Influence of thermophilic Bacillus subtilis YB7 on the biodegradation of long chain paraffinic hydrocarbons (C16H34 to C36H74)†
Abstract
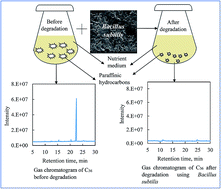
The long chain paraffinic hydrocarbons (waxes) present in crude oil pose serious issues in the upstream oil and gas industries. The waxy hydrocarbons are reflected in their resistance to biodegradation when released into the environment. Microbial degradation of these paraffins is an alternative way to address the various issues effectively and economically. The present study investigates the utilization of thermophilic Bacillus subtilis isolated from Chennai, India for the degradation of waxes, such as n-hexadecane, n-eicosane, n-tetracosane, n-octacosane, n-dotriacontane and n-hexatriacontane. Several experiments have been conducted to observe the efficiency of Bacillus subtilis at various operating temperatures and concentrations of paraffins. We inspected the primary degradability of paraffins using gas chromatography mass spectrometry and ultimate biodegradability using viscosity analysis. The degradation of paraffins at 50 °C was observed to be 60 to 77% in one day, and 78 to 98% in ten days. The performance of Bacillus subtilis at 50 °C was observed to be higher than the performance observed at 35 and 75 °C. The viscosity of paraffins has reduced with decrease in carbon number and increase in degradation time. The functional groups present after the biodegradation were investigated using Fourier transform infrared spectroscopy. We also observed that the energy required for the activation of paraffin degradation increases with decrease in enzymatic activity and increase in carbon number. The current study with the information on the percentage degradation and viscosity reduction during the degradation of various long chain paraffins will add value in the development of a robust model for microbial degradation of complex mixtures of hydrocarbon systems suitable for upstream oil and gas applications.

 Please wait while we load your content...
Please wait while we load your content...