Highly fluorescent cucurbit[8]uril–perylenemonoimide host–guest complexes as efficient fluorescent probes for N-terminal phenylalanine†
Gyan Hari Aryal,
Liming Huang * and
Kenneth W. Hunter*
* and
Kenneth W. Hunter*
Department of Microbiology and Immunology, School of Medicine, University of Nevada, Reno, NV 89557, USA. E-mail: huang@medicine.nevada.edu
First published on 25th August 2016
Abstract
We report host–guest fluorescent complexes based on cucurbit[8]uril (CB[8]) and water-soluble perylenemonoimide PMI-1. The fluorescence of CB[8]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PMI-1 complexes reverses after the displacement with other guest molecules and they could serve as selective and sensitive probes for N-terminal phenylalanine peptides.
PMI-1 complexes reverses after the displacement with other guest molecules and they could serve as selective and sensitive probes for N-terminal phenylalanine peptides.
Perylene-based dyes (e.g. perylenediimides (PDIs) and perylenemonoimides (PMIs)) have attracted great attention due to their unique opto-electronic properties and excellent photo- and thermo-stability as well as their low toxicity.1,2 These dyes have been widely used in organic light-emitting diodes,3,4 organic field-effect transistors,5,6 organic photovoltaic solar cells,7,8 and liquid crystals.9,10 PMIs have particular interest over PDIs as fluorescent probes due to their longer-wavelength emission, larger Stokes shift, and lower reduction potential as well as amenability to extend perylene core into terrylene, quaterrylene, and pentarylene structures.11,12 We are interested in design and synthesis of water-soluble PMIs that have potential applications as organic solid materials and fluorescent probes.13–16 Unfortunately, the applications of PMIs as fluorescent probes have only been minimally explored for two main reasons. First, the synthesis of water-soluble PMIs with different hydrophilic side chains in large scales is still a challenge. Second, similar to PDIs, PMIs tend to aggregate and form non-fluorescent π–π stacks in aqueous solution. To efficiently reduce aggregation of PDIs, several strategies have been developed including the introduction of substituents in the core region or large dendrons at the side chains, however these modifications require substantial synthesis and purification.17–20 Another approach to prevent aggregation of PDIs in aqueous solution is to form host–guest complexes with macrocyclic hosts such as cyclodextrin (CD) and cucurbit[8] (CB[8]), and quantum dots through non-covalent interactions.21–23 However, these supramolecular interaction strategies have only been investigated and reported for PDIs. To the best of our knowledge, tuning the optical properties of PMIs using the host–guest strategy has not been reported. In this communication, we report supramolecular host–guest complexes based on a macrocyclic host CB[8] and a water-soluble fluorescent dye PMI-1 that exhibit a significant increase in fluorescence intensity upon encapsulation of PMI-1 with CB[8] with a moderate binding affinity (Ka = ≈1.3 × 104 M−1). The fluorescence intensity reverses after the displacement of PMI-1 with other guest molecules with a binding affinity in a broad range from 103 to 1011 M−1. This allows CB[8]
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PMI-1 complexes to serve as selective and sensitive fluorescent probes for different target molecules of interest.
PMI-1 complexes to serve as selective and sensitive fluorescent probes for different target molecules of interest.
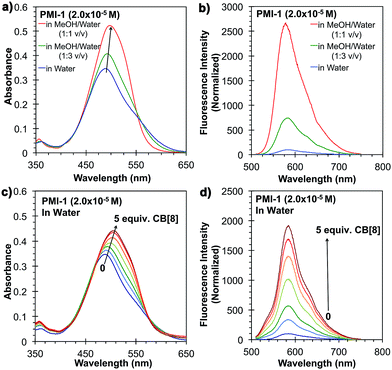
The synthesis of PMI-1 is outlined in Fig. 1a. First, compound 3 was synthesized from commercially available compound 1 in two steps with a overall yield of 76% using a previous reported procedure with some modifications.24 PMI-1 was obtained after a high-pressure reaction followed by the quaternization of the tertiary amino group of the side chain with 1-bromoethanol from 3. The quaternization of the tertiary amino group of 4 with 1-bromoethanol will not only increase the solubility of PMI-1 in polar organic solvents (e.g. methanol and DMSO) and aqueous solution (H2O and buffers), but also enhance the fluorescence of PMI-1 by eliminating intramolecular photoinduced electron transfer (PET) from the amine nitrogen lone-pair electrons to the aromatic ring.15 In addition, the free hydroxyl group on the side chain allows further modification or conjugation of PMI-1 with other molecules of interest without significant changes in spectral properties. In water, the λmax of absorption of PMI-1 is about 490 nm and the excitation of PMI-1 at 490 nm resulted in very low fluorescence with maximum emission intensity at ∼570 nm owing to self-quenching of PMI-1 aggregates in water (Fig. 2a and b). The quantum yield of PMI-1 (2 × 10−5 M) in water was determined to be around 0.01 without consideration of reabsorption due to high concentration while it is about 0.5 in pure MeOH. The fluorescence lifetime of PMI-1 in water (non-aggregated residue) and MeOH was determined to be 4.7 and 5.2 ns, respectively (Fig. S9†). In a mixed MeOH and water solvent, the absorption spectrum is red-shifted about 10 nm with an increased absorptivity. The fluorescence intensity increased approximately 8 and 27 times in the presence of 25% and 50% MeOH in water, respectively. The observation of the blue-shifted absorption combined with the significantly self-quenched fluorescence clearly indicates the formation of PMI-1 aggregates in water. The dramatic increase of the fluorescence intensity of PMI-1 in methanol or the mixture of methanol and water suggests efficient deaggregation of PMI-1.
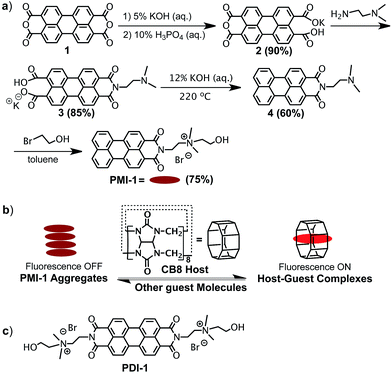 | ||
Fig. 1 (a) Synthesis of PMI-1; (b) schematic illustration of the formation of the host–guest CB[8]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PMI-1 complex; (c) the structure of PDI-1. PMI-1 complex; (c) the structure of PDI-1. | ||
The supramolecular approach to prevent aggregation of PMI-1 in water was demonstrated using a macrocyclic host cucurbit[8] (CB[8]) that contains eight repeating methylene-bridged glycoluril units with hydrophilic portals and a nonpolar hydrophobic cavity that is suitable for encapsulating the large aromatic perylene-core (Fig. 1b).23 To determine if the formation of CB[8]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PMI-1 host–guest complexes would cause deaggregation of PMI-1, 1H-NMR, ESI-MS, and UV-vis and fluorescence spectroscopy methods were used. 1H-NMR spectrum of PMI-1 (1.0 × 10−4 M) in D2O showed three aromatic peaks at 7.308, 7.460, and 7.637 ppm (Fig. S6†). Upon the addition of 5.0 equivalents of CB[8], the aromatic peaks shifted downfield to 7.386, 7.788, and to 8.046 indicating the de-aggregation of PMI-1.23 It is important to note that the downfield shift of aromatic peaks contributes to the combined effects of deaggregation and CB[8] cavity encapsulation. The presence of CB[8]
PMI-1 host–guest complexes would cause deaggregation of PMI-1, 1H-NMR, ESI-MS, and UV-vis and fluorescence spectroscopy methods were used. 1H-NMR spectrum of PMI-1 (1.0 × 10−4 M) in D2O showed three aromatic peaks at 7.308, 7.460, and 7.637 ppm (Fig. S6†). Upon the addition of 5.0 equivalents of CB[8], the aromatic peaks shifted downfield to 7.386, 7.788, and to 8.046 indicating the de-aggregation of PMI-1.23 It is important to note that the downfield shift of aromatic peaks contributes to the combined effects of deaggregation and CB[8] cavity encapsulation. The presence of CB[8]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PMI-1 1
PMI-1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex was confirmed by ESI-MS (observed m/z = 1766.2 Da, calculated m/z = 1766.2 Da) (Fig. S7†). Similar to the deaggregation study of PMI-1 in MeOH and water mixture, the addition of CB[8] to PMI-1 (2 × 10−5 M) in aqueous solution resulted in a red-spectral shift with increased absorptivity (Fig. 2c). The fluorescence intensity at λmax increases as the concentration of CB[8] increases. 5-Fold and 19-fold increases in fluorescence intensity were achieved in the presence of 1.0 and 5.0 equivalents of CB[8], respectively (Fig. 2d). In a control experiment, the addition of excess CB[7] did not cause dramatic changes in fluorescence intensity (Fig. S8†), suggesting an insufficient cavity size to encapsulate the large aromatic perylene-core. The quantum yield of PMI-1 in the presence of 5.0 equivalents of CB[8] was determined to be ∼0.22, while it was 0.01 for the free dye PMI-1 in water and 0.50 in methanol. The fluorescence lifetime of PMI-1 increased from 4.7 ns to 5.4 ns upon addition of 5.0 equivalents of CB[8] (Fig. S9†). The previous study has shown that a water-soluble PDI-1 with two positively charged side-chains can be encapsulated by CB[8] in its cavity and the binding constant was determined to be ca. 105 M−1 (Fig. 1c).23 It is known that the binding of CB[8] and PDI-1 involves two different interactions: (1) the hydrophobic interaction between the perylene-core of PDI-1 and the CB[8] cavity and (2) the electrostatic interaction between two cationic side-chains of PDI-1 and the partial negatively charged CB[8] portal.23 Compared to the structure of PDI-1, PMI-1 has a similar perylene-core that will have a hydrophobic interaction with the CB[8] cavity. In contrast, PMI-1 has only one cationic side-chain that may result in less electrostatic interaction with the partial negatively charged CB[8] portal. Thus, we anticipated that the overall bind affinity of CB[8] with PMI-1 will be lower than that of CB[8] with PDI-1. As expected, the binding affinity (Ka) of CB[8]
1 complex was confirmed by ESI-MS (observed m/z = 1766.2 Da, calculated m/z = 1766.2 Da) (Fig. S7†). Similar to the deaggregation study of PMI-1 in MeOH and water mixture, the addition of CB[8] to PMI-1 (2 × 10−5 M) in aqueous solution resulted in a red-spectral shift with increased absorptivity (Fig. 2c). The fluorescence intensity at λmax increases as the concentration of CB[8] increases. 5-Fold and 19-fold increases in fluorescence intensity were achieved in the presence of 1.0 and 5.0 equivalents of CB[8], respectively (Fig. 2d). In a control experiment, the addition of excess CB[7] did not cause dramatic changes in fluorescence intensity (Fig. S8†), suggesting an insufficient cavity size to encapsulate the large aromatic perylene-core. The quantum yield of PMI-1 in the presence of 5.0 equivalents of CB[8] was determined to be ∼0.22, while it was 0.01 for the free dye PMI-1 in water and 0.50 in methanol. The fluorescence lifetime of PMI-1 increased from 4.7 ns to 5.4 ns upon addition of 5.0 equivalents of CB[8] (Fig. S9†). The previous study has shown that a water-soluble PDI-1 with two positively charged side-chains can be encapsulated by CB[8] in its cavity and the binding constant was determined to be ca. 105 M−1 (Fig. 1c).23 It is known that the binding of CB[8] and PDI-1 involves two different interactions: (1) the hydrophobic interaction between the perylene-core of PDI-1 and the CB[8] cavity and (2) the electrostatic interaction between two cationic side-chains of PDI-1 and the partial negatively charged CB[8] portal.23 Compared to the structure of PDI-1, PMI-1 has a similar perylene-core that will have a hydrophobic interaction with the CB[8] cavity. In contrast, PMI-1 has only one cationic side-chain that may result in less electrostatic interaction with the partial negatively charged CB[8] portal. Thus, we anticipated that the overall bind affinity of CB[8] with PMI-1 will be lower than that of CB[8] with PDI-1. As expected, the binding affinity (Ka) of CB[8]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PMI-1 as determined by fluorescence titration was calculated to be ∼1.3 × 104 using a 1
PMI-1 as determined by fluorescence titration was calculated to be ∼1.3 × 104 using a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding model in the Origin 9.1 program (OriginLab Corporation) (Fig. S10†). In comparison, the Ka of CB[8]
1 binding model in the Origin 9.1 program (OriginLab Corporation) (Fig. S10†). In comparison, the Ka of CB[8]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PDI-1 as determined by fluorescence titration was calculated to be ∼7.9 × 104 using same program (Fig. S11†).
PDI-1 as determined by fluorescence titration was calculated to be ∼7.9 × 104 using same program (Fig. S11†).
The reversibility of CB[8]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PMI-1 complex was investigated with the well-known strong CB[8] binders adamantylamine (AD, Ka = 8.2 × 108 M−1)25 and methyl viologen (MV, Ka = 8.5 × 105 M−1) (Fig. 3a and b).26 When one equivalent of AD or MV was added to an aqueous solution of CB[8]
PMI-1 complex was investigated with the well-known strong CB[8] binders adamantylamine (AD, Ka = 8.2 × 108 M−1)25 and methyl viologen (MV, Ka = 8.5 × 105 M−1) (Fig. 3a and b).26 When one equivalent of AD or MV was added to an aqueous solution of CB[8]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PMI-1 (1
PMI-1 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2 × 10−5 M) complexes, the UV-vis spectrum slightly shifted toward blue that is similar to the absorbance of PMI-1 aggregates in water (Fig. S12†), and the fluorescence intensity dropped to the level of PMI-1 in the absence of CB[8] (Fig. 4). The above spectral changes suggest complete displacement of PMI-1 by CB[8] strong binders AD and MV. These studies suggested that CB[8]
1, 2 × 10−5 M) complexes, the UV-vis spectrum slightly shifted toward blue that is similar to the absorbance of PMI-1 aggregates in water (Fig. S12†), and the fluorescence intensity dropped to the level of PMI-1 in the absence of CB[8] (Fig. 4). The above spectral changes suggest complete displacement of PMI-1 by CB[8] strong binders AD and MV. These studies suggested that CB[8]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PMI-1 complexes could serve as a sensitive probe for strong binding guests. To explore the further sensing ability of CB[8]
PMI-1 complexes could serve as a sensitive probe for strong binding guests. To explore the further sensing ability of CB[8]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PMI-1 complexes, we examined several aromatic amino acids: phenylalanine (Phe), tryptophan (Trp) and tyrosine (Tyr) with lower binding affinity for CB[8] (103 to 104 M−1).27 As shown in Fig. S13 and S14,† the presence of one equivalent of Trp or Tyr resulted in ∼30% and ∼5% decrease in the fluorescence intensity of CB[8]
PMI-1 complexes, we examined several aromatic amino acids: phenylalanine (Phe), tryptophan (Trp) and tyrosine (Tyr) with lower binding affinity for CB[8] (103 to 104 M−1).27 As shown in Fig. S13 and S14,† the presence of one equivalent of Trp or Tyr resulted in ∼30% and ∼5% decrease in the fluorescence intensity of CB[8]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PMI-1 (1
PMI-1 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), but no significant change in absorbance was observed (Fig. S15 and S16†). This finding suggests displacement of PMI-1 by Trp and Tyr without formation of charge transfer 1
1), but no significant change in absorbance was observed (Fig. S15 and S16†). This finding suggests displacement of PMI-1 by Trp and Tyr without formation of charge transfer 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ternary complexes. Interestingly, in the presence of one equivalent of Phe, an ∼25% increase in fluorescence intensity and a red spectral-shift of absorption were observed suggesting the formation of 1
1 ternary complexes. Interestingly, in the presence of one equivalent of Phe, an ∼25% increase in fluorescence intensity and a red spectral-shift of absorption were observed suggesting the formation of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 charge transfer ternary complexes (Fig. S17 and S18†).23 The increase in fluorescence intensity may contribute to the combined effects of further deaggregation and quenching by phenylalanine. The formation of 1
1 charge transfer ternary complexes (Fig. S17 and S18†).23 The increase in fluorescence intensity may contribute to the combined effects of further deaggregation and quenching by phenylalanine. The formation of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexes was further investigated by 1H-NMR using equimolar concentration of Phe with CB[8]
1 complexes was further investigated by 1H-NMR using equimolar concentration of Phe with CB[8]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PMI-1. The broadening of aromatic protons of PMI-1 and Phe moiety may be due to the formation of Phe
PMI-1. The broadening of aromatic protons of PMI-1 and Phe moiety may be due to the formation of Phe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CB[8]
CB[8]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PMI-1 complexes (Fig. S25†). In comparison to tryptophan and tyrosine, the greater hydrophobicity and smaller size of the phenyl group of Phe could be the driving factor for the formation of 1
PMI-1 complexes (Fig. S25†). In comparison to tryptophan and tyrosine, the greater hydrophobicity and smaller size of the phenyl group of Phe could be the driving factor for the formation of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ternary complex.
1 ternary complex.
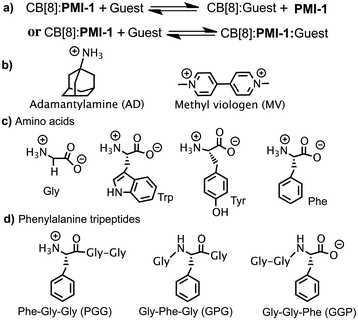 | ||
Fig. 3 (a) The scheme of the displacement of CB[8]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PMI-1; structures of (b) AD and MV; (c) amino acids; (d) phenylalanine tripeptides. PMI-1; structures of (b) AD and MV; (c) amino acids; (d) phenylalanine tripeptides. | ||
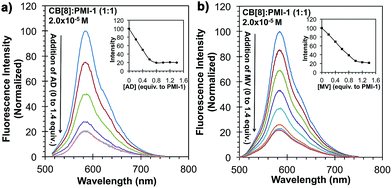 | ||
Fig. 4 Fluorescence emission of PMI-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CB[8] (1 CB[8] (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) complexes (2.0 × 10−5 M) in water in the presence of (a) AD and (b) MV. 1) complexes (2.0 × 10−5 M) in water in the presence of (a) AD and (b) MV. | ||
Host–guest complexes based on CB[8] are potentially useful for sensing and separating specific peptides. The recognition of N-terminal di- and tri-peptides using CB[8]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MBBI (tetramethylbenzobis(imidazolium)) and CB[8]
MBBI (tetramethylbenzobis(imidazolium)) and CB[8]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MV complexes has been recently reported.26–29 CB[8]
MV complexes has been recently reported.26–29 CB[8]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MV complexes have been applied to differentiate N-terminal, C-terminal, and internal tryptophan isomers.26 However it is known that MV is a toxic compound and its absorption and fluorescence emission are in the violet-blue light region. An alternative fluorescent indicator MBBI was used for recognition of tyrosine tripeptides, but CB[8]
MV complexes have been applied to differentiate N-terminal, C-terminal, and internal tryptophan isomers.26 However it is known that MV is a toxic compound and its absorption and fluorescence emission are in the violet-blue light region. An alternative fluorescent indicator MBBI was used for recognition of tyrosine tripeptides, but CB[8]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MBBI showed very little fluorescence changes in peptide recognition.28,29 Thus, the development of highly selective and sensitive fluorescent probes based on host–guest complexes of CB[8] and a fluorescence dye with longer wavelength absorption and emission for small peptides is still desirable.
MBBI showed very little fluorescence changes in peptide recognition.28,29 Thus, the development of highly selective and sensitive fluorescent probes based on host–guest complexes of CB[8] and a fluorescence dye with longer wavelength absorption and emission for small peptides is still desirable.
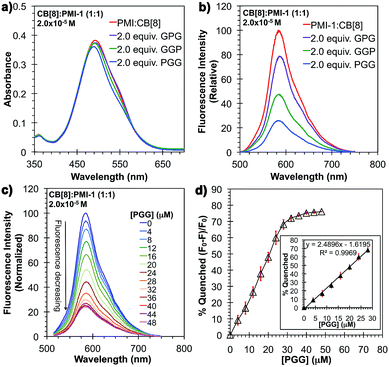
To demonstrated the molecular recognition of tripeptides using CB[8]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PMI-1 complexes, three tri-peptide sequences (Phe–Gly–Gly, Gly–Phe–Gly, Gly–Gly–Phe) of phenylalanine with the non-binding moiety glycine (Gly) were designed (Fig. 3d). As shown in Fig. 5a and b, in the presence of 2.0 equivalents of PGG, the absorption spectrum shifted ∼10 nm toward blue and the fluorescence intensity decreased to approximately 25% of the initial intensity (or 75% quenching in fluorescence intensity). This suggests the complete displacement of PMI-1 from CB[8]
PMI-1 complexes, three tri-peptide sequences (Phe–Gly–Gly, Gly–Phe–Gly, Gly–Gly–Phe) of phenylalanine with the non-binding moiety glycine (Gly) were designed (Fig. 3d). As shown in Fig. 5a and b, in the presence of 2.0 equivalents of PGG, the absorption spectrum shifted ∼10 nm toward blue and the fluorescence intensity decreased to approximately 25% of the initial intensity (or 75% quenching in fluorescence intensity). This suggests the complete displacement of PMI-1 from CB[8]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PMI-1 by 2.0 equivalents of PGG to form more stable CB[8]
PMI-1 by 2.0 equivalents of PGG to form more stable CB[8]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2PGG (1
2PGG (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) complexes. To confirm that, 1H-NMR spectra of PGG, CB[8]
2) complexes. To confirm that, 1H-NMR spectra of PGG, CB[8]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2PGG, CB[8]
2PGG, CB[8]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PMI-1 + 2PGG, and PMI-1 in D2O were further investigated, respectively (Fig. S19†). The 1H-NMR spectrum of CB[8]
PMI-1 + 2PGG, and PMI-1 in D2O were further investigated, respectively (Fig. S19†). The 1H-NMR spectrum of CB[8]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PMI-1 + 2PGG shows two sets of peaks for aromatic protons. One set of peaks is shifted to upfield that is corresponding to the protons of PGG (Fig. S19b and c†) and the other set of peaks matches with the aromatic protons of PMI-1 aggregates in D2O (Fig. S19c and d†). This suggests the complete displacement of PMI-1 by PGG. The formation of CB[8]
PMI-1 + 2PGG shows two sets of peaks for aromatic protons. One set of peaks is shifted to upfield that is corresponding to the protons of PGG (Fig. S19b and c†) and the other set of peaks matches with the aromatic protons of PMI-1 aggregates in D2O (Fig. S19c and d†). This suggests the complete displacement of PMI-1 by PGG. The formation of CB[8]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2PGG complexes was further confirmed by ESI+ of CB[8]
2PGG complexes was further confirmed by ESI+ of CB[8]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PMI-1 + 2PGG ([CB[8]
PMI-1 + 2PGG ([CB[8]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2PGG]2+: calculated 943.84, found 943.89). To further confirm the stoichiometry of the CB[8]
2PGG]2+: calculated 943.84, found 943.89). To further confirm the stoichiometry of the CB[8]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2PGG complexes, we obtained the Job plot using UV-vis spectroscopy (Fig. S27†). The Job plot shows a maximum at a molar fraction of guest equal to 0.67, indicating the 1
2PGG complexes, we obtained the Job plot using UV-vis spectroscopy (Fig. S27†). The Job plot shows a maximum at a molar fraction of guest equal to 0.67, indicating the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 host–guest complexes. In contrast, 1H-NMR spectrum of CB[8] and GPG shows a broad aromatic peak presumably due to the mixture of bound and unbound Phe moiety with CB[8] (Fig. S20†). The ESI+ indicates the presence of CB[8]
2 host–guest complexes. In contrast, 1H-NMR spectrum of CB[8] and GPG shows a broad aromatic peak presumably due to the mixture of bound and unbound Phe moiety with CB[8] (Fig. S20†). The ESI+ indicates the presence of CB[8]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2GPG and CB[8]
2GPG and CB[8]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2GGP complexes (Fig. S23 and S24†). These results shown above are consistent with the previous report that shows a binding affinity of Ka = 1.5 × 1011 M−2 for CB[8]
2GGP complexes (Fig. S23 and S24†). These results shown above are consistent with the previous report that shows a binding affinity of Ka = 1.5 × 1011 M−2 for CB[8]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2PGG (1
2PGG (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) complexes which is much higher than the binding affinity of CB[8]
2) complexes which is much higher than the binding affinity of CB[8]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PMI-1 (1.3 × 104 M−1).30 Compared with PGG, the presence of 2.0 equivalents of GGP and GPG only resulted in approximately 50% and 25% quenching in fluorescence intensity respectively, and the changes in absorbance are consistent with the changes in fluorescence (Fig. 5a and b). The selectivity of PGG over GPG was further confirmed by the 1H-NMR and fluorescence titration using mixtures of PGG and GPG with CB[8]
PMI-1 (1.3 × 104 M−1).30 Compared with PGG, the presence of 2.0 equivalents of GGP and GPG only resulted in approximately 50% and 25% quenching in fluorescence intensity respectively, and the changes in absorbance are consistent with the changes in fluorescence (Fig. 5a and b). The selectivity of PGG over GPG was further confirmed by the 1H-NMR and fluorescence titration using mixtures of PGG and GPG with CB[8]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PMI-1 complexes. As shown in Fig. S21,† the 1H-NMR spectrum of the mixture shows two sets of peaks for aromatic protons. One set of peaks is shifted to upfield that is corresponding to the protons of PGG and the other set of broad peaks is corresponding to GPG. Furthermore, the fluorescence titration study of CB[8]
PMI-1 complexes. As shown in Fig. S21,† the 1H-NMR spectrum of the mixture shows two sets of peaks for aromatic protons. One set of peaks is shifted to upfield that is corresponding to the protons of PGG and the other set of broad peaks is corresponding to GPG. Furthermore, the fluorescence titration study of CB[8]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PMI-1 in the presence of mixtures of PGG and GPG suggests more efficient displacement of PMI-1 by PGG (Fig. S26†). Based on the results shown above, we conclude that the displacement efficiency of PMI-1 from CB[8]
PMI-1 in the presence of mixtures of PGG and GPG suggests more efficient displacement of PMI-1 by PGG (Fig. S26†). Based on the results shown above, we conclude that the displacement efficiency of PMI-1 from CB[8]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PMI-1 complexes and the binding affinity of CB[8]
PMI-1 complexes and the binding affinity of CB[8]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) peptide (1
peptide (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) complexes are in the order of PGG > GGP > GPG. The N-terminal peptide PGG has the strongest binding with CB[8] presumably due to the stronger electrostatic interaction between positively charged ammonium ions adjacent to the phenyl group and the partial negatively charged CB[8] portal.23,31 The lack of electrostatic interaction with the CB[8] portal decreases the extent of dimer formation of the C-terminal peptide GGP. The weaker binding of internal peptide GPG could be due to the steric effect on the formation of the GPG dimer inside of CB[8] cavity. The fluorescence assay of N-terminal phenylalanine tri-peptide PGG was further demonstrated using CB[8]
2) complexes are in the order of PGG > GGP > GPG. The N-terminal peptide PGG has the strongest binding with CB[8] presumably due to the stronger electrostatic interaction between positively charged ammonium ions adjacent to the phenyl group and the partial negatively charged CB[8] portal.23,31 The lack of electrostatic interaction with the CB[8] portal decreases the extent of dimer formation of the C-terminal peptide GGP. The weaker binding of internal peptide GPG could be due to the steric effect on the formation of the GPG dimer inside of CB[8] cavity. The fluorescence assay of N-terminal phenylalanine tri-peptide PGG was further demonstrated using CB[8]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PMI-1 (1
PMI-1 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2.0 × 10−5 M) complexes. As shown in Fig. 5c, the fluorescence intensity gradually decreases as the concentration of PGG increases from 0 to 28 μM due to the gradual increase of self-quenched PMI-1 aggregates after the displacement of PMI-1 by PGG dimers (Fig. 5c). A linear titration curve (quenched fluorescence percentage vs. the concentration of PGG) can be obtained in the range of 0 to 28 μM and a detection limit of 4.0 μM can be achieved (Fig. 5d).
1, 2.0 × 10−5 M) complexes. As shown in Fig. 5c, the fluorescence intensity gradually decreases as the concentration of PGG increases from 0 to 28 μM due to the gradual increase of self-quenched PMI-1 aggregates after the displacement of PMI-1 by PGG dimers (Fig. 5c). A linear titration curve (quenched fluorescence percentage vs. the concentration of PGG) can be obtained in the range of 0 to 28 μM and a detection limit of 4.0 μM can be achieved (Fig. 5d).
In conclusion, we have designed and synthesized a water-soluble perylene-monoimide PMI-1 that forms non-fluorescent aggregates in aqueous solutions. The presence of a macrocyclic host CB[8] resulted the formation of host–guest CB[8]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PMI-1 complexes after the encapsulation of PMI-1 with CB[8], and the fluorescence intensity increased as high as 19-fold due to the deaggregation of PMI-1 aggregates. The fluorescence of CB[8]
PMI-1 complexes after the encapsulation of PMI-1 with CB[8], and the fluorescence intensity increased as high as 19-fold due to the deaggregation of PMI-1 aggregates. The fluorescence of CB[8]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PMI-1 can be reversed by the displacement of PMI-1 with a wide range of guest molecules with a binding affinity from 103 to 1011 M−1 such as AD, MV, and aromatic amino acids. Additionally, CB[8]
PMI-1 can be reversed by the displacement of PMI-1 with a wide range of guest molecules with a binding affinity from 103 to 1011 M−1 such as AD, MV, and aromatic amino acids. Additionally, CB[8]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PMI-1 complexes could serve as sensitive and selective fluorescence probes for N-terminal phenylalanine peptides. To the best of our knowledge, this is the first example of host–guest complexes based on CB[8] and perylenemonoimides (PMIs). We believe that the binding affinity of CB[8] with PMIs can be tuned by the structural modifications of PMIs. Our future studies will include the study of sensitivity and selectivity of CB[8]
PMI-1 complexes could serve as sensitive and selective fluorescence probes for N-terminal phenylalanine peptides. To the best of our knowledge, this is the first example of host–guest complexes based on CB[8] and perylenemonoimides (PMIs). We believe that the binding affinity of CB[8] with PMIs can be tuned by the structural modifications of PMIs. Our future studies will include the study of sensitivity and selectivity of CB[8]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PMI-1 complexes for other N-terminal peptides, the design and synthesis of PMI derivatives with different functional groups on the 9-position of the core and the side chain, and the studies of the effect of substitutes on the binding affinity with CB[8].
PMI-1 complexes for other N-terminal peptides, the design and synthesis of PMI derivatives with different functional groups on the 9-position of the core and the side chain, and the studies of the effect of substitutes on the binding affinity with CB[8].
Acknowledgements
We acknowledge the financial support from Bio-Med Scientific (Grant No. 1310043-01) and National Science Foundation for partial support.References
- S. Chen, P. Slattum, C. Wang and L. Zhang, Chem. Soc. Rev., 2015, 115, 11967–11998 CrossRef CAS PubMed.
- S. Bai, S. Debnath, N. Javid, P. W. J. M. Frederix, S. Fleming, C. Pappas and R. V. Ulijin, Langmuir, 2014, 30, 7576–7584 CrossRef CAS PubMed.
- S.-C. Lo and P. L. Burn, Chem. Rev., 2007, 107, 1097–1116 CrossRef CAS PubMed.
- T. Qin, J. Ding, L. Wang, M. Baumgarten, G. Zhou and K. Mullen, J. Am. Chem. Soc., 2009, 131, 14329–14336 CrossRef CAS PubMed.
- A. L. Briseno, S. C. B. Mannsfeld, C. Reese, J. M. Hancock, Y. Xiong, S. A. Jenekhe, Z. Bao and Y. Xia, Nano Lett., 2007, 7, 2847–2853 CrossRef CAS PubMed.
- X. Zhan, J. Zhang, S. Tang, Y. Lin, M. Zhao, J. Yang, H.-L. Zhang, Q. Peng, G. Yu and Z. Li, Chem. Commun., 2015, 51, 7156–7159 RSC.
- C. Li and H. Wonneberger, Adv. Mater., 2012, 24, 613–636 CrossRef CAS PubMed.
- D. N. Congreve, J. Lee, N. J. Thompson, E. Hontz, S. R. Yost, P. D. Reusswig, M. E. Bahlke, S. Reineke, T. Van Voorhis and M. A. Baldo, Science, 2013, 340, 334–337 CrossRef CAS PubMed.
- S.-W. Tam-Chang and L. Huang, Chem. Commun., 2008, 44, 1957–1967 RSC.
- F. Wurthner, C. R. Saha-Moller, B. Fimmel, S. Ogi, P. Leowanawat and D. Schmidt, Chem. Rev., 2016, 116, 962–1052 CrossRef PubMed.
- K.-R. Wang, D.-S. Guo, B.-P. Jiang and Y. Liu, Chem. Commun., 2012, 48, 3644–3646 RSC.
- Z. Sun, Q. Ye, C. Chi and J. Wu, Chem. Soc. Rev., 2012, 41, 7857–7889 RSC.
- L. Huang, V. J. Catalano and S.-W. Tam-Chang, Chem. Commun., 2007, 43, 2016–2018 RSC.
- L. Huang, S.-W. Tam-Chang, W. Seo and K. Rove, Adv. Mater., 2007, 19, 4149–4152 CrossRef CAS.
- L. Huang and S.-W. Tam-Chang, J. Fluoresc., 2011, 21, 213–222 CrossRef CAS PubMed.
- L. Huang and S.-W. Tam-Chang, Chem. Commun., 2011, 47, 2291–2293 RSC.
- C. Kohl, T. Weil, J. Qu and K. Mullen, Chem.–Eur. J., 2004, 10, 5297–5310 CrossRef CAS PubMed.
- S. Rehm, V. Stepanenko, X. Zhang, T. H. Rehm and F. Wurthner, Chem.–Eur. J., 2010, 16, 3372–3382 CrossRef CAS PubMed.
- T. Heek, C. Fasting, C. Rest, X. Zhang, F. Wurthner and R. Haag, Chem. Commun., 2011, 47, 3894–3896 RSC.
- B. Gao, H. Li, H. Liu, L. Zhang, Q. Bai and X. Ba, Chem. Commun., 2010, 46, 1884–1886 RSC.
- M. Zhu, G. H. Aryal, N. Zhang, H. Zhang, X. Su, R. Schmehl, X. Liu, J. Hu, J. Wei and J. Jayawickramarajah, Langmuir, 2015, 31, 578–586 CrossRef CAS PubMed.
- G. H. Aryal, L. Huang and K. W. Hunter, RSC Adv., 2016, 6, 76448–76452 RSC.
- F. Biedermann, E. Elmalen, I. Ghos, W. M. Nau and O. A. Sherman, Angew. Chem., Int. Ed., 2012, 51, 7739–7743 CrossRef CAS PubMed.
- S.-W. Tam-Chang, W. Seo and I. K. Iverson, J. Org. Chem., 2004, 69, 2719–2726 CrossRef CAS PubMed.
- L. Cao, M. Šekutor, P. Y. Zavalij, K. Mlinarić-Majerski, R. Glaser and L. Isaacs, Angew. Chem., Int. Ed., 2014, 53, 988–993 CrossRef CAS PubMed.
- M. E. Bush, N. D. Bouley and A. R. Urbach, J. Am. Chem. Soc., 2005, 127, 14511–14517 CrossRef CAS PubMed.
- P. Rajgariah and A. R. Urbach, J. Inclusion Phenom. Macrocyclic Chem., 2008, 62, 251–254 CrossRef CAS.
- F. Biedermann, U. Rauwald, M. Cziferszky, K. A. Williams, L. D. Gann, B. Y. Guo, A. R. Urabach, C. W. Bielawski and O. A. Sherman, Chem.–Eur. J., 2010, 16, 13716–13722 CrossRef CAS PubMed.
- L. C. Smith, D. G. Leach, B. E. Blaylock, O. A. Ali and A. R. Urbach, J. Am. Chem. Soc., 2015, 137, 3663–3669 CrossRef CAS PubMed.
- L. M. Heitmann, A. B. Taylor, P. J. Hart and A. R. Urbach, J. Am. Chem. Soc., 2006, 128, 12574–14581 CrossRef CAS PubMed.
- G. Ghale, V. Ramalingam, A. R. Urbach and W. M. Nau, J. Am. Chem. Soc., 2011, 133, 7528–7535 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra18012d |
| This journal is © The Royal Society of Chemistry 2016 |