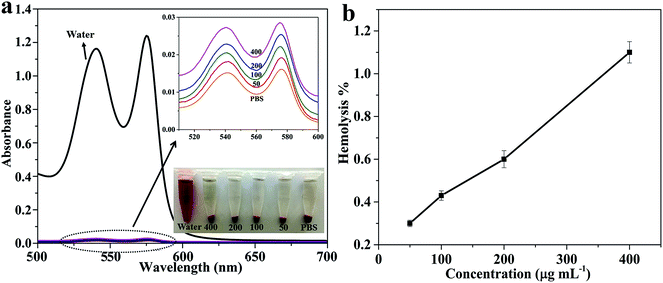
For successful drug delivery, the vectors should have excellent blood compatibility, which manifests as minimal hemolytic effects. Hemolysis assays were applied to estimate the blood adaptability of the poly(NIPAM-HEMA-SS)/MN-MWCNTs at various concentrations, as can be seen in Fig. 11. As expected, the negligible effect of the poly(NIPAM-HEMA-SS)/MN-MWCNTs is obvious at experimental concentrations over a broad range of 0 to 400 μg mL−1 (shown in the bottom-right inset of Fig. 11a). The hemolytic activities of the specimens were further quantitatively specified by evaluating the supernatant absorbance at 541 nm (hemoglobin) using UV-vis spectroscopy. At a high concentration, such as 400 μg mL−1, less than 1.1% hemolytic activity was observed for the poly(NIPAM-HEMA-SS)/MN-MWCNTs. Consequently, the negligible hemolytic activity of the poly(NIPAM-HEMA-SS)/MN-MWCNT nanocarriers confirmed the excellent biocompatibility of the vectors with blood cells, which is desirable for biological utilization.
A predetermined amount (10 mg kg−1) of poly(NIPAM-HEMA-SS)/MN-MWCNTs was intravenously injected into Balb/c mice via the tail vein. During the whole study period, all of the treated mice showed normal behavior; impaired mobility, reduced food taking, and symptoms of infection were not recognized, which indicated the negligible toxicity of the nanocarrier. The blood samples were individually obtained from ophthalmic veins before sacrifice at 4 h and 10 days after injection. The serum was separated by centrifuge at 3500 rpm for 10 min and serum levels of BUN, creatinine, ALT and AST were assayed.
Nano-materials can induce the inflammatory response and change the proper function of the immune system and, hence, hematological factors.38 Biochemical analyses were performed to determine the possible toxicity of poly(NIPAM-HEMA-SS)/MN-MWCNTs after injection.
Two significant hepatic indicators, viz. ALT and AST, were at similar levels for the mice subjected to poly(NIPAM-HEMA-SS)/MN-MWCNTs and for the control mice (Table 2).
| Chemistry | Control | Treated mice [mouse−1] | |
|---|---|---|---|
| 4 h | 10 d | ||
| Creatinine [mg dL−1] | 0.7 ± 0.08 | 0.7 ± 0.1 | 0.6 ± 0.09 |
| BUN (mM) [mg dL−1] | 36 ± 8 | 38 ± 7 | 37 ± 6 |
| ALT [U L−1] | 72 ± 10 | 77 ± 13 | 74 ± 12 |
| AST [U L−1] | 128 ± 13 | 136 ± 18 | 144 ± 16 |
Also, the results of two groups of treated mice (4 h and 10 days after injection) indicate that there are no significant variations in any indicators related to kidney functions (BUN and creatinine) compared with the control group (Table 2). On the basis of these outcomes, there are no symptoms of toxicity related to the poly(NIPAM-HEMA-SS)/MN-MWCNTs in mice at the high dosage of 10 mg kg−1 at disposal time up to 10 days, which are the indications of liver and kidney health in treated mice.
Although additional long-term studies are necessary to completely demonstrate the toxicology profiles of the poly(NIPAM-HEMA-SS)/MN-MWCNTs, our primary outcomes revealed that the poly(NIPAM-HEMA-SS)/MN-MWCNTs have acceptable biocompatibility.
The MCF7 cell line was used to evaluate the cytotoxicity of DOX–poly(NIPAM-HEMA-SS)/MN-MWCNT by incubation with DOX and DOX–poly(NIPAM-HEMA-SS)/MN-MWCNT at 37 °C and 41 °C at different concentrations. It is necessary to mention that in the hyperthermia samples, the temperature was maintained at 41 °C for 12 h and then decreased to 37 °C for 36 hours. These incubation situations, 37 °C and 41 °C, are chosen as the normal physiological temperature and intracellular temperature of cancer cells, respectively.
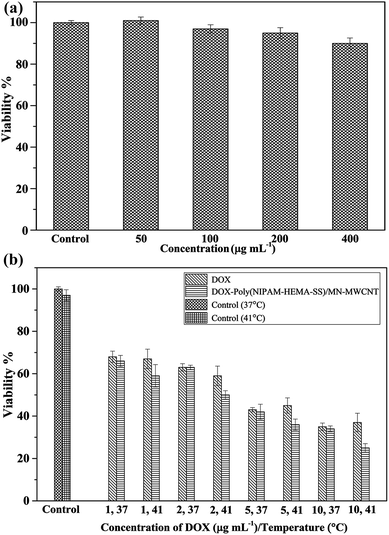
In the first step of this section, the cytotoxicity and cell viability of different concentrations of pure poly(NIPAM-HEMA-SS)/MN-MWCNT nanoparticles were studied via MTT assay. As can be seen in Fig. 12a, the control samples display no cytotoxicity at both temperatures. The pure poly(NIPAM-HEMA-SS)/MN-MWCNTs display no cytotoxicity compared with control samples toward MCF7 cells in different amounts after incubation for 48 h, suggesting the non-toxic and biocompatible properties of the nanoparticles. In the next step, the temperature responsive cytotoxicity of the DOX-loaded nanoparticles was determined in vitro on MCF7 cells in different concentrations compared with pure DOX. According to Fig. 12b, the cell viability of pure DOX was higher in comparison with that of DOX loaded nanoparticles at physiological and hyperthermia temperatures. However, the DOX–poly(NIPAM-HEMA-SS)/MN-MWCNTs show relatively temperature-sensitive effects under the same conditions. Under physiological conditions, the cytotoxicity of the DOX–poly(NIPAM-HEMA-SS)/MN-MWCNT nanoparticles was only slightly greater than of pure DOX, and they presented more cytotoxicity with increasing temperature. Meanwhile, with increasing temperature, the viability significantly decreases, so that the cell survival ratio decreased from 59 ± 1% at 37 °C to 50 ± 2% at 41 °C at a concentration of 2 μg mL−1 because of the increase of drug release under these conditions, likely as it was observed in the in vitro drug release. The IC50 values of MCF-7 cells treated with DOX at 37 °C and 41 °C were 3.66 ± 0.18 and 3.68 ± 0.21, respectively, and those of the cells treated with DOX–poly(NIPAM-HEMA-SS)/MN-MWCNTs at 37 °C and 41 °C were 3.62 ± 0.22 and 2.98 ± 0.16, respectively. As expected, DOX-loaded poly(NIPAM-HEMA-SS)/MN-MWCNTs at 41 °C displayed higher cytotoxic activities than free DOX at both temperatures and DOX–poly(NIPAM-HEMA-SS)/MN-MWCNTs at 37 °C. In addition, IC50 values were also determined for healthy cells (MCF-10A cells). The IC50 values of MCF-10A cells treated with DOX and DOX–poly(NIPAM-HEMA-SS)/MN-MWCNTs were 3.7 ± 0.14 and 4.26 ± 0.22, respectively, which represents slightly lower cytotoxic activities than the MCF-7 cell line. This reduction in cytotoxicity may be attributed to the lower temperature in MCF-10A than in the MCF-7 cell line. These results suggest that the DOX-loaded nanoparticles have greater efficiency for use as cancer treatments than pure DOX.
Below the LCST, the cellular uptake of DOX–poly(NIPAM-HEMA-SS)/MN-MWCNTs is expected to decrease because of the difference between the hydrophilicity of the nanoparticles below and above the LCST, which controls the swelling–deswelling of the polymeric shell. Above the LCST, the nanoparticles would shrink to pass the cell barriers and accumulate in the cells to release DOX inside the cells.39 We expect that the DOX–poly(NIPAM-HEMA-SS)/MN-MWCNT nanoparticles can differentiate cancer cells from healthy cells. This would result in accumulation of these vectors in cancerous cells and, accordingly, the release of DOX molecules only in the specific thermal situation of these cells, which would lead to more precise antitumor treatments and decrease the adverse effects of the loaded drug in non-cancerous cells.
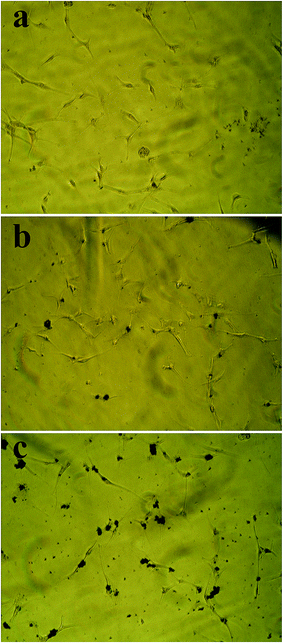
Prussian blue staining
To investigate the internalization of nanoparticles into DU145 cells, the Prussian blue staining method was used. The images taken with an inverted phase microscope at the desired concentrations of nanoparticles (Fig. 13) display concentration-related penetration of the poly(NIPAM-HEMA-SS)/MN-MWCNTs. The cellular uptake of the cells subjected to 100 μg mL−1 (Fig. 13b) was noticeably less than the cellular uptake of the cells subjected to 400 μg mL−1 (Fig. 13c). The cellular uptake increased at higher concentrations because the translocation across the cell membrane increased when the concentration was increased to 400 μg mL−1. The results confirm that the nanoparticles have the ability to pass through cell membranes and can possibly cause cell death.Conclusions
To achieve stimuli-responsive DOX delivery, thermo and reduction-responsive poly(NIPAM-HEMA-SS)/MN-MWCNT nanoparticles were synthesized. The use of stimuli-responsive polymers has many advantages, including the release and distribution of the drug in a specific target, thus reducing side effects or adverse systemic reactions. FTIR, XPS, TEM, AFM, DSC and TGA characterization confirmed that the stimuli-responsive shell is successfully coated on the MN-MWCNTs. The DOX loading efficiency and content of the poly(NIPAM-HEMA-SS)/MN-MWCNTs show acceptable values. The effects of temperature and DTT on the drug release were investigated; a dependent release profile was observed, so that the releases of DOX at higher temperature and in the presence of DTT were greater than those at physiological temperature and in the absence of DTT. The in vivo and in vitro analysis revealed that the poly(NIPAM-HEMA-SS)/MN-MWCNTs show negligible cytotoxicity, excellent biocompatibility with blood and no toxicity in the liver and kidneys of mice. DOX–poly(NIPAM-HEMA-SS)/MN-MWCNTs present significant cytotoxicity to MCF7 cells at different temperatures and concentrations. Based on these results, the DOX loaded in poly(NIPAM-HEMA-SS)/MN-MWCNTs can be adjusted by body temperature to reduce side effects.Acknowledgements
Sincere thanks are given to Prof S. Khoei and his group in Iran University of Medical Sciences (IUMS), Tehran, Iran, for generously supporting our cell viability assay experiments.References
- F. H. Nasr and S. Khoee, Eur. J. Med. Chem., 2015, 102, 132 CrossRef CAS PubMed.
- S. Khoee, A. Kavand and F. Hashemi Nasr, Polym. Int., 2015, 69, 1191 CrossRef.
- R. Q. Long and R. T. Yang, J. Am. Chem. Soc., 2001, 123, 2058 CrossRef CAS PubMed.
- M. Sabiha, R. Mostafizur, R. Saidur and S. Mekhilef, Int. J. Heat Mass Transfer, 2016, 93, 862 CrossRef CAS.
- P. Greil, Adv. Eng. Mater., 2015, 17, 124 CrossRef CAS.
- G. Risi, N. Bloise, D. Merli, A. Icaro-Cornaglia, A. Profumo, M. Fagnoni, E. Quartarone, M. Imbriani and L. Visai, RSC Adv., 2014, 4, 18683 RSC.
- Z. Chen, D. Pierre, H. He, S. Tan, C. Pham-Huy, H. Hong and J. Huang, Int. J. Pharm., 2011, 405, 153 CrossRef CAS PubMed.
- B. Permana, T. Ohba, T. Itoh and H. Kanoh, Colloids Surf., A, 2016, 490, 121 CrossRef CAS.
- R. Cheng and Y. Xue, Carbon. Nanomater. Biomed. Appl., Springer, 2016, p. 31 Search PubMed.
- K. Shikinaka, Polym. J., 2016, 48, 689–696 CrossRef CAS.
- H. Kakwere, M. P. Leal, M. E. Materia, A. Curcio, P. Guardia, D. Niculaes, R. Marotta, A. Falqui and T. Pellegrino, ACS Appl. Mater. Interfaces, 2015, 7, 10132 CAS.
- J. Pennakalathil, A. Özgün, I. Durmaz, R. L. Cetin-Atalay and D. N. S. Tuncel, J. Polym. Sci., Part A: Polym. Chem., 2015, 53, 114 CrossRef CAS.
- B. Newland, D. Leupelt, Y. Zheng, L. S. Thomas, C. Werner, M. Steinhart and W. Wang, Sci. Rep., 2015, 5, 17478 CrossRef CAS PubMed.
- U. A. Mikac, A. Sepe, S. Baumgartner and J. Kristl, Mol. Pharm., 2016, 13, 1147 CrossRef CAS PubMed.
- T. Kuang, Y. Liu, T. Gong, X. Peng, X. Hu and Z. Yu, Curr. Nanosci., 2016, 12, 38 CrossRef CAS.
- S. Bagherifard, A. Tamayol, P. Mostafalu, M. Akbari, M. Comotto, N. Annabi, M. Ghaderi, S. Sonkusale, M. R. Dokmeci and A. Khademhosseini, Adv. Healthcare Mater., 2016, 5, 175 CrossRef CAS PubMed.
- K. M. Rao, K. S. V. K. Rao and C.-S. Ha, Gels, 2016, 2, 6 CrossRef.
- W. Wang, L. Zhang, M. Liu, Y. Le, S. Lv, J. Wang and J.-F. Chen, RSC Adv., 2016, 6, 6368 RSC.
- G.-F. Luo, W.-H. Chen, Y. Liu, Q. Lei, R.-X. Zhuo and X.-Z. Zhang, Sci. Rep., 2014, 4, 6064 CrossRef CAS PubMed.
- Y.-W. Hu, Y.-Z. Du, N. Liu, X. Liu, T.-T. Meng, B.-L. Cheng, J.-B. He, J. You, H. Yuan and F.-Q. Hu, J. Controlled Release, 2015, 206, 91 CrossRef CAS PubMed.
- M. Andac, F. Plieva, A. Denizli, I. Y. Galaev and B. Mattiasson, Macromol. Chem. Phys., 2008, 209, 577 CrossRef CAS.
- C. Alvarez-Lorenzo and A. Concheiro, Chem. Commun., 2014, 50, 7743 RSC.
- Z. Su, J. Ye, Z. Qin and X. Ding, Sci. Rep., 2015, 5, 18314 CrossRef CAS PubMed.
- S.-M. Yuen, C.-C. M. Ma, Y.-Y. Lin and H.-C. Kuan, Compos. Sci. Technol., 2007, 67, 2564 CrossRef CAS.
- H. Wu, G. Liu, Y. Zhuang, D. Wu, H. Zhang, H. Yang, H. Hu and S. Yang, Biomaterials, 2011, 32, 4867 CrossRef CAS PubMed.
- M. A. F. R. Al-Khatib, M. E. S. Mirghani, I. Y. Qudsieh and I. A. Husain, J. Appl. Sci., 2010, 10, 2705 CrossRef CAS.
- J. Safari and S. Gandomi-Ravandi, RSC Adv., 2014, 4, 11486 RSC.
- T. M. Quynh, M. Yoneyamab, Y. Maki and T. Dobashi, J. Appl. Polym. Sci., 2012, 123, 2368 CrossRef CAS.
- Q. S. Zhang, L. S. Zha, J. H. Ma and B. R. Liang, J. Appl. Polym. Sci., 2007, 103, 2962 CrossRef CAS.
- P. Roach, D. J. McGarvey, M. R. Lees and C. Hoskins, Int. J. Mol. Sci., 2013, 14, 8585 CrossRef PubMed.
- G. Xu, R. Xia, H. Wang, X. Meng and Q. Zhu, Chin. Sci. Bull., 2008, 53, 2297 CAS.
- S. Khoee, Y. Bagheri and A. Hashemi, Nanoscale, 2015, 7, 4134 RSC.
- A. Demir, A. Baykal, H. Sözeri and R. Topkaya, Synth. Met., 2014, 187, 75 CrossRef CAS.
- B. Unal, A. Baykal, M. Senel and H. Sözeri, J. Inorg. Organomet., 2013, 23, 489 CrossRef CAS.
- S. Khoee and K. Hemati, Polymer, 2013, 54, 5574 CrossRef CAS.
- D.-S. Yang, D.-J. Jung and S.-H. Choi, Radiat. Phys. Chem., 2010, 79, 434 CrossRef CAS.
- F. Natalia, G. Stoychev, N. Puretskiy, I. Leonid and V. Dmitry, Eur. Polym. J., 2015, 68, 650 CrossRef CAS.
- D. F. Moyano, Y. Liu, D. Peer and V. M. Rotello, Small, 2016, 12, 76 CrossRef CAS PubMed.
- W. Xiong, W. Wang, Y. Wang, Y. Zhao, H. Chen, H. Xu and X. Yang, Colloids Surf., B, 2011, 84, 447 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2016 |